Altered Body Composition of Psoas and Thigh Muscles in Relation to Frailty and Severity of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Assessment of Disease Severity, Frailty and Muscle Quality
2.3. Magnetic Resonance Imaging (MRI) Data Acquisition
2.4. IDEAL
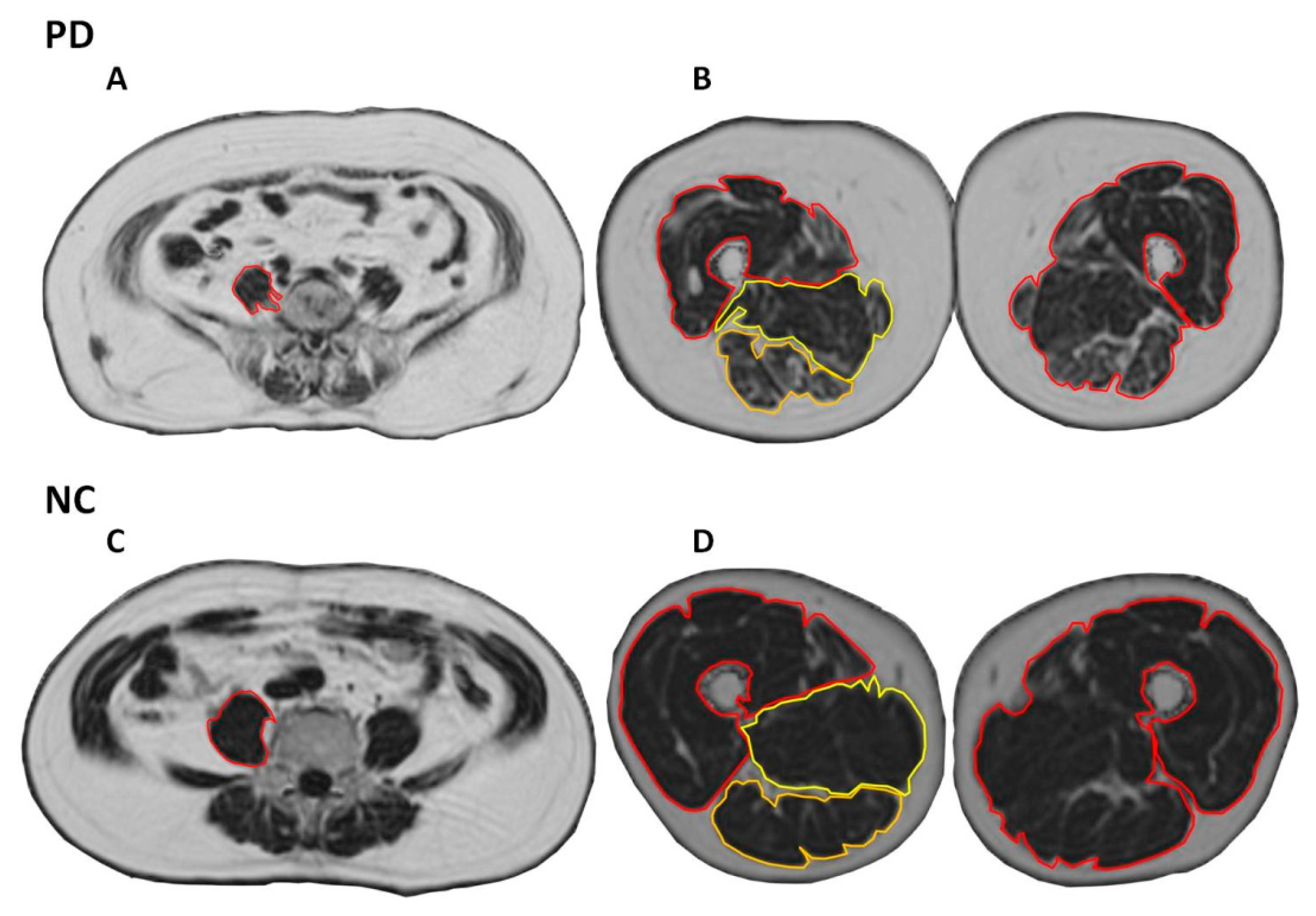
2.5. Regions of Interest (ROI)
2.6. Assessment of Cross-Sectional Area
2.7. Statistical Analysis
2.7.1. Comparison Between Normal Controls and PD Patients
2.7.2. Correlations
3. Results
3.1. Demographic Characteristics of the Participants:
3.2. Frailty
3.3. IDEAL
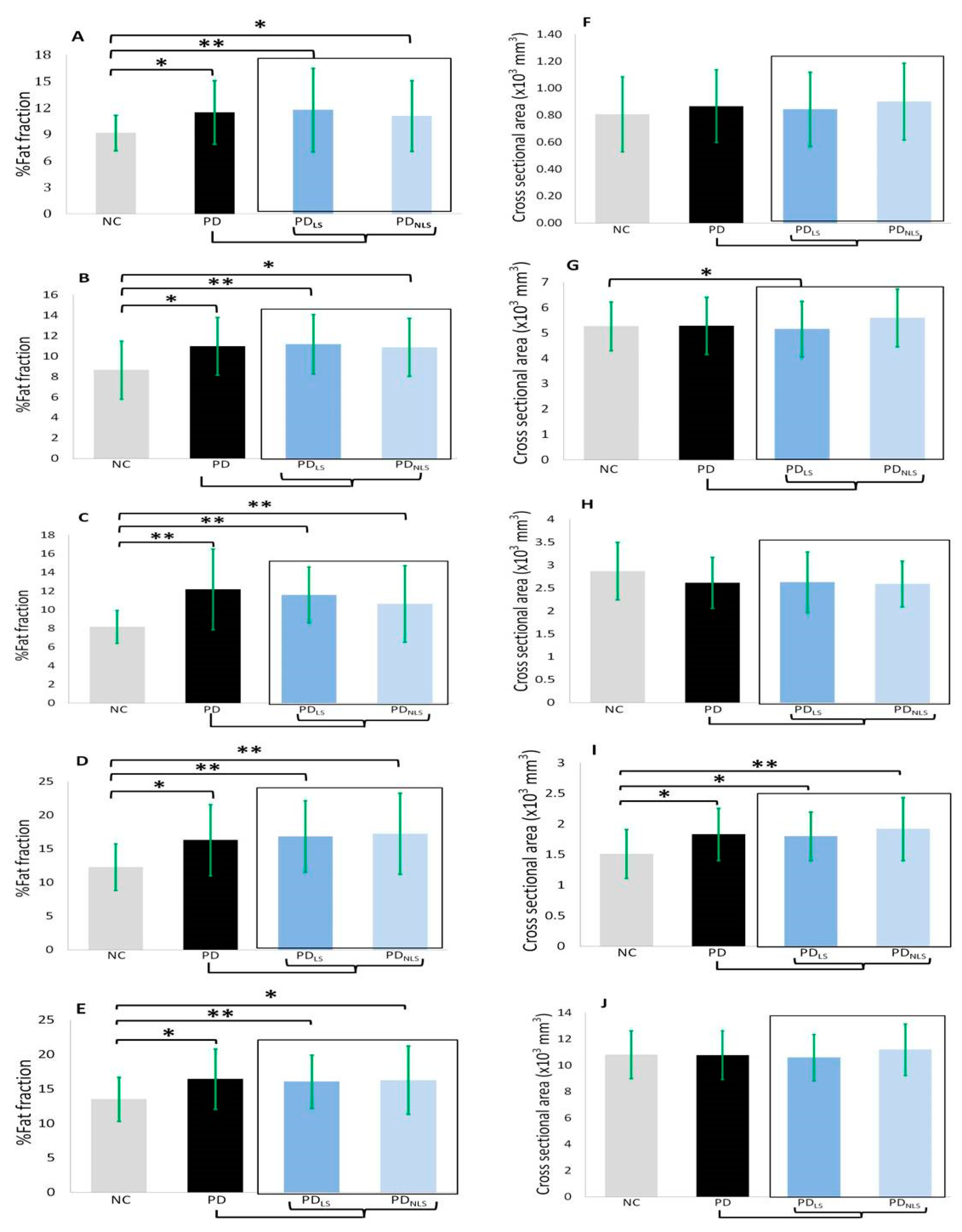
3.3.1. The Percentage of Fat Content
3.3.2. Cross-Sectional Areas
3.4. Analysis in Gender Groups
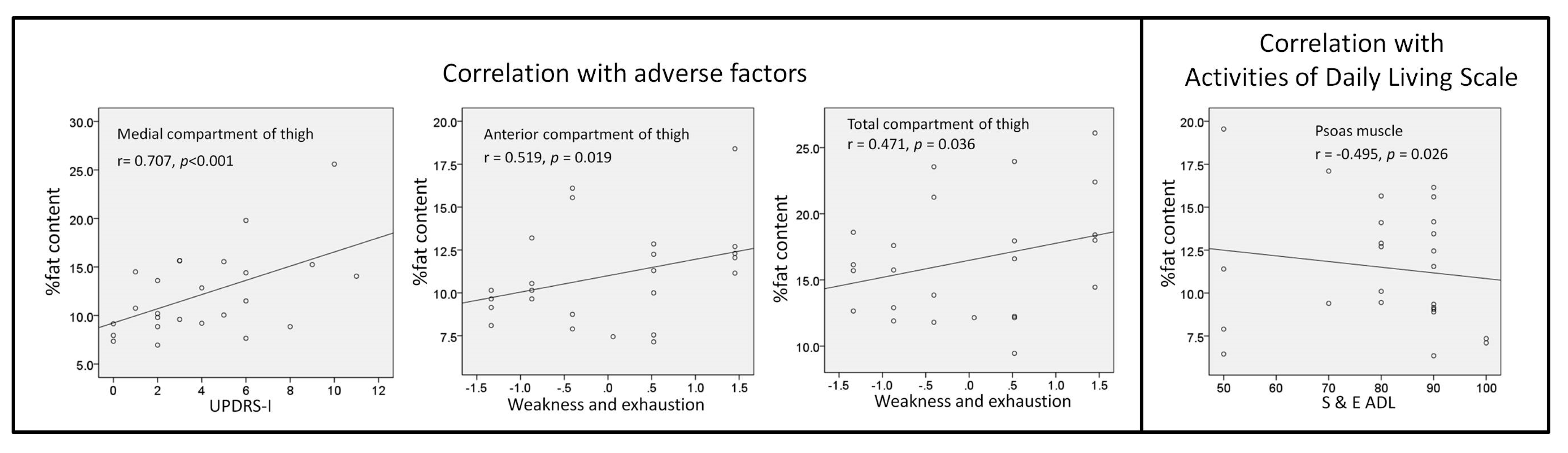
3.5. Correlations
3.5.1. Correlations among the Percentage of Fat Content, Disease Severity, and Frailty
3.5.2. Correlations among Compartment Area, Disease Severity, and Frailty
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kakinuma, S.; Nogaki, H.; Pramanik, B.; Morimatsu, M. Muscle weakness in Parkinson’s disease: Isokinetic study of the lower limbs. Eur. Neurol. 1998, 39, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Lapsley, J.; Kluger, B.M.; Schenkman, M. Quadriceps muscle weakness, activation deficits, and fatigue with Parkinson disease. Neurorehabil. Neural Repair 2012, 26, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, D.; Müller, H.P.; Ludolph, A.C.; Dupuis, L.; Kassubek, J. Body fat distribution in Parkinson’s disease: An MRI-based body fat quantification study. Parkinsonism Relat. Disord. 2016, 33, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.N.; Sherman, S.J.; VanWyck, D. Frailty in Parkinson’s disease and its clinical implications. Parkinsonism Relat. Disord. 2008, 14, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Drey, M.; Hasmann, S.E.; Krenovsky, J.; Hobert, M.A.; Straub, S.; Elshehabi, M.; von Thaler, A.; Fallgatter, A.J.; Eschweiler, G.W.; Suenkel, U.; et al. Associations between early markers of Parkinson’s disease and sarcopenia. Front. Aging Neurosci 2017, 9, 53. [Google Scholar] [CrossRef]
