Spatio-Temporal Variation in Nutrient Profiles and Exchange Fluxes at the Sediment-Water Interface in Yuqiao Reservoir, China
Abstract
1. Introduction
2. Materials and Methods
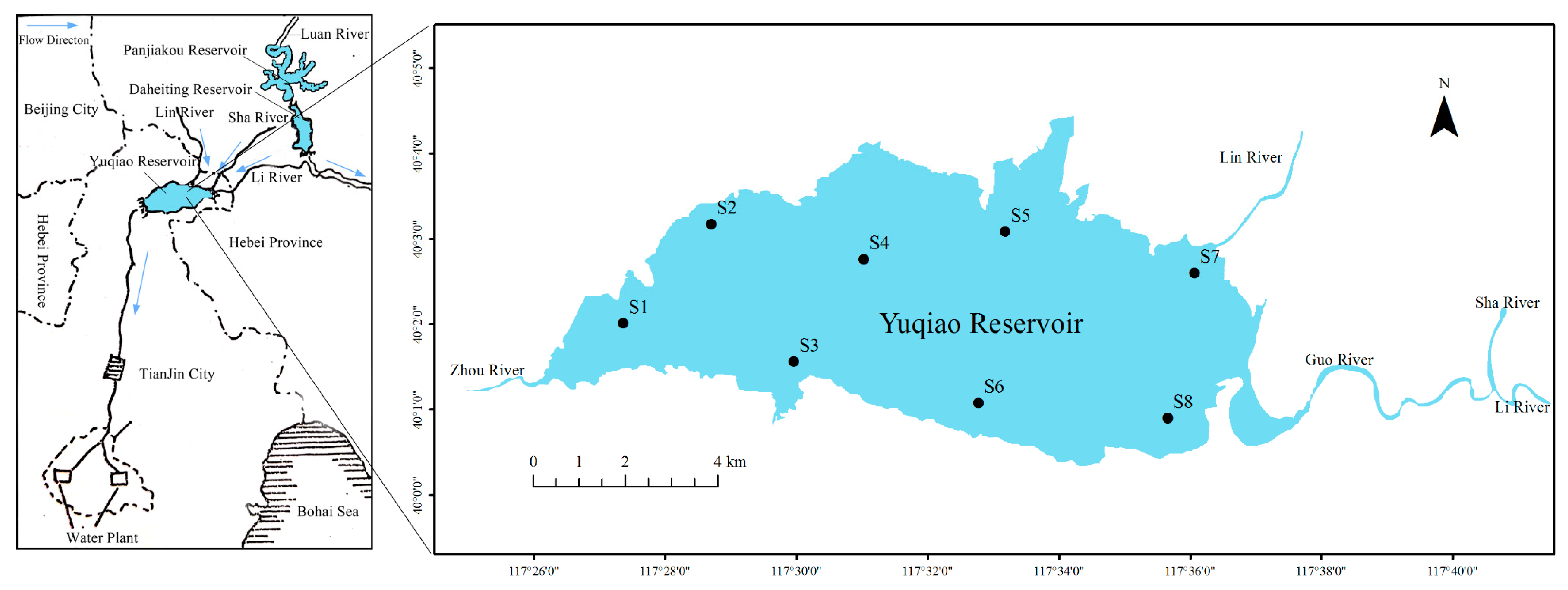
2.1. Site Description
2.2. Field Sampling
2.3. Incubation of Sediment Cores
2.4. Sample Analysis
2.5. Statistical Analysis
3. Results
3.1. Nutrients in the Surface Sediment and Water
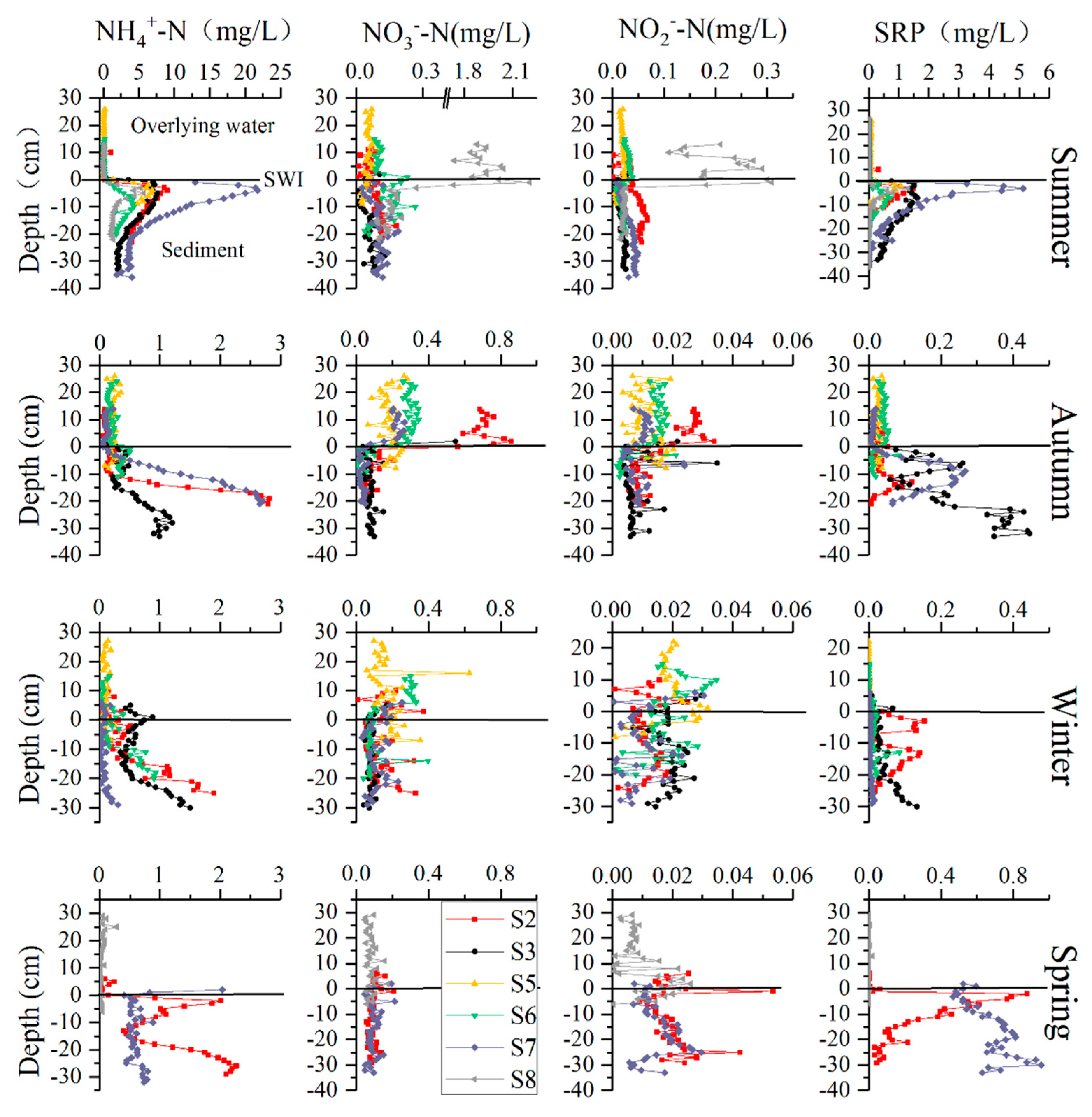
3.2. DIN and SRP Profiles at the SWI
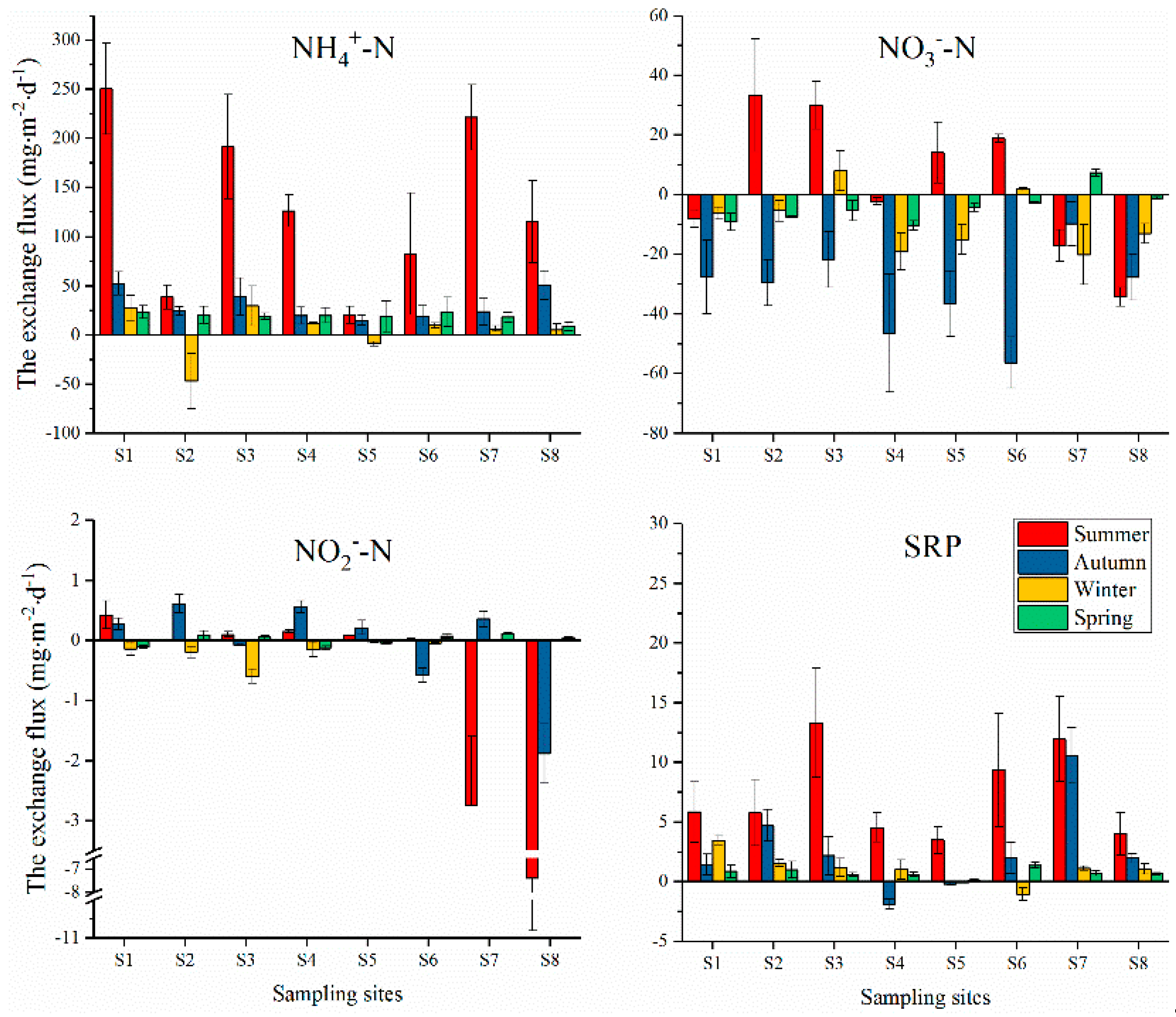
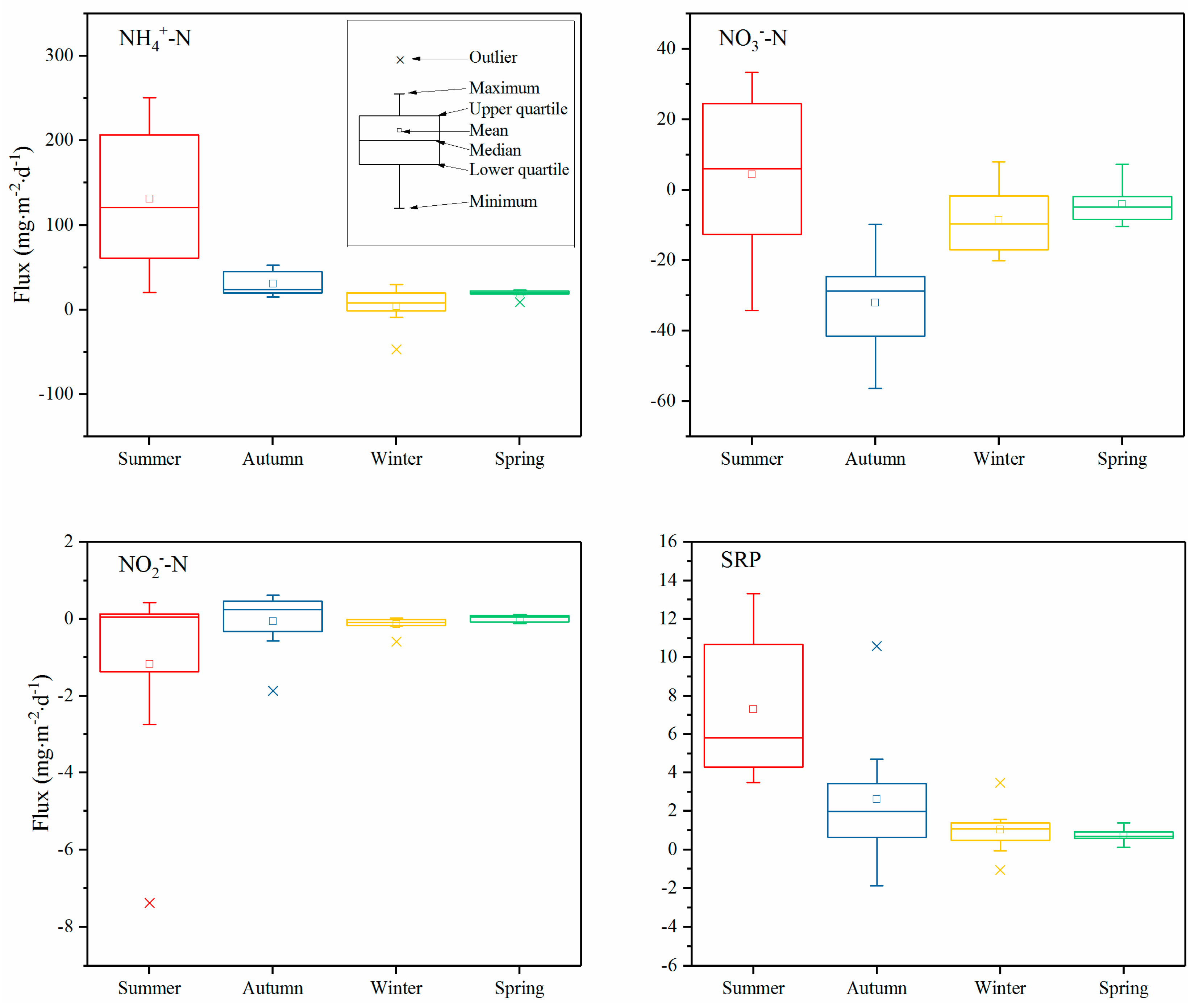
3.3. Fluxes of DIN and SRP at the SWI
4. Discussion
4.1. Characteristics of the Surface Sediments
4.2. Characteristics of Nutrients in the Pore Water
4.3. Nutrient Fluxes at the SWI
4.4. Estimation of Annual DIN and SRP Fluxes in Yuqiao Reservoir
4.5. Countermeasures and Suggestions for Eutrophication Control of Yuqiao Reservoir
5. Conclusions
- High TN, TP, and TOC contents occurred in the sediments at Yuqiao Reservoir compared with other eutrophic lakes and reservoirs. Concentrations of NH4+-N and SRP in the pore water are generally higher than those in the overlying water in contrast to those of NO3−-N and NO2−-N. NH4+-N was the main form of DIN in the pore water.
- The fluxes of NH4+-N, NO3−-N, NO2−-N, and SRP indicate that the sediment was the source of NH4+-N and SRP, while it was a sink for NO3−-N and NO2−-N. The fluxes of NH4+-N, NO3−-N, and SRP were higher in the summer compared to other seasons. At the annual scale, the sediment released 1133.15 and 92.46 tons of N and P, respectively, to the overlying water, which indicates that the internal release of N and P plays an important role in maintaining the eutrophic status of Yuqiao Reservoir. Technical measures such as sediment dredging should be adopted to control the internal load.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemley, D.A.; Adams, J.B. Eutrophication. In Encyclopedia of Ecology, 2nd ed.; Elsevier: Oxford, UK, 2018; pp. 86–90. [Google Scholar]
- Nowlin, W.H.; Evarts, J.L.; Vanni, M.J. Release rates and potential fates of nitrogen and phosphorus from sediments in a eutrophic reservoir. Freshw. Biol. 2005, 50, 301–322. [Google Scholar] [CrossRef]
