Economic Impact of Pharmacist-Participated Medication Management for Elderly Patients in Nursing Homes: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Quality Assessment
2.4. Data Extraction and Analysis
3. Results
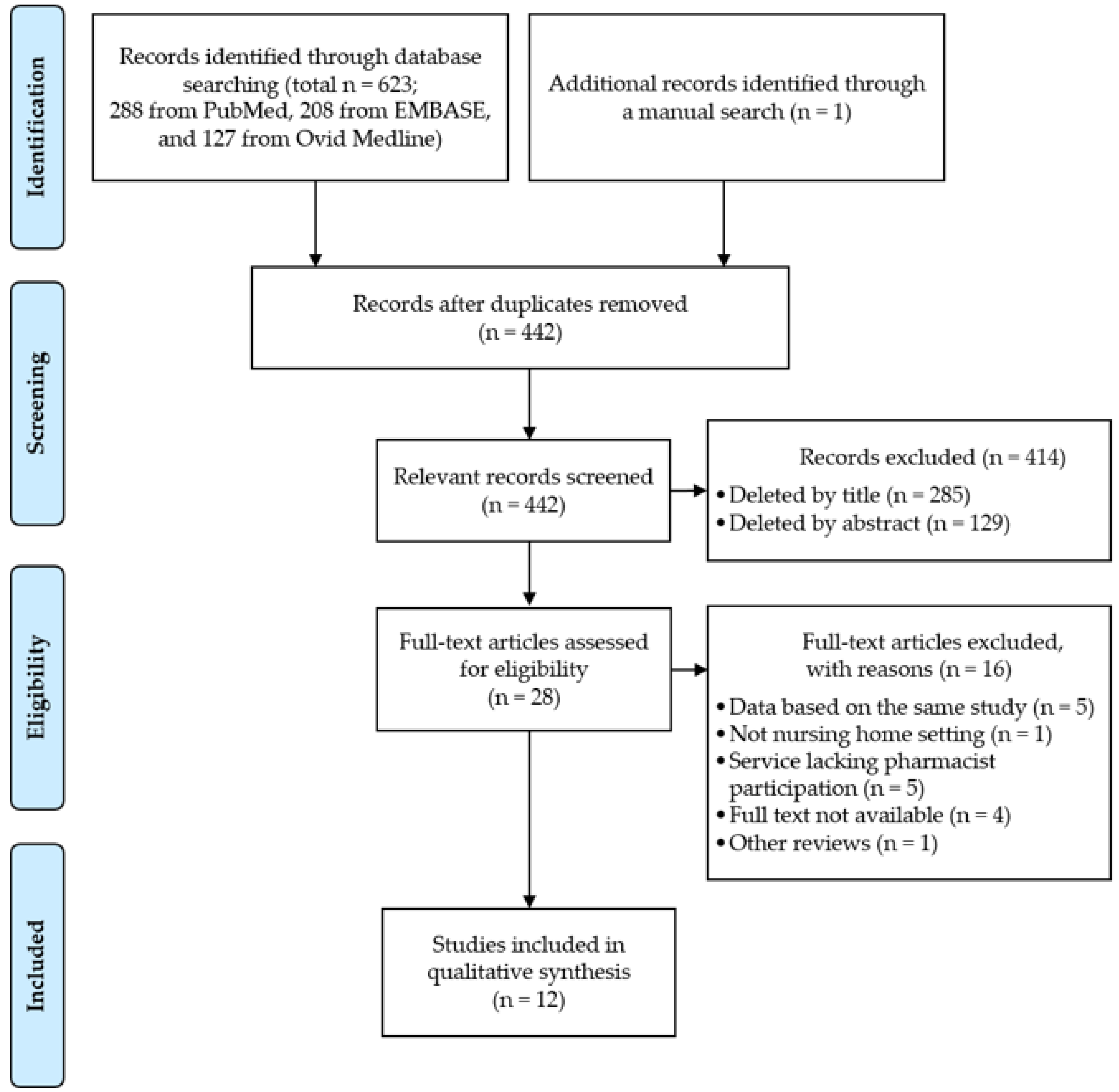
3.1. Search Results
3.2. Quality Assessment
3.3. Literature Evaluation
3.3.1. Effect of Interprofessional Networks
3.3.2. Effect of Interprofessional Coordination
3.3.3. Effect of Interprofessional Teamwork
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hagen, S. Rising Demand for Long-Term Services and Supports for Elderly People; Congressional Budget Office: Washington DC, USA, 2013. Available online: https://www.cbo.gov/sites/default/files/113th-congress-2013–2014/reports/44363-ltc.pdf. (accessed on 2 May 2019).