- RB, M.; Barden, H.S.; Bisek, J.P.; Hanson, J. Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am. J. Clin. Nutr. 1990, 51, 1106–1112. [Google Scholar]
- Gallagher, D.; Kuznia, P.; Heshka, S.; Albu, J.; Heymsfield, S.B.; Goodpaster, B.; Visser, M.; Harris, T.B. Adipose tissue in muscle: A novel depot similar in size to visceral adipose tissue. Am. J. Clin. Nutr. 2005, 81, 903–910. [Google Scholar] [CrossRef]
- Costa, D.N.; Pedrosa, I.; McKenzie, C.; Reeder, S.B.; Rofsky, N.M. Body MRI using IDEAL. AJR Am. J. Roentgenol. 2008, 190, 1076–1084. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinicopathological study of 100 cases. J. Neurol. Neurosurg. Psychiatr. 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T.; Bressman, S.; Dürr, A.; Higgins, J.; Klockgether, T.; Myers, R.H. State of the art review: Molecular diagnosis of inherited movement disorders. Movement Disorders Society task force on molecular diagnosis. Mov. Disord. 2003, 18, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; WPoewe ORPoewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement disorder society task force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.S.; England, A.C. Projection technique for evaluating surgery in Parkinson’s disease. In Third Symposium on Parkinson’s Disease; E and S Livingstone: Edinburgh, UK, 1969. [Google Scholar]
- Chan, D.C.; Tsou, H.H.; Yang, R.S.; Tsauo, J.Y.; Chen, C.Y.; Hsiung, C.A.; Kuo, K.N. A pilot randomized controlled trial to improve geriatric frailty. BMC Geriatr. 2012, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Healthspan 2014, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Doherty, K.M.; van de Warrenburg, B.P.; Peralta, M.C.; Silveira-Moriyama, L.; Azulay, J.P.; Gershanik, O.S.; Bloem, B.R. Postural deformities in Parkinson’s disease. Lancet Neurol. 2011, 10, 538–549. [Google Scholar] [CrossRef]