- Orihel, D.M.; Hadas, O.; Pinkas, R.; Viner-Mozzini, Y.; Sukenik, A. Internal nutrient loading may increase microcystin concentrations in freshwater lakes by promoting growth of Microcystis populations. Ann. Limnol.-Int. J. Limnol. 2013, 49, 225–235. [Google Scholar] [CrossRef][Green Version]
- Yu, L.; Kong, F.X.; Zhang, M.; Yang, Z.; Shi, X.L.; Du, M.Y. The dynamics of microcystis genotypes and microcystin production and associations with environmental factors during blooms in Lake Chaohu, China. Toxins 2014, 6, 3238–3257. [Google Scholar] [CrossRef]
- Bhadha, J.H.; Jawitz, J.W.; Min, J.H. Phosphorus mass balance and internal load in an impacted subtropical isolated wetland. Water Air Soil Pollut. 2011, 218, 619–632. [Google Scholar] [CrossRef]
- Ozkundakci, D.; Hamilton, D.P.; Scholes, P. Effect of intensive catchment and in-lake restoration procedures on phosphorus concentrations in a eutrophic lake. Ecol. Eng. 2010, 36, 396–405. [Google Scholar] [CrossRef]
- Ignatieva, N.V. Nutrient exchange across the sediment-water interface in the eastern Gulf of Finland. Boreal Environ. Res. 1999, 4, 295–305. [Google Scholar]
- Callender, E.; Hammond, D.E. Nutrient exchange across the sediment-water interface in the Potomac River Estuary. Estuar. Coast. Shelf Sci. 1982, 15, 395–413. [Google Scholar] [CrossRef]
- Serpa, D.; Falcao, M.; Duarte, P.; da Fonseca, L.C.; Vale, C. Evaluation of ammonium and phosphate release from intertidal and subtidal sediments of a shallow coastal lagoon (Ria Formosa-Portugal): A modelling approach. Biogeochemistry 2007, 82, 291–304. [Google Scholar] [CrossRef]
- Watson, P.G.; Frickers, P.E.; Howland, R.J.M. Benthic fluxes of nutrients and some trace metals in the Tamar estuary, SW England. Netherland J. Aquat. Ecol. 1993, 27, 135–146. [Google Scholar] [CrossRef]
- Zabel, M.; Dahmke, A.; Schulz, H.D. Regional distribution of diffusive phosphate and silicate fluxes through the sediment-water interface: The eastern South Atlantic. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 1998, 45, 277–300. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Yin, K.; Ying, L.; Zhang, D.; Yang, Y.; Huang, X. Pore water nutrient characteristics and the fluxes across the sediment in the Pearl River estuary and adjacent waters, China. Estuar. Coast. Shelf Sci. 2013, 133, 182–192. [Google Scholar] [CrossRef]
