Public Health Risks Associated with Heavy Metal and Microbial Contamination of Drinking Water in Australia
Abstract
1. Introduction
2. Lead Levels in Drinking Water in Australia and Their Origin
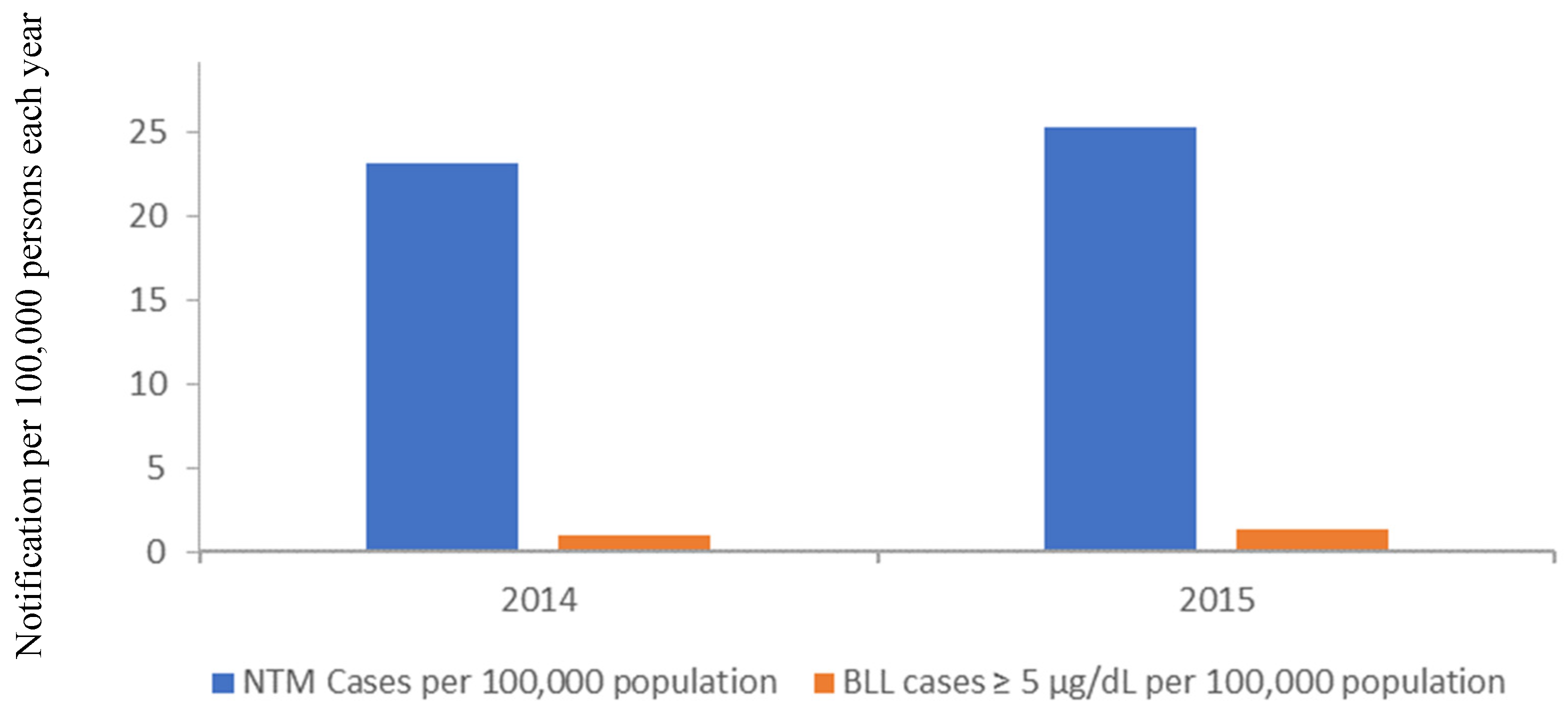
3. Contribution of Lead in Drinking Water to Overall Lead Body Burdens
4. Opportunistic Premise Plumbing Pathogens (OPPPs)
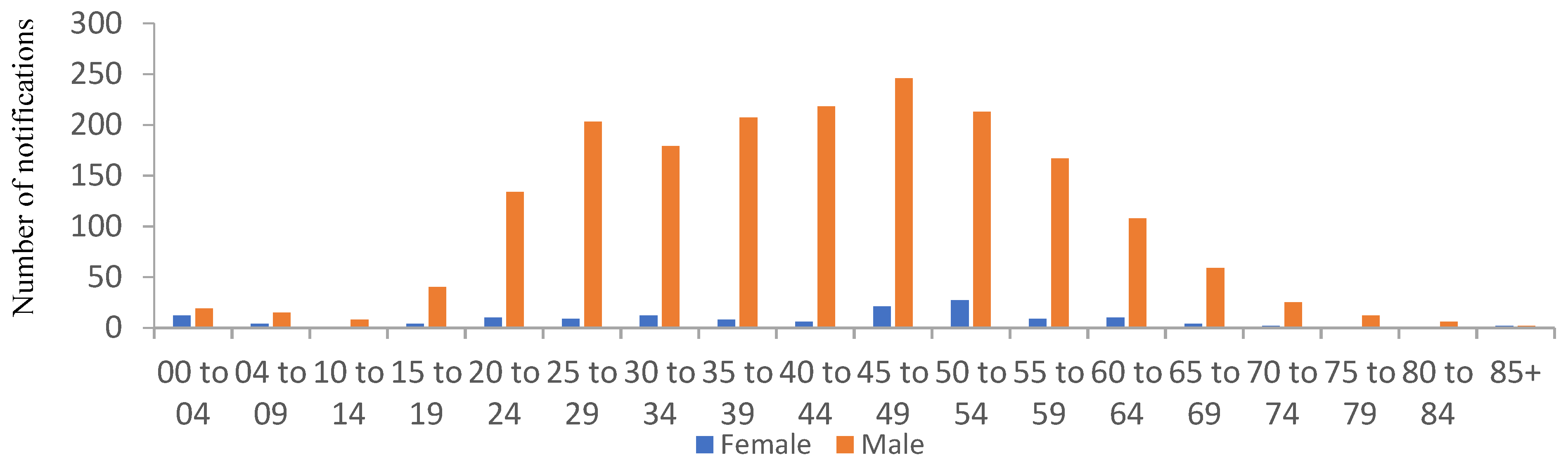
Non-Tuberculous Mycobacterium (NTM)
5. Plumbing Material
Plumbing Material Quality and Compliance
6. Conclusions and Recommendations
- National and global data does not support that brass fittings are the only source of lead contamination, and should be viewed in respect to the associated human health risk;
- Replacement of existing Watermarked fittings introduces a significant capital cost;
- The potential perceived benefits from the replacement of brass fittings is not balanced against the potential for increased microbial risks from OPPPs;
- The risk of lead exposure is much higher in rainwater compared to municipal water supplies and changes in regulation should reflect this.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Neal, M. Brass Fittings Leaching Lead in Drinking Fountains, But Daily use in Households a Larger Concern (ABC News 2018). Available online: https://www.abc.net.au/news/2018-07-06/lead-leached-by-brass-shuts-fountains-but-what-about-homes/9886518 (accessed on 13 September 2019).
- Laschon, E. Perth Children’s Hospital Lead Contamination in Water Pipes Supplying Site, Audit Finds (ABC News 2017). Available online: https://www.abc.net.au/news/2017-04-24/building-commission-audit-into-perth-childrens-hospital/8466882 (accessed on 13 September 2019).
- Kines, L. Greater Victoria Schoolds Get Lead out of Water with New Drinking Fountains (Times Colonist, 2018). Available online: https://www.timescolonist.com/news/local/greater-victoria-schools-get-lead-out-of-water-with-new-drinking-fountains-1.23422846 (accessed on 13 September 2019).
- Edwards, M. Fetal death and reduced birth rates associated with exposure to lead-contaminated drinking water. Environ. Sci. Technol. 2014, 48, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Triantafyllidou, S.; Best, D. Elevated blood lead in young children due to lead-contaminated drinking water: Washington, dc, 2001–2004. Environ. Sci. Technol. 2009, 43, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Attisha, M.; Lachance, J.; Sadler, R.C.; Champney Schnepp, A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am. J. Public Health 2016, 106, 283–290. [Google Scholar] [CrossRef]
- enHealth. enHealth Guidance Statement: Lead in Drinking Water from Some Plumbing Products; enHealth: Canberra, Australia, 2018.
