Spirometric Pulmonary Restriction in Herbicide-Exposed U.S. Vietnam War Veterans
Abstract
1. Introduction
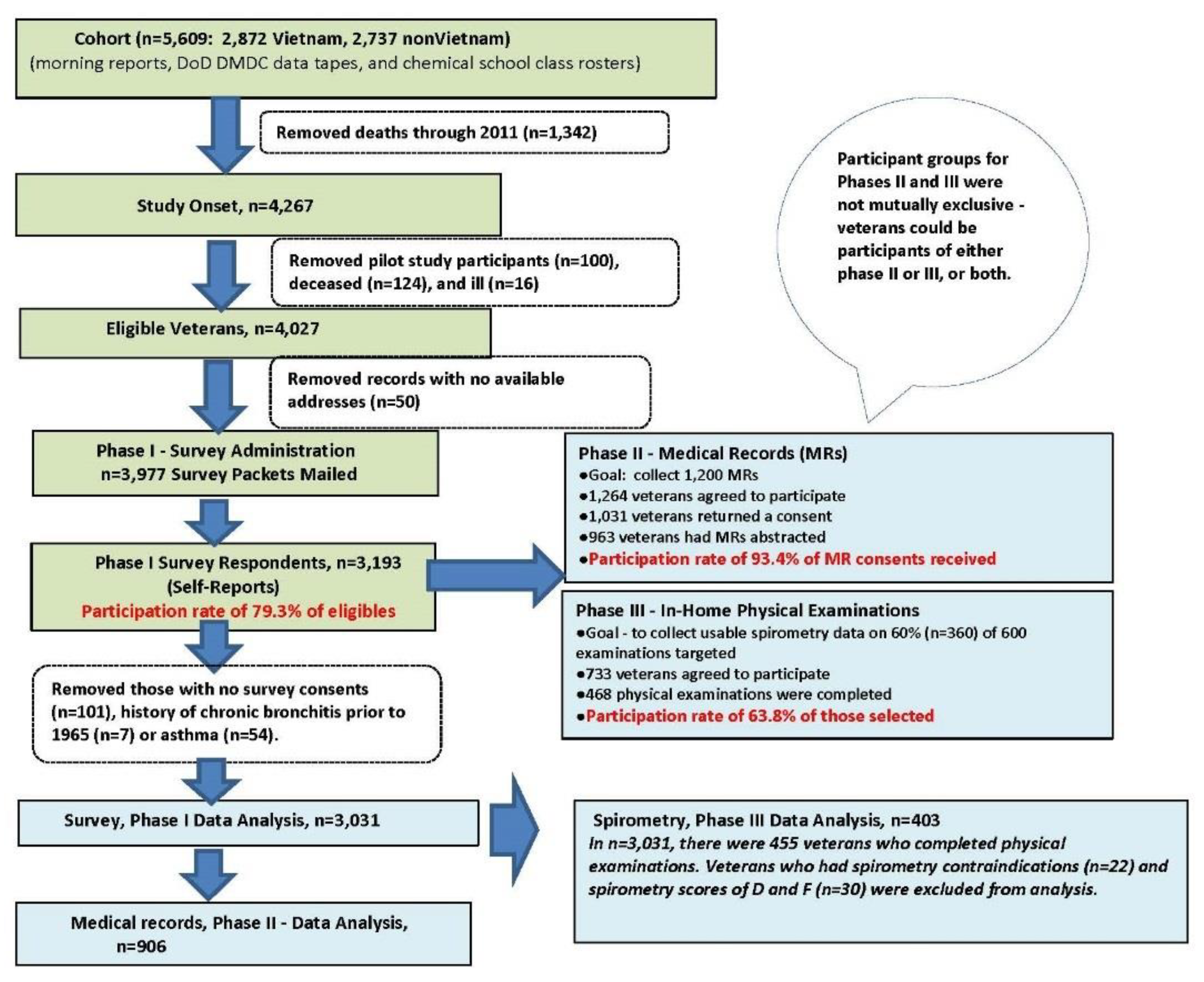
2. Materials and Methods
2.1. Pulmonary Function Measures
2.2. Other Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Veterans and Agent Orange: Update 11 (2018); The National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Townsend, M.C. Occupational and Environmental Lung Disorders Committee. Spirometry in the occupational health setting—2011 update. J. Occup. Environ. Med. 2011, 53, 569–584. [Google Scholar] [PubMed]
- Miguel-Reyes, J.L.; Gochicoa-Rangel, L.; Pérez-Padilla, R.; Torre-Bouscoulet, L. Functional respiratory assessment in interstitial lung disease. Rev. Investig. Clin. 2015, 67, 5–14. [Google Scholar] [PubMed]
- Kim, H.R.; Shin, D.Y.; Chung, K.H. A review of current studies on cellular and molecular mechanisms underlying pulmonary fibrosis induced by chemicals. Environ. Health Toxicol. 2018, 33, e2018014. [Google Scholar] [CrossRef] [PubMed]
- Zacharisen, M.C.; Fink, J.N. Hypersensitivity pneumonitis and related conditions in the work environment. Immunol. Allergy Clin. N. Am. 2011, 31, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Wysong, K.; Phillips, J.A.; Hammond, S. Hypersensitivity pneumonitis. Workplace Hlth. Saf. 2016, 64, 284. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Holguin, F.; Pavlin, B.I.; Ferdinands, J.M. Risk factors for prevalence of and mortality related to restriction on spirometry: Findings from the First National Health and Nutrition Examination Survey and follow-up. Int. J. Tuberc. Lung. Dis. 2005, 9, 613–621. [Google Scholar] [PubMed]
- Mannino, D.M.; Buist, A.S.; Petty, T.L.; Enright, P.L.; Redd, S.C. Lung function and mortality in the United States: Data from the First National Health and Nutrition Examination Survey follow-up study. Thorax 2003, 58, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Kurth, L.; Hnizdo, E. Change in prevalence of restrictive lung impairment in the U.S. population and associated risk factors: The National Health and Nutrition Examination Survey (NHANES) 1988–1994 and 2007–2010. Multidiscip. Respir. Med. 2015, 10, 7. [Google Scholar] [CrossRef]
- Scarlata, S.; Pedone, C.; Fimognari, F.L.; Bellia, V.; Forastiere, F.; Incalzi, R.A. Restrictive pulmonary dysfunction at spirometry and mortality in the elderly. Resp. Med. 2008, 102, 1349–1354. [Google Scholar] [CrossRef]
