Incidence of the Acute Symptom of Chronic Periodontal Disease in Patients Undergoing Supportive Periodontal Therapy: A 5-Year Study Evaluating Climate Variables
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Oral Examination
2.3. Questionnaires
2.4. Climate Data
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Madianos, P.N.; Bobetsis, Y.A.; Kinane, D.F. Generation of inflammatory stimuli: How bacteria set up inflammatory responses in the gingiva. J. Clin. Periodontol. 2005, 32, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.P.V.; Boches, S.K.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Paster, B.J. Comparisons of Subgingival Microbial Profiles of Refractory Periodontitis, Severe Periodontitis and Periodontal Health using the Human Oral Microbe Identification Microarray (HOMIM). J. Periodontol. 2009, 80, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C.; Kornman, K.S. The pathogenesis of human periodontitis: An introduction. Periodontology 2000 1997, 14, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Benakanakere, M.; Kinane, D.F. Innate cellular responses to the periodontal biofilm. Front. Oral Biol. 2012, 15, 41–55. [Google Scholar]
- JSP Clinical Practice Guideline for the Periodontal Treatment. 2015. Available online: www.perio.jp/publication/upload_file/guideline_perio_plan2015_en.pdf (accessed on 25 July 2019).
- Sanz-Miralles, E.C.; Twigg, J.; Bravo, M.; Manresa, C. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Parameter on Periodontal Maintenance. J. Periodontol. 2000, 71, 849–850. [CrossRef]
- Cohen, R.E. Research, Science and Therapy Committee, American Academy of Periodontology. Position paper: Periodontal maintenance. J. Periodontol. 2003, 74, 1395–1401. [Google Scholar]
- Gaunt, F.; Devine, M.; Pennington, M.; Vernazza, C.; Gwynnett, E.; Steen, N.; Heasman, P.; Vernazza, C. The cost-effectiveness of supportive periodontal care for patients with chronic periodontitis. J. Clin. Periodontol. 2008, 35, 67–82. [Google Scholar] [CrossRef]
- Silva, G.L.M.; Soares, R.V.; Zenóbio, E.G. Periodontal Abscess during Supportive Periodontal Therapy: A Review of the Literature. J. Contemp. Dent. Pract. 2008, 9, 82–91. [Google Scholar] [CrossRef]
- Herrera, D.; Roldán, S.; Sanz, M. The periodontal abscess: A review. J. Clin. Periodontol. 2000, 27, 377–386. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Wang, B.; Yang, H.; Ban, J.; Liu, F.; Li, T. Acute effects of temperature exposure on blood pressure: An hourly level panel study. Environ. Int. 2019, 124, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Palma, A.; Stegmayr, B.; Wauthier, M.; Torres, A.; Argilés, À. Blood Pressure Seasonality in Hemodialysis Patients from Five European Cities of Different Latitudes. Kidney Blood Press. Res. 2018, 43, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, N.G.; Zenchenko, T.A.; Boyko, E.R. Annual blood pressure dynamics and weather sensitivity in women. Ter. Arkhiv. 2017, 89, 56. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, J.M.; Dosil Díaz, O.; Taboada Hidalgo, J.J.; Fernández, J.R.; Sánchez-Santos, L. Influence of weather in the incidence of acute myocardial infarction in Galicia. Med. Clin. (Barc.) 2015, 145, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Su, H. Effects of climatic temperature stress on cardiovascular diseases. Eur. J. Intern. Med. 2010, 21, 164–167. (In Spain) [Google Scholar] [CrossRef] [PubMed]
- Shanks, N.J.; Papworth, G. Environmental factors and heatstroke. Occup. Med. 2001, 51, 45–49. [Google Scholar] [CrossRef]
- Tamasauskiene, L.; Rastenyte, D.; Radisauskas, R.; Tamosiunas, A.; Tamasauskas, D.; Vaiciulis, V.; Kranciukaite-Butylkiniene, D.; Milinaviciene, E. Relationship of meteorological factors and acute stroke events in Kaunas (Lithuania) in 2000–2010. Environ. Sci. Pollut. Res. 2017, 24, 9286–9293. [Google Scholar] [CrossRef]