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of motor dysfunction in Parkinson’s Disease as the rationale for drug treatment and rehabilitation. Parkinson Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef]
- Brown, P.; Corcos, D.M.; Rothwell, J.C. Does parkinsonian action tremor contribute to muscle weakness in Parkinson’s disease? Brain 1997, 120, 401–408. [Google Scholar] [CrossRef]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Batista, F.S.; Gomes, G.A.; Neri, A.L.; Guariento, M.E.; Cintra, F.A.; Sousa Mda, L.; D’Elboux, M.J. Relationship between lower-limb muscle strength and frailty among elderly people. Sao Paulo Med. J. 2012, 130, 102–108. [Google Scholar] [CrossRef]
- Kwon, Y.N.; Yoon, S.S. Sarcopenia: Neurological point of view. J. Bone Metab. 2017, 24, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Maden-Wilkinson, T.M.; Degens, H.; Jones, D.A.; McPhee, J.S. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J. Musculoskelet. Neuronal Interact. 2013, 13, 320–328. [Google Scholar] [PubMed]
- Borkan, G.A.; Gerzof, S.G.; Robbins, A.H.; Hults, D.E.; Silbert, C.K.; Silbert, J.E. Assessment of abdominal fat content by computed tomography. Am. J. Clin. Nutr. 1982, 36, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Pineda, A.R.; Wen, Z.; Shimakawa, A.; Yu, H.; Brittain, J.H.; Gold, G.E.; Beaulieu, C.H.; Pelc, N.J. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): Application with fast spin-echo imaging. Magn. Resonance Med. 2005, 54, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; McKenzie, C.A.; Pineda, A.R.; Yu, H.; Shimakawa, A.; Brau, A.C.; Hargreaves, B.A.; Gold, G.E.; Brittain, J.H. Water–Fat separation with IDEAL Gradient-Echo imaging. J. Magn. Resonance Imaging 2007, 25, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Engelke, K.; Museyko, O.; Wang, L.; Laredo, J.-D. Quantitative analysis of skeletal muscle by computed tomography imagingdState of the art. J. Orthop. Transl. 