- Backman, H.; Eriksson, B.; Hedman, L.; Stridsman, C.; Jansson, S.; Sovijärvi, A.; Lindberg, A.; Rönmark, E.; Lundbäck, B. Restrictive spirometric pattern in the general adult population: Methods of defining the condition and consequences on prevalence. Resp. Med. 2016, 120, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Sherrill, D.L.; Venker, C.; Ceccato, C.M.; Halonen, M.; Martinez, F.D. Morbidity and mortality associated with the restrictive spirometric pattern: A longitudinal study. Thorax 2010, 65, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Health status of Vietnam veterans. II. Physical health. The Centers for Disease Control Vietnam Experience Study. JAMA 1988, 259, 2708–2714. [Google Scholar] [CrossRef]
- Boehmer, T.K.; Flanders, W.D.; McGeehin, M.A.; Boyle, C.; Barrett, D.H. Postservice mortality in Vietnam veterans: 30-year follow-up. Arch. Intern. Med. 2004, 164, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Dalager, N.A.; Needham, L.L.; Patterson, D.G., Jr.; Lees, P.S.J.; Yates, K.; Matanoski, G.M. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am. J. Ind. Med. 2006, 49, 875–884. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.; Cox, B.; Broughton, J.; Tong, D. The mortality and cancer experience of New Zealand Vietnam war veterans: A cohort study. BMJ Open 2013, 3, e003379. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.; McBride, D.; Broughton, J.; Tong, D. Health conditions in a cohort of New Zealand Vietnam veterans: Hospital admissions between 1988 and 2009. BMJ Open 2015, 5, e008409. [Google Scholar] [CrossRef] [PubMed]
- Dalager, N.A.; Kang, H.K. Mortality among Army Chemical Corps Vietnam veterans. Am. J. Ind. Med. 1997, 31, 719–726. [Google Scholar] [CrossRef]
- Ketchum, N.S.; Michalek, J.E. Postservice mortality of Air Force veterans occupationally exposed to herbicides during the Vietnam War: 20-year follow-up results. Mil. Med. 2005, 170, 406–413. [Google Scholar] [CrossRef]
- Thomas, T.L.; Kang, H.K. Mortality and morbidity among Army Chemical Corps Vietnam veterans: A preliminary report. Am. J. Ind Med. 1990, 18, 665–673. [Google Scholar] [CrossRef]
- O’Toole, B.I.; Marshall, P.P.; Grayson, D.A.; Schureck, R.J.; Dobson, M.; Ffrench, M.; Pulvertaft, B.; Meldrum, L.; Bolton, J.; Vennard, J. The Australian Vietnam Veterans Health Study: II. Self-reported health of veterans compared with the Australian population. Int. J. Epidemiol. 1996, 25, 319–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Toole, B.I.; Catts, S.V.; Outram, S.; Pierse, K.R.; Cockburn, J. The physical and mental health of Australian Vietnam veterans 3 decades after the War and its relation to military service, combat, and post-traumatic stress disorder. Am. J. Epidemiol. 