- Yin, H.B.; Zhu, J.C.; Tang, W.Y. Management of nitrogen and phosphorus internal loading from polluted river sediment using Phoslock (R) and modified zeolite with intensive tubificid oligochaetes bioturbation. Chem. Eng. J. 2018, 353, 46–55. [Google Scholar] [CrossRef]
- Cermelj, B.; Bertuzzi, A.; Faganeli, J. Modelling of pore water nutrient distribution and benthic fluxes in shallow coastal waters (Gulf of Trieste, Northern Adriatic). Water Air Soil Pollut. 1997, 99, 435–443. [Google Scholar] [CrossRef]
- Jin, D.Y.; Huang, Y.J. The environmental problems and controlling countermeasures of Tianjin Yuqiao Reservoir. Res. Environ. Sci. 2004, 17, 77–79. (In Chinese) [Google Scholar]
- Johnston, S.G.; Burton, E.D.; Keene, A.F.; Bush, R.T.; Sullivan, L.A.; Isaacson, L. Pore water sampling in acid sulfate soils: a new peeper method. J. Environ. Qual. 2009, 38, 2474–2477. [Google Scholar] [CrossRef]
- Xu, D.; Wu, W.; Ding, S.M.; Sun, Q.; Zhang, C.S. A high-resolution dialysis technique for rapid determination of dissolved reactive phosphate and ferrous iron in pore water of sediments. Sci. Total Environ. 2012, 421, 245–252. [Google Scholar] [CrossRef]
- Fan, C.X.; Zhang, L.; Yang, L.Y.; Huang, W.Y.; Xu, P.Z. Simulation of internal loadings of nitrogen and phosphorus in a Lake. Oceanol. Limnol. Sin. 2002, 33, 370–378. (In Chinese) [Google Scholar]
- Li, B.; Ding, S.M.; Fan, C.X.; Zhong, J.C.; Zhao, B.; Yin, H.B.; Zhang, L. Estimation of releasing fluxes of sediment nitrogen and phosphorus in Fubao Bay in Dianchi Lake. Environ. Sci. 2008, 29, 114–120. (In Chinese) [Google Scholar]
- Jin, X.C.; Tu, Q.Y. The Standard Methods in Lake Eutrophication Investigation, 2nd ed.; China Environmental Science Press: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Grasshoff, K.; Erhardt, M.; Kremling, K. Methods of Seawater Analysis. In Marine Pollution Bulletin, 2nd revised and extended edition; Verlag Chemie GmbH: Weiheim, Germany, 1984; Volume 15, pp. 313–348. [Google Scholar]
- Ni, L.X.; Li, D.D.; Su, L.L.; Xu, J.J.; Li, S.Y.; Ye, X.; Geng, H.; Wang, P.F.; Li, Y.; Li, Y.P.; et al. Effects of algae growth on cadmium remobilization and ecological risk in sediments of Taihu Lake. Chemosphere 2016, 151, 37–44. [Google Scholar] [CrossRef]
- Tendaupenyu, P.; Magadza, C.H.D. Nutrient concentrations in the surface sediments of Lake Chivero, Zimbabwe: A shallow, hypereutrophic, subtropical artificial lake. Lakes Reserv. Res. Manag. 2017, 22, 297–309. [Google Scholar] [CrossRef]