2018, 15, 91–103. [Google Scholar]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Barichella, M.; Pinelli, G.; Iorio, L.; Cassani, E.; Valentino, A.; Pusani, C.; Ferri, V.; Bolliri, C.; Pasqua, M.; Pezzoli, G.; et al. Sarcopenia and dynapenia in patients with Parkinsonism. JAMDA 2016, 17, 640–646. [Google Scholar] [PubMed]
- Takahashi, T.; Sugie, M.; Nara, M.; Koyama, T.; Obuchi, S.P.; Harada, K.; Kyo, S.; Ito, H. Femoral muscle mass relates to physical frailty components in community-dwelling older people. Geriatr. Gerontol. Int. 2017, 17, 1636–1641. [Google Scholar] [CrossRef]
- Revillaa, M.; Jiménez-Jiménez, F.; Villa, L.; Hernández, E.; Ortí-Pareja, M.; Gasalla, T.; Rico, H. Body composition in Parkinson’s disease: A study with dual-energy X-ray absorptiometry. Parkinsonism Relat. Disord. 1998, 4, 137–142. [Google Scholar] [CrossRef]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Harmer, P.; Fitzgerald, K.; Eckstrom, E.; Stock, R.; Galver, J.; Maddalozzo, G.; Batya, S.S. Tai Chi and postural stability in patients with Parkinson’s Disease. N. Engl. J. Med. 2012, 366, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Hsu, R.W.; Lin, Z.R.; Fan, C.H. Effects of circuit exercise and Tai Chi on body composition in middle-aged and older women. Geriatr. Gerontol. Int. 2015, 15, 282–288. [Google Scholar] [CrossRef] [PubMed]
- MA, B. Sex differences in body composition. Adv. Exp. Med. Biol. 2017, 1043, 9–27. [Google Scholar]
| Clinical Demographics | PD (n = 25) | Control (n = 20) | p |
| Age (year) | 63.60 ± 5.54 | 63.00 ± 4.09 | 0.688 |
| Sex | |||
| Male | 5 | 4 | |
| Female | 20 | 16 | |
| UPDRS I | 4.04 ± 3.12 | ||
| UPDRS II | 10.68 ± 5.41 | ||
| UPDRS III | 27.92 ± 14.17 | ||
| UPDRS total | 42.64 ± 20.24 | ||
| Modified H & Y (maximum stage is 5) | 2.02 ± 1.08 | ||
| S & E (minimum points is 0 suggesting vegetative functions) | 80.40 ± 15.41 | ||
| Disease duration (years) | 1.70 ± 2.15 | ||