- MacGregor, D.M. Effect of weather on attendance with injury at a paediatric emergency department. Emerg. Medi. J. EMJ 2003, 20, 204–205. [Google Scholar] [CrossRef]
- Alahmad, B.; Shakarchi, A.; AlSeaidan, M.; Fox, M. The effects of temperature on short-term mortality risk in Kuwait: A time-series analysis. Environ. Res. 2019, 171, 278–284. [Google Scholar] [CrossRef]
- Ehelepola, N.D.B.; Ariyaratne, K.; Jayaratne, A. The association between local meteorological changes and exacerbation of acute wheezing in Kandy, Sri Lanka. Glob. Health Action 2018, 11, 1482998. [Google Scholar] [CrossRef]
- Kunikullaya, K.U.; Vijayaraghava, A.; Asha, P.; Kunnavil, R.; Muralimohan, B. Meteorological parameters and pollutants on asthma exacerbation in Bangalore, India—An ecological retrospective time-series study. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 133–141. [Google Scholar] [CrossRef]
- O’Hare, C.; O’Sullivan, V.; Flood, S.; Kenny, R.A. Seasonal and meteorological associations with depressive symptoms in older adults: A geo-epidemiological study. J. Affect. Disord. 2016, 191, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Bullock, B.; Murray, G.; Meyer, D. Highs and lows, ups and downs: Meteorology and mood in bipolar disorder. PLoS ONE 2017, 12, e0173431. [Google Scholar] [CrossRef] [PubMed]
- Vergés, J.; Montell, E.; Tomàs, E.; Cumelles, G.; Castañeda, G.; Marti, N.; Möller, I. Weather conditions can influence rheumatic diseases. Proc. West. Pharmacol. Soc. 2004, 47, 134–136. [Google Scholar] [PubMed]
- Strusberg, I.; Mendelberg, R.C.; Serra, A.H.; Strusberg, A.M. Influence of weather conditions on rheumatic pain. J. Rheumatol. 2002, 29, 335–338. [Google Scholar]
- Macfarlane, T.V.; McBeth, J.; Jones, G.T.; Nicholl, B.; Macfarlane, G.J. Whether the weather influences pain? Results from the EpiFunD study in North West England. Rheumatology 2010, 49, 1513–1520. [Google Scholar] [CrossRef]
- Edefonti, V.C.; Bravi, F.; Cioffi, I.; Capuozzo, R.; Ammendola, L.; Abate, G.; DeCarli, A.; Ferraroni, M.; Farella, M.; Michelotti, A. Chronic pain and weather conditions in patients suffering from temporomandibular disorders: A pilot study. Community Dent. Oral Epidemiol. 2012, 40, 56–64. [Google Scholar] [CrossRef]
- Kloss-Brandstätter, A.; Hächl, O.; Leitgeb, P.C.; Buchner, A.; Coassin, S.; Rasse, M.; Kronenberg, F.; Kloss, F.R. Epidemiologic evidence of barometric pressure changes inducing increased reporting of oral pain. Eur. J. Pain. 2011, 15, 844–880. [Google Scholar] [CrossRef]
- Takeuchi, N.; Ekuni, D.; Tomofuji, T.; Morita, M. Relationship between Acute Phase of Chronic Periodontitis and Meteorological Factors in the Maintenance Phase of Periodontal Treatment: A Pilot Study. Int. J. Environ. Res. Public Health 2015, 12, 9119–9130. [Google Scholar] [CrossRef]
- Oberg, A.L.; Ferguson, J.A.; McIntyre, L.M.; Horner, R.D. Incidence of stroke and season of the year: Evidence of an association. Am. J. Epidemiol. 2000, 152, 558–564. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R. Supportive periodontal therapy. Periodontol 2000 2004, 36, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Development of a Classification System for Periodontal Diseases and Conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Kasai, S.; Imamura, K.; Ihara, Y.; Kita, D.; Ota, K.; Sekino, J.; Nakagawa, T.; Saito, A. Changes in antimicrobial susceptibility profile and prevalence of quinolone low-sensitive strains in subgingival plaque from acute periodontal lesions after systemic administration of sitafloxacin. Microb. Pathog. 2015, 79, 41–46. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, S.; Kinsella, A.; O’Callaghan, E. The effects of daily weather variables on psychosis admissions to psychiatric hospitals. Int. J. Biometeorol. 2013, 57, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Japan Meteorological Agency. Available online: https://www.jma.go.jp/jma/index.html (accessed on 25 July 2019).