- Wu, T.F.; Qin, B.Q.; Brookes, J.D.; Yan, W.M.; Ji, X.Y.; Feng, J. Spatial distribution of sediment nitrogen and phosphorus in Lake Taihu from a hydrodynamics-induced transport perspective. Sci. Total Environ. 2019, 650, 1554–1565. [Google Scholar] [CrossRef]
- Zhou, F.X.; Gao, X.L.; Zhang, Y.; Yuan, H.M.; Song, J.M.; Liu, K.; Yang, B.; Zhuang, W. Potential mobility of inorganic nutrients and its controls at the sediment water interface in the main path of Kuroshio Current off eastern Taiwan. Mar. Pollut. Bull. 2017, 119, 270–276. [Google Scholar] [CrossRef]
- Knosche, R. Organic sediment nutrient concentrations and their relationship with the hydrological connectivity of floodplain waters (River Havel, NE Germany). Hydrobiologia 2006, 560, 63–76. [Google Scholar] [CrossRef]
- Chai, B.B. Study on Pollutants Release from Sediments and the Multi-Phase Interface Effect; Xi’an University of Architecture and Technology: Xi’An, China, 2008; pp. 38–51. (In Chinese) [Google Scholar]
- Lerat, Y.; Lasserre, P.; Lecorre, P. Seasonal-Changes in Pore Water Concentrations of Nutrients and Their Diffusive Fluxes at the Sediment Water Interface. J. Exp. Mar. Biol. Ecol. 1990, 135, 135–160. [Google Scholar] [CrossRef]
- Garcia-Robledo, E.; Corzo, A. Effects of macroalgal blooms on carbon and nitrogen biogeochemical cycling in photoautotrophic sediments: An experimental mesocosm. Mar. Pollut. Bull. 2011, 62, 1550–1556. [Google Scholar] [CrossRef]
- Denis, L.; Grenz, C. Spatial variability in oxygen and nutrient fluxes at the sediment-water interface on the continental shelf in the Gulf of Lions (NW Mediterranean). Oceanol. Acta 2003, 26, 373–389. [Google Scholar] [CrossRef]
- Jiang, X.; Wen, S.L.; Yao, S.C.; Zheng, X.L.; Kang, D.J.; Zhong, J.C. Environmental significance of phosphorus existing forms in the sediments of Yuqiao Reservoir in Tianjin. J. Lake Sci. 2018, 30, 628–639. (In Chinese) [Google Scholar]
- Gunnars, A.; Blomqvist, S. Phosphate exchange across the sediment-water interface when shifting from anoxic to oxic conditions—An experimental comparison of freshwater and brackish-marine systems. Biogeochemistry 1997, 37, 203–226. [Google Scholar] [CrossRef]