| Levodopa equivalent dose (mg/day) | 482.58 ± 359.98 | ||
| Height (cm) | 157.31 ± 5.44 | 157.20 ± 6.18 | 0.951 |
| Body weight (kg) | 62.06 ± 9.93 | 57.77 ± 11.25 | 0.182 |
| Body Mass Index (BMI) | 25.09 ± 3.80 | 23.14 ± 3.51 | 0.091 |
| Assessments of Frailty | |||
| Body weight loss (0: no; 1: yes) | 0.42 ± 0.58 | 1.23 ± 0.44 | 0.048 * |
| Weakness | 1.46 ± 1.14 | 0.15 ± 0.38 | 0.000 * |
| Exhaustion | 1.42 ± 1.10 | 0.15 ± 0.38 | 0.000 * |
| Physical activity (× 102 kcals/week) | 2.17 ± 0.93 | 3.03 ± 1.91 | 0.081 |
| Slowness (five-meter walking time; second) | 7.96 ± 4.45 | 5.36 ± 0.58 | 0.048 * |
| Grip strength of Right hand (kg) | 18.32 ± 7.32 | 22.85 ± 7.04 | 0.029 * |
| Grip strength of Left hand (kg) | 15.73 ± 4.99 | 22.72 ± 7.42 | 0.000 * |
| Patients with Parkinson’s Disease | |||||
| Fat Fraction (%) | Normal Controls | PD | PDLS | PDNLS | p |
| Psoas muscles | 9.16 ± 2.01 §# | 11.49 ± 3.59 | 11.77 ± 4.71 § | 11.08 ± 4.01 # | 0.011 * |
| Anterior compartment of thigh | 8.65 ± 2.83 §# | 10.97 ± 2.81 | 11.17 ± 2.91 § | 10.87 ± 2.84 # | 0.019 * |
| Medial compartment of thigh | 8.18 ± 1.77 §# | 12.19 ± 4.34 | 11.59 ± 2.99 § | 10.62 ± 4.10 # | 0.001 * |
| Posterior compartment of thigh | 12.27 ± 3.46 §# | 16.30 ± 5.28 | 16.83 ± 5.31 § | 17.26 ± 6.05 # | 0.010 * |
| Total thigh | 13.51 ± 3.19 §# | 16.45 ± 4.37 | 16.05 ± 3.84 § | 16.27 ± 4.93 # | 0.019 * |
| Cross-Sectional Area (mm3) | |||||
| Psoas muscle | 806.80 ± 276.63 | 867.38 ± 269.67 | 844.49 ± 275.11 | 901.71 ± 284.93 | 0.672 |
| Anterior compartment of thigh | 5267.33 ± 960.91 § | 5288.28 ± 1129.28 | 5152.86 ± 1094.41 § | 5601.82 ± 1140.72 | 0.246 |
| Medial compartment of thigh | 2872.52 ± 626.79 §# | 2615.18 ± 556.09 | 2628.61 ± 660.64§ | 2590.92 ± 501.83 # | 0.062 |
| Posterior compartment of thigh | 1511.22 ± 399.79 §# | 1832.25 ± 426.36 | 1800.75 ± 394.60 § | 1919.07 ± 513.87# | 0.061 |
| Total thigh | 10,809.89 ± 1814.65 § | 10,781.20 ± 1843.18 | 10,590.24 ± 1771.51 § | 11,200.29 ± 1956.08 | 0.076 |