- Abraham, B.; Ledolter, J. Statistical Methods for Forecasting; Wiley: New York, NY, USA, 1983; pp. 225–229, 336–355. [Google Scholar]
- Kitano, Y.; Yamada, T.J. The relationship between explosive cyclon through northern japan and pacific blocking. Jpn. J. Civ. Eng. 2016, 72, 121–126. [Google Scholar]
- Sato, J. Whether change and pain. Jpn. J. Spinal Surg. 2015, 29, 153–156. [Google Scholar] [CrossRef]
- Sato, J. Possible mechanism of weather related pain. Jpn. J. Biometeor. 2003, 40, 219–224. [Google Scholar]
- Sato, J.; Takanari, K.; Omura, S.; Mizumura, K. Effects of lowering barometric pressure on guarding behavior, heart rate and blood pressure in a rat model of neuropathic pain. Neurosci. Lett. 2001, 299, 17–20. [Google Scholar] [CrossRef]
- Jentsch, H.F.R.; März, D.; Krüger, M. The effects of stress hormones on growth of selected periodontitis related bacteria. Anaerobe 2013, 24, 49–54. [Google Scholar] [CrossRef]
- Hodges, F.R. Barodontalgia at 12,000 feet. J. Am. Dent. Assoc. 1978, 97, 66–68. [Google Scholar] [CrossRef]
- Fledelius, H.C. High atmospheric pressure and myopic shift in caisson workers. Lancet 2003, 361, 362. [Google Scholar] [CrossRef]
- Muhm, J.M.; Rock, P.B.; McMullin, D.L.; Jones, S.P.; Lu, I.; Eilers, K.D.; Space, D.R.; McMullen, A. Effect of Aircraft-Cabin Altitude on Passenger Discomfort. N. Engl. J. Med. 2007, 357, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.J.; Youn, C.E.; Haltiner, A.M.; Watson, N.F. Do Weather-Related Ambient Atmospheric-Pressure Changes Influence Sleep Disordered Breathing? J. Clin. Sleep Med. 2010, 6, 152–156. [Google Scholar] [PubMed]
- Brown, H.K.; Simpson, J.A.; Murchison, J.T.; Simpson, A.J. The influence of meteorological variables on the development of deep venous thrombosis. Thromb. Haemost. 2009, 102, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Zadik, Y. Barodontalgia. J. Endod. 2009, 35, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Panagiotakos, D.; Picciotto, S.; Katsouyanni, K.; Löwel, H.; Jacquemin, B.; Lanki, T.; Stafoggia, M.; Bellander, T.; Koenig, W.; et al. Air Temperature and Inflammatory Responses in Myocardial Infarction Survivors. Epidemiology 2008, 19, 391–400. [Google Scholar] [CrossRef]