- Jensen, H.S.; Mortensen, P.B.; Andersen, F.O.; Rasmussen, E.; Jensen, A. Phosphorus Cycling in a Coastal Marine Sediment, Aarhus Bay, Denmark. Limnol. Oceanogr. 1995, 40, 908–917. [Google Scholar] [CrossRef]
- Petranich, E.; Covelli, S.; Acquavita, A.; De Vittor, C.; Faganeli, J.; Contin, M. Benthic nutrient cycling at the sediment-water interface in a lagoon fish farming system (northern Adriatic Sea, Italy). Sci. Total Environ. 2018, 644, 137–149. [Google Scholar] [CrossRef]
- Fan, C.X.; Zhang, L.; Qin, B.Q.; Hu, W.P.; Gao, G.; Wang, J.J. Migration mechanism of biogenic elements and their quantification on the sediment-water interface of Lake Taihu:1. Spatial variation of the ammonium release rates and its source and sink fluxes. J. Lake Sci. 2004, 16, 10–20. (In Chinese) [Google Scholar]
- Wang, Z.Q.; Li, B.; Liang, R.J.; Wang, L.Z. Comparative study on endogenous release of nitrogen and phosphorus in Nansi Lake, China. Acta Sci. Circumstantiae 2013, 33, 487–493. (In Chinese) [Google Scholar]
- Huang, T.L.; Liu, F.; Shi, J.C. Distribution features and diffusion fluxes of nutrient in interstitial water of a source water reservoir. Chin. J. Environ. Eng. 2016, 10, 4357–4363. (In Chinese) [Google Scholar]
- Bowden, W.B. A N-15 Isotope-Dilution Study of Ammonium Production and Consumption in a Marsh Sediment. Limnol. Oceanogr. 1984, 29, 1004–1015. [Google Scholar] [CrossRef]
- Rocha, C. Rhythmic ammonium regeneration and flushing in intertidal sediments of the Sado estuary. Limnol. Oceanogr. 1998, 43, 823–831. [Google Scholar] [CrossRef]
- Gomez, E.; Durillon, C.; Rofes, G.; Picot, B. Phosphate adsorption and release from sediments of brackish lagoons: pH, O2 and loading influence. Water Res. 1999, 33, 2437–2447. [Google Scholar] [CrossRef]
- Mu, D.; Yuan, D.K.; Feng, H.; Xing, F.W.; Teo, F.Y.; Li, S.Z. Nutrient fluxes across sediment-water interface in Bohai Bay Coastal Zone, China. Mar. Pollut. Bull. 2017, 114, 705–714. [Google Scholar] [CrossRef]
- Wu, Y.H.; Wen, Y.J.; Zhou, J.X.; Wu, Y.Y. Phosphorus release from lake sediments: Effects of pH, temperature and dissolved oxygen. Ksce J. Civ. Eng. 2014, 18, 323–329. [Google Scholar] [CrossRef]
- Liu, Z.W.; Hu, J.R.; Zhong, P.; Zhang, X.F.; Ning, J.J.; Larsen, S.E.; Chen, D.Y.; Gao, Y.M.; He, H.; Jeppesen, E. Successful restoration of a tropical shallow eutrophic lake: Strong bottom-up but weak top-down effects recorded. Water Res. 2018, 146, 88–97. [Google Scholar] [CrossRef]