| Fat Fraction (%) | Normal Controls | PDLS | p | PDNLS | p |
| Psoas muscles | 9.16 ± 2.01 | 11.77 ± 4.71 | 0.003 * | 11.08 ± 4.01 # | 0.038 * |
| Anterior compartment of thigh | 8.65 ± 2.83 | 11.17 ± 2.91 | 0.002 * | 10.87 ± 2.84 # | 0.016 * |
| Medial compartment of thigh | 8.18 ± 1.77 | 11.59 ± 2.99 | 0.000 * | 10.62 ± 4.10 # | 0.007 * |
| Posterior compartment of thigh | 12.27 ± 3.46 | 16.83 ± 5.31 | 0.000 * | 17.26 ± 6.05 # | 0.000 * |
| Total thigh | 13.51 ± 3.19 | 16.05 ± 3.84 | 0.008 * | 16.27 ± 4.93 # | 0.026 * |
| Cross-Sectional area (mm3) | Normal Controls | PDLS | p | PDNLS | p |
| Psoas muscles | 806.80 ± 276.63 | 844.49 ± 275.11 | 0.637 | 901.71 ± 284.93 | 0.584 |
| Anterior compartment of thigh | 5267.33 ± 960.91 | 5151.86 ± 1094.41 | 0.027 * | 5601.82 ± 1140.72 | 0.945 |
| Medial compartment of thigh | 2872.52 ± 626.79 | 2628.61 ± 660.64 | 0.031 * | 2590.92 ± 501.83 | 0.017 * |
| Posterior compartment of thigh | 1511.22 ± 399.79 | 1800.75 ± 394.60 | 0.042 * | 1919.07 ± 513.87 | 0.007 * |
| Total thigh | 10,809.89 ± 1,814.65 | 10,590.24 ± 1,771.51 | 0.004 * | 11,200.29 ± 1,956.08 | 0.272 |
| Female/Male groups | PD, female, n = 20 | NC, female, n = 16 | p | PD, male, n = 5 | NC, male, n = 4 | p |
| Age | 63.80 ± 5.46 | 62.38 ± 3.63 | 0.377 | 62.8 ± 6.42 | 65.50 ± 5.45 | 0.525 |
| BMI | 25.27 ± 3.65 | 22.76 ± 3.72 | 0.053 | 24.42 ± 4.78 | 24.69 ± 2.20 | 0.920 |
| Fat Fraction (%) | p | p | ||||
| Psoas muscles | 11.89 ± 3.75 | 9.14 ± 2.28 | 0.014 * | 9.90 ± 2.53 | 9.25 ± 0.96 | 0.645 |
| Anterior compartment of thigh | 11.55 ± 2.82 | 9.16 ± 2.79 | 0.016 * | 8.64 ± 1.11 | 6.61 ± 2.15 | 0.108 |
| Medial compartment of thigh | 12.86 ± 4.48 | 8.61 ± 1.71 | 0.001 * | 9.48 ± 2.50 | 6.46 ± 0.31 | 0.053 |
| Posterior compartment of thigh | 17.21 ± 5.37 | 12.87 ± 3.64 | 0.009 * | 12.63 ± 3.02 | 9.88 ± 0.21 | 0.119 |
| Total thigh | 17.66 ± 4.00 | 14.44 ± 2.52 | 0.008 * | 11.59 ± 1.24 | 9.88 ± 0.31 | 0.263 |
| Cross-Sectional Area (mm3) | p | p | ||||