- Schäuble, C.L.; Hampel, R.; Breitner, S.; Ruckerl, R.; Phipps, R.; Diaz-Sanchez, D.; Devlin, R.B.; Carter, J.D.; Soukup, J.; Silbajoris, R.; et al. Short-term effects of air temperature on blood markers of coagulation and inflammation in potentially susceptible individuals. Occup. Environ. Med. 2012, 69, 670–678. [Google Scholar] [CrossRef]
- D’Amato, G.; Holgate, S.T.; Pawankar, R.; Ledford, D.K.; Cecchi, L.; Al-Ahmad, M.; Al-Enezi, F.; Al-Muhsen, S.; Ansotegui, I.; Baena-Cagnani, C.E.; et al. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organ. J. 2015, 8, 1–52. [Google Scholar] [CrossRef]
- Proud, D.; Bailey, G.; Naclerio, R.; Reynolds, C.; Cruz, A.; Eggleston, P.; Lichtenstein, L.; Togias, A. Tryptase and histamine as markers to evaluate mast cell activation during the responses to nasal challenge with allergen, cold, dry air, and hyperosmolar solutions. J. Allergy Clin. Immunol. 1992, 89, 1098–1110. [Google Scholar] [CrossRef]
- Tseng, C.M.; Chen, Y.T.; Ou, S.M.; Hsiao, Y.H.; Li, S.Y.; Wang, S.J.; Yang, A.C.; Chen, T.J.; Perng, D.W. The Effect of Cold Temperature on Increased Exacerbation of Chronic Obstructive Pulmonary Disease: A Nationwide Study. PLoS ONE 2013, 8, e57066. [Google Scholar] [CrossRef]
- Falagas, M.E.; Theocharis, G.; Spanos, A.; Vlara, L.A.; Issaris, E.A.; Panos, G.; Peppas, G. Effect of meteorological variables on the incidence of respiratory tract infections. Respir. Med. 2008, 102, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.W.; Chan, K.P.; Hui, D.S.; Goddard, J.R.; Shaw, J.G.; Reid, D.W.; Yang, I.A. Acute exacerbation of COPD. Respirology 2016, 21, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, L.; Kan, H.; Xu, J.; Chen, R.; Liu, Y.; Wang, W. Effects of Meteorological Factors on Daily Hospital Admissions for Asthma in Adults: A Time-Series Analysis. PLoS ONE 2014, 9, e102475. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, C.; Hu, W.; Turner, L.R.; Su, H.; Tong, S. Extreme temperatures and emergency department admissions for childhood asthma in Brisbane, Australia. Occup. Environ. Med. 2013, 70, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Alonso, B.; de Arriba, L.; Santa Cruz, I.; Serrano, C.; Sanz, M. Acute periodontal lesions. Periodontology 2000 2014, 65, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Amar, S. Identification of Proteins Differentially Expressed in Human Monocytes Exposed to Porphyromonas gingivalis and Its Purified Components by High-Throughput Immunoblotting. Infect. Immun. 2006, 74, 1204–1214. [Google Scholar] [CrossRef]
- Gokyu, M.; Kobayashi, H.; Nanbara, H.; Sudo, T.; Ikeda, Y.; Suda, T.; Izumi, Y. Thrombospondin-1 Production Is Enhanced by Porphyromonas gingivalis Lipopolysaccharide in THP-1 Cells. PLoS ONE 2014, 9, e115107. [Google Scholar] [CrossRef]
- Barksby, H.E.; Nile, C.J.; Jaedicke, K.M.; Taylor, J.J.; Preshaw, P.M. Differential expression of immunoregulatory genes in monocytes in response to Porphyromonas gingivalis and Escherichia coli lipopolysaccharide. Clin. Exp. Immunol. 2009, 156, 479–487. [Google Scholar] [CrossRef]
- Thurnheer, T.; Belibasakis, G.N.; Bostanci, N. Colonisation of gingival epithelia by subgingival biofilms in vitro: Role of “red complex” bacteria. Arch. Oral Boil. 2014, 59, 977–986. [Google Scholar] [CrossRef]
- Ahl, D.R.; Hilgeman, J.L.; Snyder, J.D. Periodontal emergencies. Dent. Clin. N. Am. 1986, 30, 459–472. [Google Scholar]
- Lewis, A.M.; Meechan, C.; Macfarlane, T.W.; Lamey, P.J.; Kay, E. Presentation and antimicrobial treatment of acute orofacial infections in general dental practice. Br. Dent. J. 1989, 166, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.L.; Flanary, D.B.; Newell, D.H. The prevalence of periodontal abscess. J. Indiana Dent. Assoc. 1994, 73, 18–23. [Google Scholar] [PubMed]
- Kaldahl, W.B.; Kalkwarf, K.L.; Patil, K.D.; Molvar, M.P.; Dyer, J.K. Long-Term Evaluation of Periodontal Therapy: I. Response to 4 Therapeutic Modalities. J. Periodontol. 1996, 67, 93–102. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.E.; Lainson, P.A.; Spivey, J.D. Tooth Loss Due to Periodontal Abscess: A Retrospective Study. J. Periodontol. 