2009, 170, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Cypel, Y.; Kang, H. Mortality patterns of Army Chemical Corps veterans who were occupationally exposed to herbicides in Vietnam. Ann. Epidemiol. 2010, 20, 339–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cypel, Y.S.; Hines, S.E.; Davey, V.J.; Eber, S.M.; Schneiderman, A.I. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam era US Army Chemical Corps veterans: A retrospective cohort study. Am. J. Ind. Med. 2018, 61, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.W.; Ryu, S.Y.; Ohrr, H.; Hong, J.S. Agent Orange exposure and risk of death in Korean Vietnam veterans: Korean Veterans Health Study. Int. J. Epidemiol. 2014, 43, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.W.; Hong, J.S.; Ohrr, H.; Yi, J.J. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: The Korean veterans health study. Environ. Res. 2014, 133, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Cypel, Y.S.; Kress, A.M.; Eber, S.M.; Schneiderman, A.I.; Davey, V.J. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J. Occup. Environ. Med. 2016, 58, 1127–1136. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardization of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Enright, P.L.; Skloot, G.S.; Cox-Ganser, J.M.; Udasin, I.G.; Herbert, R. Quality of spirometry performed by 13,599 participants in the World Trade Center Worker and Volunteer Medical Screening Program. Respir. Care 2010, 55, 303–309. [Google Scholar]
- Ferguson, G.T.; Enright, P.L.; Buist, S.A.; Higgins, M.W. Office spirometry for lung health assessment in adults. A consensus statement from the National Lung Health Education Program. Chest 2000, 117, 1146–1161. [Google Scholar] [CrossRef]
- Kreiss, K. Work-related spirometric restriction in flavoring manufacturing workers. Am. J. Ind. Med. 2014, 57, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kreiss, K.; Fedan, K.B.; Nasrullah, M.; Kim, T.J.; Materna, B.L.; Prudhomme, J.C.; Enright, P.L. Longitudinal lung function declines among California flavoring manufacturing workers. Am. J. Ind. Med. 2012, 55, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr. Opin. Cardiol. 2006, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Waist Circumference and Waist-hip Ratio: Report of a WHO Expert Consultation. Geneva. 8–11 December 2008. Available online: http://apps.who.int/iris/bitstream/10665/44583/9789241501491_eng.pdf (accessed on 30 October 2017).