- Victorian School Building Authority. Building Quality Standards Handbook; Department of Education and Training, State of Victoria: Melbourne, Australia, 2019.
- Feazel, L.M.; Baumgartner, L.K.; Peterson, K.L.; Frank, D.N.; Harris, J.K.; Pace, N.R. Opportunistic pathogens enriched in showerhead biofilms. Proc. Natl. Acad. Sci. USA 2009, 106, 16393–16399. [Google Scholar] [CrossRef]
- Chern, E.C.; King, D.; Haugland, R.; Pfaller, S. Evaluation of quantitative polymerase chain reaction assays targeting mycobacterium avium, m. Intracellulare, and m. Avium subspecies paratuberculosis in drinking water biofilms. J. Water Health 2015, 13, 131–139. [Google Scholar] [CrossRef]
- Donohue, M.J.; Mistry, J.H.; Donohue, J.M.; O’Connell, K.; King, D.; Byran, J.; Covert, T.; Pfaller, S. Increased frequency of nontuberculous mycobacteria detection at potable water taps within the united states. Environ. Sci. Technol. 2015, 49, 6127–6133. [Google Scholar] [CrossRef]
- Falkinham, J.O. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg. Infect. Dis. 2011, 17, 419–424. [Google Scholar] [CrossRef]
- Ichijo, T.; Izumi, Y.; Nakamoto, S.; Yamaguchi, N.; Nasu, M. Distribution and respiratory activity of mycobacteria in household water system of healthy volunteers in japan. PLoS ONE 2014, 9, e110554. [Google Scholar] [CrossRef]
- Nishiuchi, Y.; Tamura, A.; Kitada, S.; Taguri, T.; Matsumoto, S.; Tateishi, Y.; Yoshimura, M.; Ozeki, Y.; Matsumura, N.; Ogura, H.; et al. Mycobacterium avium complex organisms predominantly colonize in the bathtub inlets of patients’ bathrooms. Jpn. J. Infect. Dis. 2009, 62, 182–186. [Google Scholar]
- Briancesco, R.; Semproni, M.; Della Libera, S.; Sdanganelli, M.; Bonadonna, L. Non-tuberculous mycobacteria and microbial populations in drinking water distribution systems. Annali dell’Istituto Superiore di Sanita 2010, 46, 254–258. [Google Scholar]
- Thomson, R.; Tolson, C.; Carter, R.; Coulter, C.; Huygens, F.; Hargreaves, M. Isolation of nontuberculous mycobacteria (ntm) from household water and shower aerosols in patients with pulmonary disease caused by ntm. J. Clin. Microbiol. 2013, 51, 3006–3011. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, Y.; Iwamoto, T.; Maruyama, F. Infection sources of a common non-tuberculous mycobacterial pathogen, mycobacterium avium complex. Front. Med. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Health Care; National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare; NHMRC: Canberra, Australia, 2010.
- Conger, N.G.; O’Connell, R.J.; Laurel, V.L.; Olivier, K.N.; Graviss, E.A.; Williams-Bouyer, N.; Zhang, Y.; Brown-Elliott, B.A.; Wallace, R.J., Jr. Mycobacterium simae outbreak associated with a hospital water supply. Infect. Control Hosp. Epidemiol. 2004, 25, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Blanc, D.S.; Nahimana, I.; Petignat, C.; Wenger, A.; Bille, J.; Francioli, P. Faucets as a reservoir of endemic pseudomonas aeruginosa colonization/infections in intensive care units. Intensive Care Med. 2004, 30, 1964–1968. [Google Scholar] [CrossRef]
- Whiley, H.; Keegan, A.; Giglio, S.; Bentham, R. Mycobacterium avium complex—The role of potable water in disease transmission. J. Appl. Microbiol. 2012, 113, 223–232. [Google Scholar] [CrossRef]
- D’Antonio, S.; Orazi, D.; Magini, E.; Altieri, A.; Alma, M.G.; Puglisi, G.; Sanguigni, I.; De santis, R.; Paone, G. Non tuberculous mycobacteria (ntm) contamination in a hospital water supply network: Association with pulmonary infection in respiratory wards. Eur. Respir. J. 2015, 46, PA2682. [Google Scholar]
- Wilks, S.A.; Michels, H.; Keevil, C.W. The survival of escherichia coli o157 on a range of metal surfaces. Int. J. Food Microbiol. 2005, 105, 445–454. [Google Scholar] [CrossRef]
- Michels, H.; Moran, W.; Michel, J. Antimicrobial properties of copper alloy surfaces, with a focus on hospital-acquired infections. Int. J. Met. 2008, 2, 47–56. [Google Scholar] [CrossRef]
- Bezanson, G.; Burbridge, S.; Haldane, D.; Marrie, T. Insitu colonization of polyvinyl-chloride, brass, and copper by legionella-pneumophila. Can. J. Microbiol. 1992, 38, 328–330. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Drinking Water Guidelines 6: National Water Quality Management Strategy/National Health and Medical Research Council, National Resource Management Ministerial Council; National Health and Medical Research Council: Canberra, ACT, Australia, 2011.