- Nizzoli, D.; Bartoli, M.; Cooper, M.; Welsh, D.T.; Underwood, G.J.C.; Viaroli, P. Implications for oxygen, nutrient fluxes and denitrification rates during the early stage of sediment colonisation by the polychaete Nereis spp. in four estuaries. Estuar. Coast. Shelf Sci. 2007, 75, 125–134. [Google Scholar] [CrossRef]
- Yin, S.X.; Chen, D.; Chen, L.M.; Edis, R. Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils. Soil Biol. Biochem. 2002, 34, 1131–1137. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, X.C.; Yao, Y.; Li, L.H.; Wu, F.C. Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China. Water Res. 2008, 42, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Billen, G. Coupled biogeochemical cycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- Morse, J.W.; Morin, J. Ammonium interaction with coastal marine sediments: Influence of redox conditions on K*. Mar. Chem. 2005, 95, 107–112. [Google Scholar] [CrossRef]
- Eckert, W.; Nishri, A.; Parparova, R. Factors regulating the flux of phosphate at the sediment-water interface of a subtropical calcareous lake: A simulation study with intact sediment cores. Water Air Soil Pollut. 1997, 99, 401–409. [Google Scholar] [CrossRef]
- Martins, G.; Terada, A.; Ribeiro, D.C.; Corral, A.M.; Brito, A.G.; Smets, B.F.; Nogueira, R. Structure and activity of lacustrine sediment bacteria involved in nutrient and iron cycles. FEMS Microbiol. Ecol. 2011, 77, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.X.; Peng, S.; Tian, Y.M.; Zhang, H.Y. Effects of dissolved oxygen and nutrient loading on phosphorus fluxes at the sediment-water interface in the Hai River Estuary, China. Mar. Pollut. Bull. 2018, 130, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Glud, R.N.; Berg, P.; Fossing, H.; Jorgensen, B.B. Effect of the diffusive boundary layer on benthic mineralization and O2 distribution: A theoretical model analysis. Limnol. Oceanogr. 2007, 52, 547–557. [Google Scholar] [CrossRef]
- Wang, J.J.; Shen, J.; Zhang, L.; Fan, C.X.; Li, W.C.; Pan, J.Z. Sediment-water nutrient fluxes and the effects of oxygen in Lake Dianchi and Lake Fuxian, Yunnan Province. J. Lake Sci. 2010, 22, 640–648. (In Chinese) [Google Scholar]