| Psoas muscles | 770.25 ± 150.54 | 700.47 ± 174.58 | 0.207 | 1255.91 ± 304.67 | 1232.11 ± 175.48 | 0.894 |
| Anterior compartment of thigh | 4940.69 ± 864.43 | 4931.28 ± 652.32 | 0.971 | 6678.60 ± 1040.19 | 6611.50 ± 842.03 | 0.920 |
| Medial compartment of thigh | 2469.79 ± 449.27 | 2702.78 ± 584.89 | 0.185 | 3246.75 ± 541.33 | 3551.56 ± 95.60 | 0.309 |
| Posterior compartment of thigh | 1784.91 ± 391.55 | 1383.51 ± 251.04 | 0.001 * | 2021.60 ± 553.58 | 2022.05 ± 512.19 | 0.999 |
| Total thigh | 10,290.29 ± 1410.12 | 10,200.82 ± 1282.27 | 0.845 | 12,744.46 ± 2217.25 | 13,246.16 ± 1655.87 | 0.719 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-K.; Chen, H.-L.; Lu, C.-H.; Chen, M.-H.; Chiang, P.-L.; Chen, Y.-S.; Lin, W.-C. Altered Body Composition of Psoas and Thigh Muscles in Relation to Frailty and Severity of Parkinson’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 3667. https://doi.org/10.3390/ijerph16193667
Wang C-K, Chen H-L, Lu C-H, Chen M-H, Chiang P-L, Chen Y-S, Lin W-C. Altered Body Composition of Psoas and Thigh Muscles in Relation to Frailty and Severity of Parkinson’s Disease. International Journal of Environmental Research and Public Health. 2019; 16(19):3667. https://doi.org/10.3390/ijerph16193667
Chicago/Turabian StyleWang, Cheng-Kang, Hsiu-Ling Chen, Cheng-Hsien Lu, Meng-Hsiang Chen, Pi-Ling Chiang, Yueh-Sheng Chen, and Wei-Che Lin. 2019. "Altered Body Composition of Psoas and Thigh Muscles in Relation to Frailty and Severity of Parkinson’s Disease" International Journal of Environmental Research and Public Health 16, no. 19: 3667. https://doi.org/10.3390/ijerph16193667
APA StyleWang, C.-K., Chen, H.-L., Lu, C.-H., Chen, M.-H., Chiang, P.-L., Chen, Y.-S., & Lin, W.-C. (2019). Altered Body Composition of Psoas and Thigh Muscles in Relation to Frailty and Severity of Parkinson’s Disease. International Journal of Environmental Research and Public Health, 16(19), 3667. https://doi.org/10.3390/ijerph16193667