- Ford, E.S.; Cunningham, T.J.; Mercado, C.I. Lung function and metabolic syndrome: Findings of National Health and Nutrition Examination Survey 2007–2010. J. Diabetes 2014, 6, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, J.L.; Wolfe, W.H.; Patterson, D.G.; Needham, L.L.; Michalek, J.E.; Miner, J.C.; Peterson, M.R.; Philips, D.L. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam veterans of Operation Ranch Hand. J. Toxicol. Environ. Health 1989, 27, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, W.H.; Michalek, J.E.; Miner, J.C.; Pirkle, J.L.; Caudill, S.P.; Patterson, D.G., Jr.; Needham, L.L. Determinants of TCDD half-life in veterans of Operation Ranch Hand. J. Toxicol. Environ. Health 1994, 41, 481–488. [Google Scholar] [CrossRef]
- SAS Institute Inc. Base SAS 9.4 Procedures Guide-SAS/STAT, 2nd ed.; SAS Institute, Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Allison, P.D. Logistic Regression Using the SAS System. Theory and Application; SAS Institute Inc.: Cary, NC, USA, 1999. [Google Scholar]
- Forde, A.T.; Crookes, D.M.; Suglia, S.F.; Demmer, R.T. The Weathering Hypothesis as an explanation for racial disparities in health: A systematic review. Ann. Epidemiol. 2019, 33, 1–18. [Google Scholar] [CrossRef]
- Chen, R.; Tunstall-Pedoe, H.; Bolton-Smith, C.; Hannah, M.K.; Morrison, C. Association of dietary antioxidants and waist circumference with pulmonary function and airway obstruction. Am. J. Epidemiol. 2001, 153, 157–163. [Google Scholar] [CrossRef]
- Lazarus, R.; Gore, C.J.; Booth, M.; Owen, N. Effects of body composition and fat distribution on ventilatory function in adults. Am. J. Clin. Nutr. 1998, 68, 35–41. [Google Scholar] [CrossRef]
- Maiolo, C.; Mohamed, E.I.; Carbonelli, M.G. Body composition and respiratory function. Acta. Diabetol. 2003, 40, S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Breland, J.Y.; Phibbs, C.S.; Hoggatt, K.J.; Washington, D.L.; Lee, J.; Haskell, S.; Uchendu, U.S.; Saechao, F.S.; Zephyrin, L.C.; Frayne, S.M. The obesity epidemic in the Veterans Health Administration: Prevalence among key populations of women and men veterans. J. Gen. Intern. Med. 2017, 32, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Dalager, N.A.; Needham, L.L.; Patterson, D.G., Jr.; Matanoski, G.M.; Kanchanaraksa, S.; Lees, P.S.J. US army chemical corps Vietnam veterans health study: Preliminary results. Chemosphere 2001, 43, 943–949. [Google Scholar] [CrossRef]
- Lathrop, G.D.; Wolfe, W.H.; Albanese, R.A.; Moynahan, P.M. Air Force Health Study (Project Ranch Hand II). In An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Baseline Morbidity Study Results; Prepared for The Surgeon General, United States Air Force, Washington DC; USAF School of Aerospace Medicine, Aerospace Medical Division: Brooks Air Force Base, TX, USA, 24 February 1984. [Google Scholar]