1997, 68, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Laino, L.; Herford, A.S.; Lauritano, F.; Giudice, G.L.; Fama, F.; Santoro, R.; Troiano, G.; Iannello, G.; et al. Oral Health Impact Profile in Celiac Patients: Analysis of Recent Findings in a Literature Review. Gastroenterol. Res. Pract. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Terranova, A.; Briguglio, F.; De Stefano, R.; Famà, F.; D’Amico, C.; Amoroso, G.; Marino, S.; Gorassini, F.; Mastroieni, R.; et al. Diabetes: Oral Health Related Quality of Life and Oral Alterations. BioMed Res. Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L. Chlorhexidine Gel Use in the Oral District: A Systematic Review. Gels 2019, 5, 31. [Google Scholar] [CrossRef]
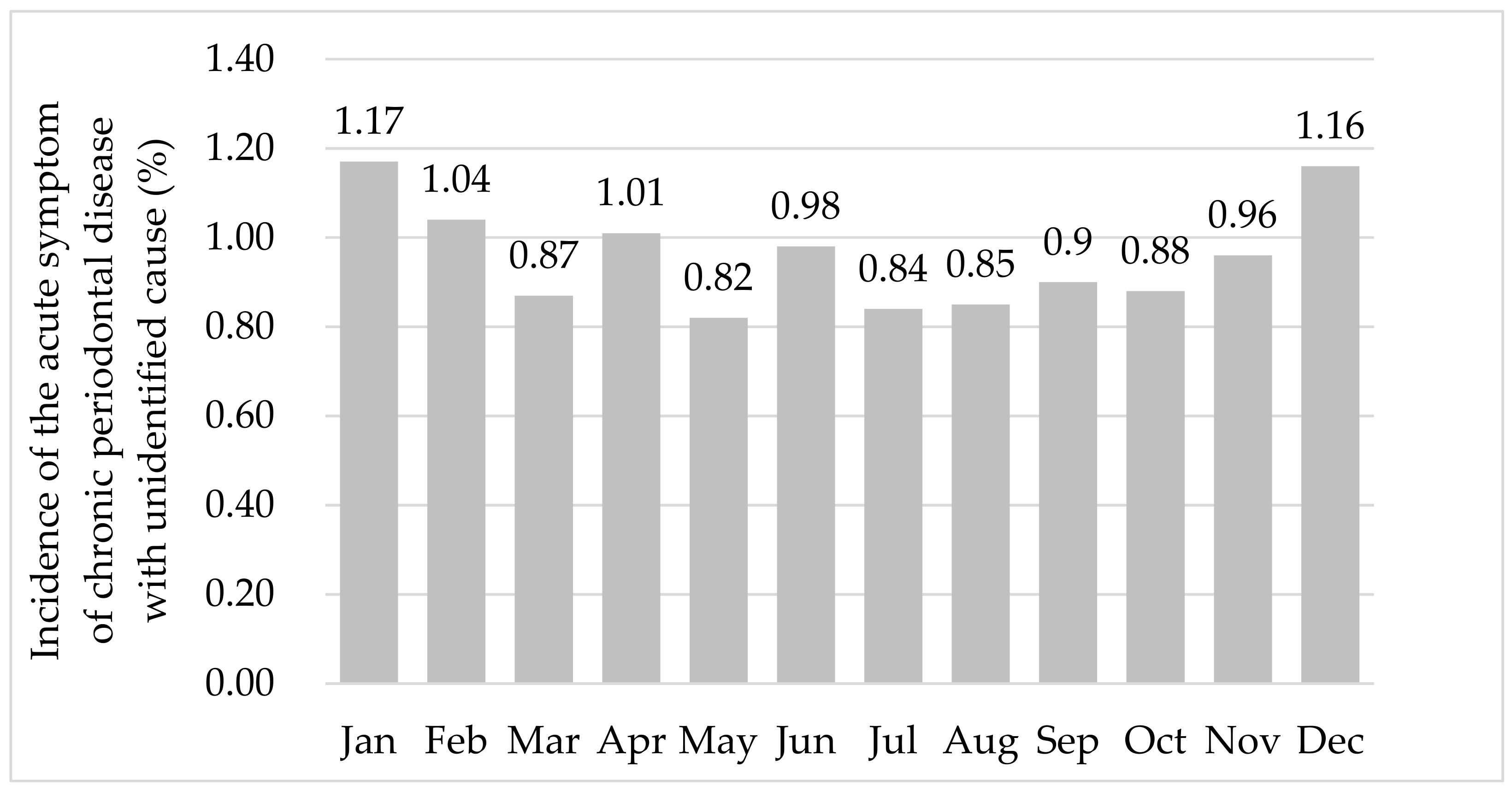

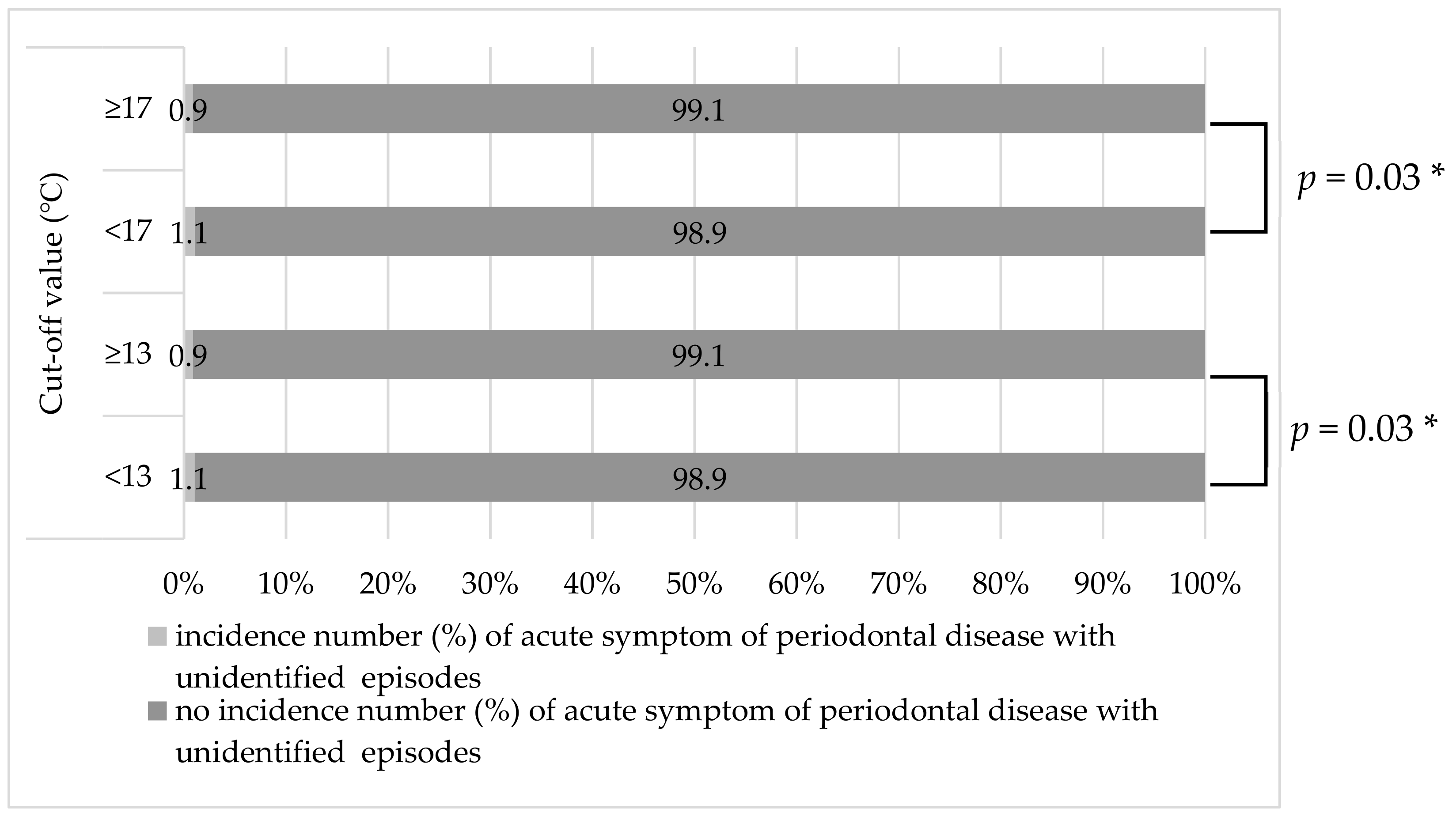
| Age (Years) | Male | Female | Total |
|---|---|---|---|
| Number of Patients Who Had Received Supportive Periodontal Therapy | |||
| 20–29 | 198 | 327 | 525 |
| 30–39 | 294 | 932 | 1226 |
| 40–49 | 897 | 2170 | 3067 |
| 50–59 | 1623 | 4856 | 6479 |
| 60–69 | 4657 | 11,160 | 15,817 |
| 70–79 | 4685 | 10,047 | 14,732 |
| ≥80 | 2348 | 3665 | 6013 |
| Total | 14,702 | 33,157 | 47,859 |
| Number of Patients with Acute Symptom of Chronic Periodontal Disease with Unidentified Cause | |||
| 20–29 | 1 | 2 | 3 |
| 30–39 | 1 | 4 | 5 |
| 40–49 | 9 | 16 | 25 |
| 50–59 | 19 | 26 | 45 |
| 60–69 | 46 | 103 | 149 |
| 70–79 | 62 | 116 | 178 |
| ≥80 | 16 | 37 | 53 |
| Total | 154 | 304 | 458 |
| Parameter | Mean ± SD | Lag Time a | r | p Value * |
|---|---|---|---|---|
| Wind speed (m/s) | ||||
| Mean daily wind speed | 3.0 ± 1.2 | Omitted | ||
| Maximum daily wind speed | 6.6 ± 2.5 | Omitted | ||
| Minimum daily wind speed | 0.6 ± 0.6 | Omitted | ||
| Barometric Pressure (hPa) | ||||
| Mean daily barometric pressure | 1013.5 ± 7.0 | 3 | 0.242 | 0.896 |
| Maximum daily barometric pressure | 1016.3 ± 7.0 | Omitted | ||
| Minimum daily barometric pressure | 1010.7 ± 7.2 | 3 | 0.260 | 0.884 |
| Daily range of barometric pressure | 5.5 ± 3.3 | Omitted | ||
| Maximum hourly increase in barometric pressure | 0.8 ± 0.4 | Omitted | ||
| Maximum hourly decrease in barometric pressure | 1.0 ± 0.4 | 2 | 0.043 | 0.123 |