- Harvey, P.J.; Handley, H.K.; Taylor, M.P. Widespread copper and lead contamination of household drinking water, new south wales, australia. Environ. Res. 2016, 151, 275–285. [Google Scholar] [CrossRef]
- Hayes, C.R.; Skubala, N.D. Is there still a problem with lead in drinking water in the european union? J. Water Health 2009, 7, 569–580. [Google Scholar] [CrossRef]
- Chubaka, C.E.; Whiley, H.; Edwards, J.W.; Ross, K.E. Lead, zinc, copper, and cadmium content of water from south australian rainwater tanks. Int. J. Environ. Res. Public Health 2018, 15, 1551. [Google Scholar] [CrossRef] [PubMed]
- Magyar, M.I.; Mitchell, V.G.; Ladson, A.R.; Diaper, C. Lead and other heavy metals: Common contaminants of rainwater tanks in melbourne. In Proceedings of the Water Down Under 2008; Engineers Australia: Modbury, SA, Australia, 2008; pp. 409–417. [Google Scholar]
- Murphy, B. Statement from australia’s chief medical officer, professor brendan murphy, on lead in drinking water from some plumbing products and the enhealth guidelines. In The Community Should be Reassured that Our Drinking Water Is Safe; Australian Government Depatment of Health: Canberra, Australia, 2018. [Google Scholar]
- Brown, M.J.; Margolis, S. Lead in Drinking Water and Human Blood Lead Levels in the United States; U.S Department of Health & Human Services: Atlanta, GA, USA, 2012.
- Kelsall, L.M.; de Gooyer, T.E.; Carey, M.; Vaughan, L.; Ansari, Z. Blood lead levels in the adult victorian population: Results from the victorian health monitor. Aust. N. Z. J. Public Health 2013, 37, 233–237. [Google Scholar] [CrossRef] [PubMed]
- The US Environmental Protection Agency. Report on the Environment: Blood Lead Level; US EPA: DC, USA, 2012.
- Falq, G.; Zeghnoun, A.; Pascal, M.; Vernay, M.; Le Strat, Y.; Garnier, R.; Olichon, D.; Bretin, P.; Castetbon, K.; Frery, N. Blood lead levels in the adult population living in france the french nutrition and health survey (enns 2006–2007). Environ. Int. 2011, 37, 565–571. [Google Scholar] [CrossRef]
- Bushnik, T.; Haines, D.; Levallois, P.; Levesque, J.; Van Oostdam, J.; Viau, C. Lead and bisphenol a concentrations in the canadian population. Health Rep. 2010, 21, 7–18. [Google Scholar]
- Munksgaard, N.C.; Taylor, M.P.; Mackay, A. Recognising and responding to the obvious: The source of lead pollution at mount isa and the likely health impacts. Med. J. Aust. 2010, 193, 131–132. [Google Scholar] [CrossRef]
- Lyle, D.M.; Phillips, A.R.; Balding, W.A.; Burke, H.; Stokes, D.; Corbett, S.; Hall, J. Dealing with lead in broken hill—Trends in blood lead levels in young children 1991–2003. Sci. Total Environ. 2006, 359, 111–119. [Google Scholar] [CrossRef]
- Green, D.; Sullivan, M.; Cooper, N.; Dean, A.; Marquez, C. A pilot study of children’s blood lead levels in mount isa, queensland. Int. J. Environ. Res. Public Health 2017, 14, 1567. [Google Scholar] [CrossRef]
- Burke, H.; Balding, B.; Lyle, D. Reducing lead exposure in children in broken hill. NSW Public Health Bull. 2003, 14, 52–54. [Google Scholar] [CrossRef]
- Victoria Department of Health and Human Service. Surveillance of Notifiable Infectious Diseases in Victoria, 2011–2014; Branch, H.P., Ed.; State of Victoria: Melbourne, Victoria, 2018.