- Cha, E.S.; Lee, Y.K.; Moon, E.K.; Kim, Y.B.; Lee, Y.-J.; Jeong, W.C.; Cho, E.Y.; Lee, I.J.; Hur, J.; Ha, M.; et al. Paraquat application and respiratory health effects among South Korean farmers. Occup. Environ. Med. 2012, 69, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Schenker, M.B.; Stoecklin, M.; Lee, K.; Lupercio, R.; Zeballos, R.J.; Enright, P.; Hennessy, T.; Beckett, L.A. Pulmonary function and exercise-associated changes with chronic low-level paraquat exposure. Am. J. Resp. Crit. Care Med. 2004, 170, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Casado, I.; Pena, G.; Gil, F.; Villanueva, E.; Pla, A. Low level of exposure to pesticides leads to lung dysfunction in occupationally exposed subjects. Inhal. Toxicol. 2008, 20, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Pieris-John, R.J.; Ruberu, D.K.; Wickremasinghe, A.R.; van-der-Hoek, W. Low-level exposure to organophosphate pesticides leads to restrictive lung dysfunction. Resp. Med. 2005, 99, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mukherjee, S.; Roychoudhury, S.; Siddique, S.; Lahiri, T.; Ray, M.R. Chronic exposures to cholinesterase-inhibiting pesticides adversely affect respiratory health of agricultural workers in India. J. Occup. Health 2009, 51, 488–497. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society. Lung function testing: Selection of reference values and interpretative strategies. Am. Rev. Respir. Dis 1991, 144, 1202–1218. [Google Scholar] [CrossRef]
- Ford, E.S.; Mannino, D.M.; Wheaton, A.G.; Giles, W.H.; Presley-Cantrell, L.; Croft, L.B. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States. Findings from the National Health and Nutrition Examination Surveys from 1988–1994 to 2007–2010. Chest 2013, 143, 1395–1406. [Google Scholar] [CrossRef]
| Characteristic a | All (n = 403) | Exposed (Herbicide Sprayers) (n = 191, 47.39%) | Unexposed (Herbicide Nonsprayers) (n = 212, 52.61%) | p Value |
|---|---|---|---|---|
| Age (yrs), at time of study n (%) | ||||
| 59–64 | 121 (30.0) | 49 (25.7) | 72 (34.0) | 0.076 |
| 65–69 | 218 (54.1) | 105 (55.0) | 113 (53.3) | |
| 70+ | 64 (15.9) | 37 (19.4) | 27 (12.7) | |
| Age (yrs) mean (± SD) | 66.4 (± 3.85) | 66.8 (± 3.85) | 66.0 (± 3.82) | 0.053 * |
| Race/ethnicity n (%) | ||||
| White | 301 (74.7) | 133 (69.6) | 168 (79.3) | 0.078 |
| Black | 65 (16.1) | 38 (19.9) | 27 (12.7) | |
| Other nonwhite | 37 (9.2) | 20 (10.5) | 17 (8.0) | |
| Vietnam service status n (%) | <0.0001 * | |||
| In-theater (Vietnam) | 272 (67.5) | 162 (84.8) | 110 (51.9) | |
| Outside SEA (non-Vietnam) | 131 (32.5) | 29 (15.2) | 102 (48.1) | |
| Officer status n (%) | 0.23 | |||
| Yes | 62 (15.4) | 25 (13.1) | 37 (17.5) | |
| No (enlisted) | 341 (84.6) | 166 (86.9) | 175 (82.6) | |
| Military service duration (mos) n (%) | 0.017 * | |||
| 18–23 | 96 (23.8) | 41 (21.5) | 55 (25.9) | |
| 24–36 | 218 (54.1) | 96 (50.3) | 122 (57.6) | |
| > 36 | 89 (22.1) | 54 (28.3) | 35 (16.5) | |
| Cigarette smoking n (%) | 0.14 | |||
| Current | 55 (13.9) | 29 (15.5) | 26 (12.4) | |
| Former | 208 (52.4) | 104 (55.6) | 104 (49.5) | |
| Nonsmoker | 134 (33.8) | 54 (28.9) | 80 (38.1) | |
| Missing | 6 | |||
| BMI (kg/m2) n (%) | 0.58 | |||
| < 24.9 | 66 (16.4) | 34 (17.8) | 32 (15.1) | |
| 25.0–29.9 | 151 (37.5) | 67 (35.1) | 84 (39.6) | |
| ≥ 30 | 186 (46.2) | 90 (47.1) | 96 (45.3) | |
| Waist: hip ratio n (%) | 0.61 | |||
| ≥ 0.90 | 341 (84.8) | 163 (85.8) | 178 (84.0) | |
| < 0.90 | 61 (15.2) | 27 (14.2) | 34 (16.0) | |
| Missing | 1 | |||
| Waist circumference (cm) n (%) | 0.63 | |||
| ≥ 102 | 189 (46.9) | 92 (48.7) | 97 (45.8) | |
| < 102 | 214 (53.1) | 99 (51.8) | 115 (54.3) | |
| Height (cm) mean (± SD) | 175.8 (± 7.52) | 175.8 (± 7.47) | 175.9 (± 7.58) | 0.89 |