| Total hours of sunlight (h) | 13.7 ± 9.6 | Omitted | ||
| Total daily rainfall (mm) | 3.5 ± 10.0 | Omitted | ||
| Temperature (°C) | ||||
| Mean daily temperature | 16.3 ± 8.6 | 1 | 0.009 | 0.027 |
| Maximum daily temperature | 20.7 ± 8.8 | Omitted | ||
| Minimum daily temperature | 12.3 ± 8.9 | Omitted | ||
| Daily range of temperature | 8.4 ± 2.9 | Omitted | ||
| Maximum hourly increase in temperature | 2.1 ± 0.8 | Omitted | ||
| Maximum hourly decrease in temperature | 1.5 ± 0.7 | Omitted | ||
| Humidity (%) | ||||
| Mean daily humidity | 67.0 ± 11.4 | Omitted | ||
| Maximum daily humidity | 85.5 ± 8.7 | Omitted | ||
| Minimum daily humidity | 46.1 ± 14.5 | Omitted | ||
| Daily humidity range | 39.4 ± 11.5 | Omitted |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saho, H.; Takeuchi, N.; Ekuni, D.; Morita, M. Incidence of the Acute Symptom of Chronic Periodontal Disease in Patients Undergoing Supportive Periodontal Therapy: A 5-Year Study Evaluating Climate Variables. Int. J. Environ. Res. Public Health 2019, 16, 3070. https://doi.org/10.3390/ijerph16173070
Saho H, Takeuchi N, Ekuni D, Morita M. Incidence of the Acute Symptom of Chronic Periodontal Disease in Patients Undergoing Supportive Periodontal Therapy: A 5-Year Study Evaluating Climate Variables. International Journal of Environmental Research and Public Health. 2019; 16(17):3070. https://doi.org/10.3390/ijerph16173070
Chicago/Turabian StyleSaho, Hikari, Noriko Takeuchi, Daisuke Ekuni, and Manabu Morita. 2019. "Incidence of the Acute Symptom of Chronic Periodontal Disease in Patients Undergoing Supportive Periodontal Therapy: A 5-Year Study Evaluating Climate Variables" International Journal of Environmental Research and Public Health 16, no. 17: 3070. https://doi.org/10.3390/ijerph16173070
APA StyleSaho, H., Takeuchi, N., Ekuni, D., & Morita, M. (2019). Incidence of the Acute Symptom of Chronic Periodontal Disease in Patients Undergoing Supportive Periodontal Therapy: A 5-Year Study Evaluating Climate Variables. International Journal of Environmental Research and Public Health, 16(17), 3070. https://doi.org/10.3390/ijerph16173070