- Hemond, H.F.; Lin, K. Nitrate suppresses internal phosphorus loading in an eutrophic lake. Water Res. 2010, 44, 3645–3650. [Google Scholar] [CrossRef]
- Rasmussen, H.; Jorgensen, B.B. Microelectrode studies of seasonal oxygen-uptake in a coastal sediment—Role of molecular-diffusion. Mar. Ecol. Prog. Ser. 1992, 81, 289–303. [Google Scholar] [CrossRef]
- Rysgaard, S.; Christensen, P.B.; Nielsen, L.P. Seasonal-Variation in nitrification and denitrification in estuarine sediment colonized by benthic microalgae and bioturbating infauna. Mar. Ecol. Prog. Ser. 1995, 126, 111–121. [Google Scholar] [CrossRef]
- Tyler, A.C.; McGlathery, K.J.; Anderson, I.C. Benthic algae control sediment-water column fluxes of organic and inorganic nitrogen compounds in a temperate lagoon. Limnol. Oceanogr. 2003, 48, 2125–2137. [Google Scholar] [CrossRef]
- Valdemarsen, T.; Kristensen, E.; Holmer, M. Metabolic threshold and sulfide-buffering in diffusion controlled marine sediments impacted by continuous organic enrichment. Biogeochemistry 2009, 95, 335–353. [Google Scholar] [CrossRef]
| Site | Sediment | Surface Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TN | TP | TOC | Water Content | TN | TP | NH4+-N | NO3−-N | NO2−-N | SRP | |
| mg/kg | mg/kg | % | % | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| S1 | 3208.72 | 555.69 | 3.04 | 74.62 | 3.55 | 0.40 | 0.43 | 0.61 | 0.005 | 0.07 |
| S2 | 3521.97 | 578.51 | 3.38 | 73.92 | 2.13 | 0.27 | 0.54 | 0.38 | 0.007 | 0.09 |
| S3 | 3763.91 | 599.87 | 4.27 | 79.41 | 2.73 | 0.19 | 0.39 | 0.44 | 0.006 | 0.00 1 |
| S4 | 3880.25 | 817.74 | 4.68 | 80.86 | 4.11 | 0.34 | 0.38 | 0.72 | 0.005 | 0.06 |
| S5 | 2507.14 | 630.17 | 2.90 | 65.84 | 2.57 | 0.24 | 0.42 | 0.47 | 0.003 | 0.03 |
| S6 | 2023.53 | 639.90 | 1.84 | 68.79 | 2.67 | 0.20 | 0.49 | 0.50 | 0.004 | 0.01 |
| S7 | 2538.69 | 552.71 | 2.46 | 59.96 | 2.28 | 0.18 | 0.36 | 0.42 | 0.005 | 0.01 |
| S8 | 2179.71 | 532.88 | 2.19 | 65.64 | 3.68 | 0.31 | 0.49 | 0.59 | 0.004 | 0.03 |
| Source | Location | Season | NH4+-N | NO3−-N | NO2−-N | SRP | Method |
|---|---|---|---|---|---|---|---|
| (mg·m−2·Day−1) | (mg·m−2·Day−1) | (mg·m−2·Day−1) | (mg·m−2·Day−1) | ||||
| Fan et al. 2004 [35] | Lake Taihu, China | Summer | 34.1 ± 20.8 | — | — | — | Sediment core incubation |
| Winter | −16.0 ± 17.6 | — | — | — | |||