- World Health Organization. Quantitative Microbial Risk Assessment: Application for Water Safety Management; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- The US Environmental Protection Agency. Lead: Human Exposure and Health Risk Assessment for Selected Case Studies; The US Environmental Protection Agency: Research Triangle Park, NC, USA, 2007; Volume 2.
- Zartarian, V.; Xue, J.; Tornero-Velez, R.; Brown, J. Children’s lead exposure: A multimedia modeling analysis to guide public health decision-making. Environ. Health Perspect. 2017, 125, 097009. [Google Scholar] [CrossRef]
- Spagnolo, A.M.; Orlando, P.; Perdelli, F.; Cristina, M.L. Hospital water and prevention of waterborne infections. Rev. Med. Microbiol. 2016, 27, 25–32. [Google Scholar] [CrossRef]
- Falkinham, J.O., III; Hilborn, E.D.; Arduino, M.J.; Pruden, A.; Edwards, M.A. Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, mycobacterium avium, and pseudomonas aeruginosa. Environ. Health Perspect. (Online) 2015, 123, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.M. Surveillance for waterborne disease outbreaks associated with drinking water—United states, 2013–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1216–1221. [Google Scholar] [CrossRef]
- Collier, S.A.; Stockman, L.J.; Hicks, L.A.; Garrison, L.E.; Zhou, F.; Beach, M.J. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol. Infect. 2012, 140, 2003–2013. [Google Scholar] [CrossRef]
- Bentham, R.; Whiley, H. Quantitative microbial risk assessment and opportunist waterborne infections⁻ are there too many gaps to fill? Int. J. Environ. Res. Public Health 2018, 15, 1150. [Google Scholar] [CrossRef] [PubMed]
- Karakousis, P.C.; Moore, R.D.; Chaisson, R.E. Mycobacterium avium complex in patients with hiv infection in the era of highly active antiretroviral therapy. Lancet 2004, 4, 557–565. [Google Scholar] [CrossRef]