| COPD n (%) | <0.0001 * | |||
| Yes | 65 (16.4) | 47 (25.3) | 18 (8.5) | |
| No | 332 (83.6) | 139 (74.7) | 193 (91.5) | |
| Missing | 6 | |||
| Hypertension n (%) | 0.011 * | |||
| Yes | 289 (71.9) | 148 (77.9) | 141 (66.5) | |
| No | 113 (28.1) | 42 (22.1) | 71 (33.5) | |
| Missing | 1 | |||
| Diabetes n (%) | 0.056 | |||
| Yes | 75 (18.6) | 43 (22.5) | 32 (15.1) | |
| No | 328 (81.4) | 148 (77.5) | 180 (84.9) | |
| Spirometry Parameters | All Veterans (n = 403) | Exposed (Herbicide Sprayers) (n = 191) | Unexposed (Herbicide Nonsprayers) (n = 212) | p Value |
|---|---|---|---|---|
| FEV1 mean (± SD) | 2.89 (± 0.66) | 2.85 (± 0.65) | 2.93 (± 0.66) | 0.23 |
| FVC mean (± SD) | 3.97 (± 0.81) | 3.90 (± 0.78) | 4.04 (± 0.83) | 0.082 |
| FEV1/FVC mean (± SD) | 72.78 (± 8.33) | 73.01 (± 8.47) | 72.57 (± 8.22) | 0.60 |
| % of predicted, FEV1 mean (± SD) | 89.96 (± 18.94) | 89.55 (± 19.63) | 90.32 (± 18.33) | 0.69 |
| % of predicted, FVC mean (± SD) | 92.24 (± 16.42) | 91.37 (± 16.32) | 93.03 (± 16.51) | 0.31 |
| Prevalence of abnormality | ||||
| Spirometric restriction a % (95% CI) | 12.7 (9.8, 16.3) | 15.7 (10.6, 20.9) | 9.91 (5.9, 13.9) | 0.081 |
| Mixed process of spirometric restriction and obstruction b % (95% CI) | 4.5 (2.8, 7.0) | 4.7 (1.7, 7.7) | 4.3 (1.5, 7.0) | 0.82 |
| Independent Variables b | aOR (95% CI) |
|---|---|
| Spray status (ref = nonsprayer) | 1.64 (0.82, 3.29) |
| Age, at time of study, 65–69 yrs (ref =59–64 yrs) | 1.16 (0.51, 2.61) |
| Age, 70+ yrs | 2.03 (0.65, 6.37) |
| Race/ethnicity, black (ref = white) | 3.04 (1.36, 6.79) * |
| Race/ethnicity, other nonwhite | 2.78 (0.96, 8.06) |
| Vietnam (ref = non-Vietnam) | 0.88 (0.40, 1.95) |
| Rank (ref = Officer) | 0.57 (0.21, 1.52) |
| Military duration, 24–36 mos (ref =18–23 mos) | 0.70 (0.33, 1.50) |
| Military duration, >36 mos | 0.68 (0.25, 1.90) |
| Cigarette smoker, current (ref = nonsmoker) | 1.43 (0.50, 4.14) |
| Cigarette smoker, former | 1.30 (0.63, 2.70) |
| Waist circumference (≥102 cm versus <102 cm (ref)) | 2.46 (1.25, 4.85) * |
| COPD (ref = no COPD) | 0.83 (0.36, 1.94) |
| Height, cm | 1.04 (1.00, 1.09) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cypel, Y.; Hines, S.E.; Davey, V.J.; Eber, S.M.; Schneiderman, A.I. Spirometric Pulmonary Restriction in Herbicide-Exposed U.S. Vietnam War Veterans. Int. J. Environ. Res. Public Health 2019, 16, 3131. https://doi.org/10.3390/ijerph16173131
Cypel Y, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Spirometric Pulmonary Restriction in Herbicide-Exposed U.S. Vietnam War Veterans. International Journal of Environmental Research and Public Health. 2019; 16(17):3131. https://doi.org/10.3390/ijerph16173131
Chicago/Turabian StyleCypel, Yasmin, Stella E. Hines, Victoria J. Davey, Stephanie M. Eber, and Aaron I. Schneiderman. 2019. "Spirometric Pulmonary Restriction in Herbicide-Exposed U.S. Vietnam War Veterans" International Journal of Environmental Research and Public Health 16, no. 17: 3131. https://doi.org/10.3390/ijerph16173131
APA StyleCypel, Y., Hines, S. E., Davey, V. J., Eber, S. M., & Schneiderman, A. I. (2019). Spirometric Pulmonary Restriction in Herbicide-Exposed U.S. Vietnam War Veterans. International Journal of Environmental Research and Public Health, 16(17), 3131. https://doi.org/10.3390/ijerph16173131