| Li et al. 2008 [19] | Lake Dianchi, China | Autumn | 22.94–163.12 | — | — | 0.90–2.06 | Sediment core incubation |
| Wang et al. 2013 [36] | Lake Nansihu, China | Summer | 3.1–10.3 | — | — | 0.3–2.7 | Sediment core incubation |
| Petranich et al. 2018 [34] | Marano and Grado Lagoon, Italy | Summer | 24.3 | 22.32 | 0.874 | 2.28 | In-situ benthic chambers |
| Autumn | 10.98 | 32.24 | 0.506 | 0.285 | |||
| Winter | 3.96 | −8.68 | −0.138 | −0.19 | |||
| Denis and Grenz 2003 [30] | Gulf of Lions, NW Mediterranean | Spring | −0.4–3.67 | 1.61–17.55 | −1.7–0.1 | −0.64–2.76 | Sediment core incubation |
| Huang et al. 2016 [37] | Zhoucun Reservoir, China | Autumn | 62.83–133.23 | — | — | 0.36–1.27 | Diffusive (Fick’s Law) |
| This study | Yuqiao Reservoir, China | Spring | 19.00 ± 4.63 | −4.13 ± 5.58 | 0.01 ± 0.09 | 0.74 ± 0.36 | Sediment core incubation |
| Summer | 130.98 ± 84.03 | 4.35 ± 23.78 | −1.17 ± 2.71 | 7.29 ± 3.76 | |||
| Autumn | 30.57 ± 14.79 | −31.96 ± 14.46 | −0.06 ± 0.83 | 2.61 ± 3.75 | |||
| Winter | 4.38 ± 24.09 | −8.60 ± 10.03 | −0.14 ± 0.20 | 1.05 ± 1.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Wu, T.; Yang, J.; Jiang, X.; Zhong, J. Spatio-Temporal Variation in Nutrient Profiles and Exchange Fluxes at the Sediment-Water Interface in Yuqiao Reservoir, China. Int. J. Environ. Res. Public Health 2019, 16, 3071. https://doi.org/10.3390/ijerph16173071
Wen S, Wu T, Yang J, Jiang X, Zhong J. Spatio-Temporal Variation in Nutrient Profiles and Exchange Fluxes at the Sediment-Water Interface in Yuqiao Reservoir, China. International Journal of Environmental Research and Public Health. 2019; 16(17):3071. https://doi.org/10.3390/ijerph16173071
Chicago/Turabian StyleWen, Shuailong, Tao Wu, Jie Yang, Xue Jiang, and Jicheng Zhong. 2019. "Spatio-Temporal Variation in Nutrient Profiles and Exchange Fluxes at the Sediment-Water Interface in Yuqiao Reservoir, China" International Journal of Environmental Research and Public Health 16, no. 17: 3071. https://doi.org/10.3390/ijerph16173071
APA StyleWen, S., Wu, T., Yang, J., Jiang, X., & Zhong, J. (2019). Spatio-Temporal Variation in Nutrient Profiles and Exchange Fluxes at the Sediment-Water Interface in Yuqiao Reservoir, China. International Journal of Environmental Research and Public Health, 16(17), 3071. https://doi.org/10.3390/ijerph16173071