- Prince, D.S.; Peterson, D.D.; Steiner, R.M.; Gottlieb, J.E.; Scott, R.; Israel, H.L.; Figueroa, W.G.; Fish, J.E. Infection with mycobacterium avium complex in patients without predisposing conditions. N. Engl. J. Med. 1989, 321, 863–868. [Google Scholar] [CrossRef]
- Lindeboom, J.A.; Kuijper, E.J.; Bruijnesteijn van Coppenraet, E.S.; Lindeboom, R.; Prins, J.M. Surgical excision versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children: A multicenter, randomized, controlled trial. Clin. Infect. Dis. 2007, 44, 1057–1064. [Google Scholar] [CrossRef]
- Olivier, K.N.; Weber, D.J.; Wallace, R.J.; Faiz, A.R.; Lee, J.-H.; Zhang, Y.; Brown-Elliot, B.A.; Handler, A.; Wilson, R.W.; Schechter, M.S.; et al. Nontuberculous mycobacteria: Multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 167, 828–834. [Google Scholar] [CrossRef]
- Shah, N.M.; Davidson, J.A.; Anderson, L.F.; Lalor, M.K.; Kim, J.; Thomas, H.L.; Lipman, M.; Abubakar, I. Pulmonary mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in england, wales and northern ireland, 2007–2012. BMC Infect. Dis. 2016, 16, 195. [Google Scholar] [CrossRef]
- Ringshausen, F.C.; Wagner, D.; de Roux, A.; Diel, R.; Hohmann, D.; Hickstein, L.; Welte, T.; Rademacher, J. Prevalence of nontuberculous mycobacterial pulmonary disease, germany, 2009–2014. Emerg. Infect. Dis. 2016, 22, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Geiter, L.J.; Snider, D.E., Jr. The epidemiology of nontuberculous mycobacterial diseases in the united states. Results from a national survey. Am. Rev. Respir. Dis. 1987, 135, 1007–1014. [Google Scholar] [PubMed]
- Cassidy, P.M.; Hedberg, K.; Saulson, A.; McNelly, E.; Winthrop, K.L. Nontuberculous mycobacterial disease prevalence and risk factors: A changing epidemiology. Clin. Infect. Dis. 2009, 49, e124–e129. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Shaw, P.A.; Strickland, D.; Jackson, L.A.; Raebel, M.A.; Blosky, M.A.; Montes de Oca, R.; Shea, Y.R.; Seitz, A.E.; Holland, S.M.; et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am. J. Respir. Crit. Care Med. 2010, 182, 970–976. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Baxter, R.; Liu, L.; Varley, C.D.; Curtis, J.R.; Baddley, J.W.; McFarland, B.; Austin, D.; Radcliffe, L.; Suhler, E.; et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann. Rheum. Dis. 2013, 72, 37–42. [Google Scholar] [CrossRef]
- Haverkort, F. National atypical mycobacteria survey, 2000. Commun. Dis. Intell. Q. Rep. 2003, 27, 180–189. [Google Scholar]
- Whiley, H.; Keegan, A.; Fallowfield, H.; Bentham, R. Detection of legionella, L. Pneumophila and mycobacterium avium complex (mac) along potable water distribution pipelines. Int. J. Environ. Res. Public Health 2014, 11, 7393–7495. [Google Scholar] [CrossRef]
- Cristina, M.L.; Spagnolo, A.M.; Casini, B.; Baggiani, A.; Del Giudice, P.; Brusaferro, S.; Poscia, A.; Moscato, U.; Perdelli, F.; Orlando, P. The impact of aerators on water contamination by emerging gram-negative opportunists in at-risk hospital departments. Infect. Control Hosp. Epidemiol. 2014, 35, 122–129. [Google Scholar] [CrossRef]
- Gebert, M.J.; Delgado-Baquerizo, M.; Oliverio, A.M.; Webster, T.M.; Nichols, L.M.; Honda, J.R.; Chan, E.D.; Adjemian, J.; Dunn, R.R.; Fierer, N. Ecological analyses of mycobacteria in showerhead biofilms and their relevance to human health. mBio 2018, 9, e01614-18. [Google Scholar] [CrossRef]
- Mehtar, S.; Wiid, I.; Todorov, S.D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and mycobacterium tuberculosis isolated from healthcare facilities in the western cape: An in-vitro study. J. Hosp. Infect. 2008, 68, 45–51. [Google Scholar] [CrossRef]
- Varkey, A. Antibacterial properties of some metals and alloys in combating coliforms in contaminated water. Sci. Res. Essays 2011, 5, 3834–3839. [Google Scholar]
- Morvay, A.; Decun, M.; Scurtu, M.; Sala, C.; Morar, A.; Sarandan, M. Biofilm formation on materials commonly used in household drinking water systems. Water Sci. Technol. Water Supply 2011, 11, 252–257. [Google Scholar] [CrossRef]
- Loret, J.-F.; Dumoutier, N. Non-tuberculous mycobacteria in drinking water systems: A review of prevalence data and control means. Int. J. Hyg. Environ. Health 2019, 222, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Mullis, S.N.; Falkinham, J.O., 3rd. Adherence and biofilm formation of mycobacterium avium, mycobacterium intracellulare and mycobacterium abscessus to household plumbing materials. J. Appl. Microbiol. 2013, 115, 908–914. [Google Scholar] [CrossRef] [PubMed]
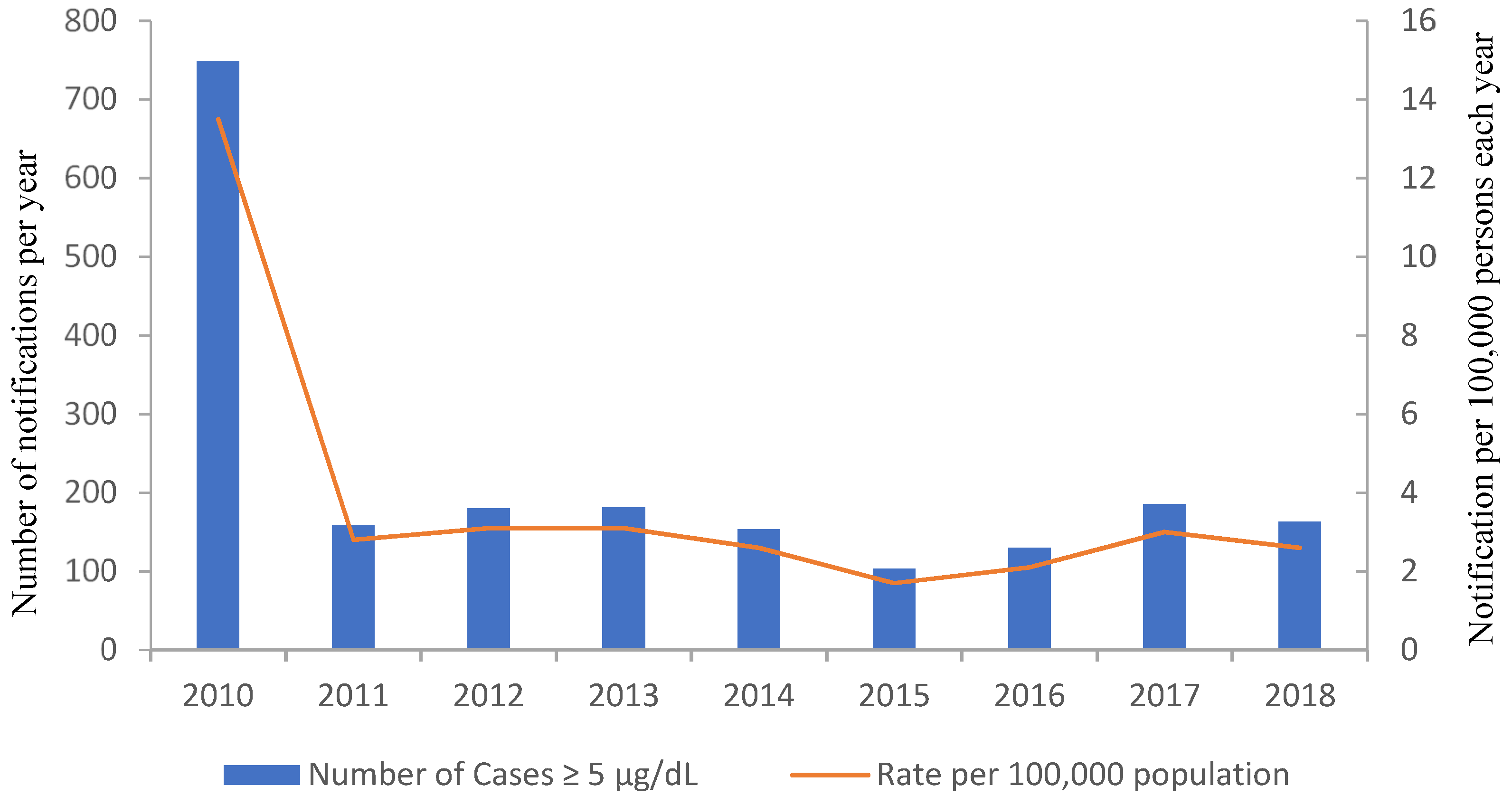
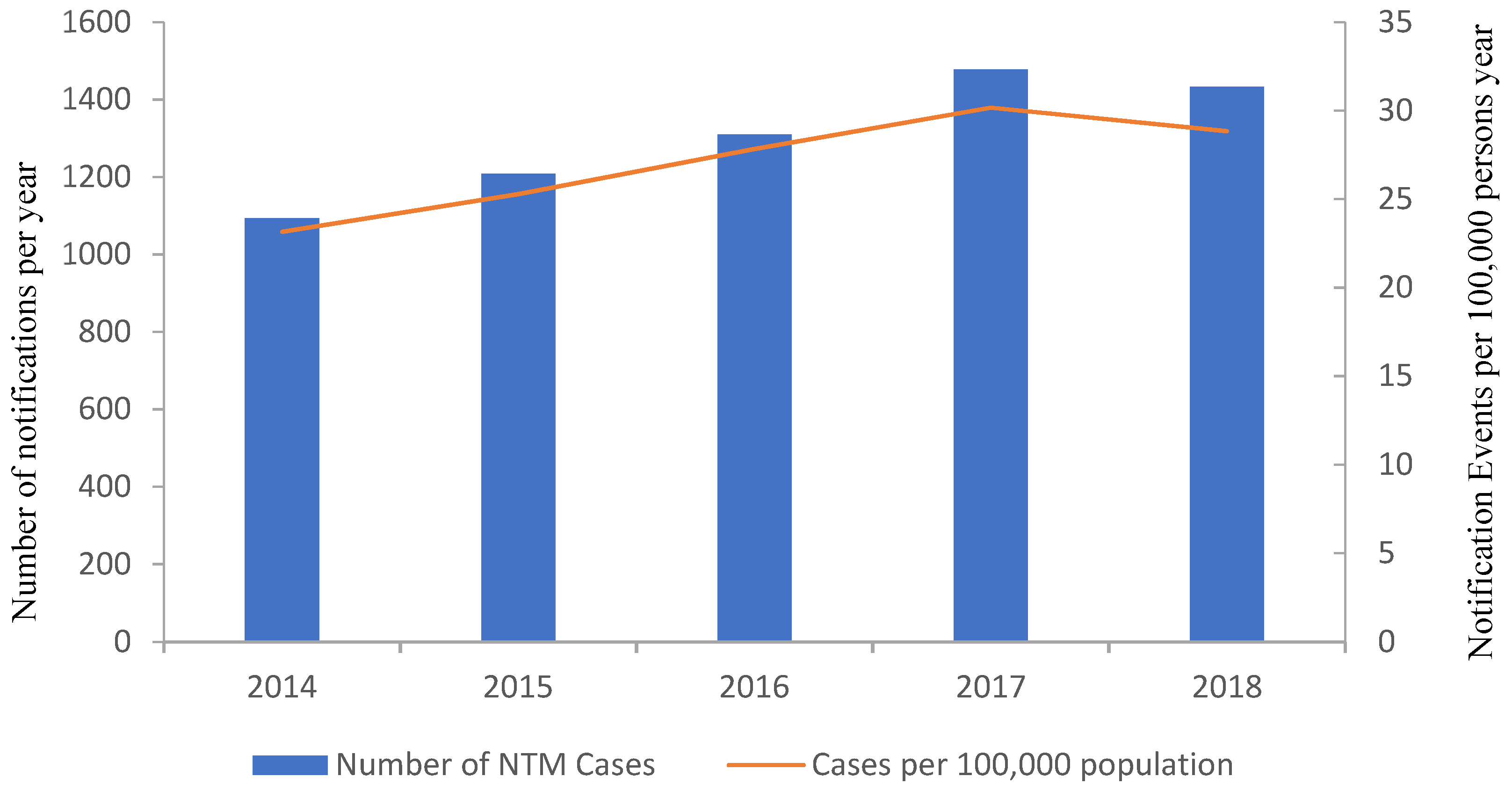
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molino, P.J.; Bentham, R.; Higgins, M.J.; Hinds, J.; Whiley, H. Public Health Risks Associated with Heavy Metal and Microbial Contamination of Drinking Water in Australia. Int. J. Environ. Res. Public Health 2019, 16, 3982. https://doi.org/10.3390/ijerph16203982
Molino PJ, Bentham R, Higgins MJ, Hinds J, Whiley H. Public Health Risks Associated with Heavy Metal and Microbial Contamination of Drinking Water in Australia. International Journal of Environmental Research and Public Health. 2019; 16(20):3982. https://doi.org/10.3390/ijerph16203982
Chicago/Turabian StyleMolino, Paul J, Richard Bentham, Michael J Higgins, Jason Hinds, and Harriet Whiley. 2019. "Public Health Risks Associated with Heavy Metal and Microbial Contamination of Drinking Water in Australia" International Journal of Environmental Research and Public Health 16, no. 20: 3982. https://doi.org/10.3390/ijerph16203982
APA StyleMolino, P. J., Bentham, R., Higgins, M. J., Hinds, J., & Whiley, H. (2019). Public Health Risks Associated with Heavy Metal and Microbial Contamination of Drinking Water in Australia. International Journal of Environmental Research and Public Health, 16(20), 3982. https://doi.org/10.3390/ijerph16203982