- Rolland, Y.; Aquino, J.P.; Andrieu, S.; Beard, J.; Benetos, A.; Berrut, G.; Coll-Planas, L.; Dartigues, J.F.; Dong, B.; Forette, F.; et al. Identification of the main domains for quality of care and clinical research in nursing homes. J. Nutr. Health Aging 2011, 15, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Jackson, S.H. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 2004, 57, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Alldred, D.P.; Kennedy, M.C.; Hughes, C.; Chen, T.F.; Miller, P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst. Rev. 2016, 2, CD009095. [Google Scholar] [CrossRef] [Green Version]
- Cool, C.; Cestac, P.; Laborde, C.; Lebaudy, C.; Rouch, L.; Lepage, B.; Vellas, B.; Barreto, P.S.; Rolland, Y.; Lapeyre-Mestre, M. Potentially inappropriate drug prescribing and associated factors in nursing homes. J. Am. Med. Dir. Assoc. 2014, 15, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Boscardin, W.J.; Steinman, M.A.; Schwartz, J.B. Patterns of chronic co-morbid medical conditions in older residents of U.S. nursing homes: Differences between the sexes and across the agespan. J. Nutr. Health Aging 2014, 18, 429–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opondo, D.; Eslami, S.; Visscher, S.; de Rooij, S.E.; Verheij, R.; Korevaar, J.C.; Abu-Hanna, A. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: A systematic review. PLoS ONE 2012, 7, e43617. [Google Scholar] [CrossRef] [PubMed]
- Tommelein, E.; Mehuys, E.; Petrovic, M.; Somers, A.; Colin, P.; Boussery, K. Potentially inappropriate prescribing in community-dwelling older people across Europe: A systematic literature review. Eur. J. Clin. Pharmacol. 2015, 71, 1415–1427. [Google Scholar] [CrossRef]
- Morin, L.; Laroche, M.L.; Texier, G.; Johnell, K. Prevalence of Potentially Inappropriate Medication Use in Older Adults Living in Nursing Homes: A Systematic Review. J. Am. Med. Dir. Assoc. 2016, 17, 861–869. [Google Scholar] [CrossRef]
- Centers for Medicare and Medicaid Services. State Operations Manual, Appendix PP: Guidance to Surveyors for Long Term Care Facilities (Rev 107, 04-04-14); Centers for Medicare and Medicaid: Baltimore, MD, USA, 2009.
- Peris-Marti, J.F.; Fernandez-Villalba, E.; Bravo-Jose, P.; Saez-Lleo, C.; Garcia-Mina Freire, M. Reflection on the pharmaceutical service in nursing homes; understanding reality to cover needs. Farm. Hosp. 2016, 40, 302–315. [Google Scholar]
- Doty, P. Cost-Effectiveness of Home and Community-Based Long-Term Care Services; Department of Health and Human Services: Washington DC, USA, 1999. Available online: http://aspe.hhs.gov/system/files/pdf/73916/costeff.pdf (accessed on 2 May 2019).
- Bootman, J.L.; Harrison, D.L.; Cox, E. The health care cost of drug-related morbidity and mortality in nursing facilities. Arch. Intern. Med. 1997, 157, 2089–2096. [Google Scholar] [CrossRef]
- Kaye, H.S.; Harrington, C.; LaPlante, M.P. Long-term care: Who gets it, who provides it, who pays, and how much? Health (Millwood) 2010, 29, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Trygstad, T.K.; Christensen, D.B.; Wegner, S.E.; Sullivan, R.; Garmise, J.M. Analysis of the North Carolina long-term care polypharmacy initiative: A multiple-cohort approach using propensity-score matching for both evaluation and targeting. Clin. Ther. 2009, 31, 2018–2037. [Google Scholar] [CrossRef] [PubMed]
- Verrue, C.; Mehuys, E.; Boussery, K.; Adriaens, E.; Remon, J.P.; Petrovic, M. A pharmacist-conducted medication review in nursing home residents: Impact on the appropriateness of prescribing. Acta. Clin. Belg. 2012, 67, 423–429. [Google Scholar] [PubMed]
- Willan, A.R.; Briggs, A.H. The Statistical Analysis of Cost-Effectiveness Data; Wiley: Chichester, UK, 2006. [Google Scholar]
- Deeks, J.J.; Dinnes, J.; D’Amico, R.; Sowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G.; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating non-randomised intervention studies. Health Technol. Assess. 2003, 7, 1–173. [Google Scholar] [CrossRef]
- Thomas, B.H.; Ciliska, D.; Dobbins, M.; Micucci, S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evid. Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Barr, H.; Koppel, I.; Reeves, S.; Hammick, M.; Freeth, D.S. Effective Interprofessional Education: Argument, Assumption and Evidence; Wiley: Oxford, UK, 2005. [Google Scholar]
- Reeves, S.; Lewin, S.; Espin, S.; Zwarenstein, M. Interprofessional Teamwork for Health and Social Care; Blackwell: London, UK, 2010. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cooper, J.W., Jr. Consultant pharmacist drug therapy recommendations from monthly drug regimen reviews in a geriatric nursing facility: A two-year study and cost analysis. J. Nutr. Health Aging 1997, 1, 181–184. [Google Scholar] [CrossRef]
- Burns, A.; Furniss, L.; Cooke, J.; Lloyd Craig, S.K.; Scobie, S. Pharmacist medication review in nursing homes: A cost analysis. Int. J. Geriatr. Psychopharmacol. 2000, 2, 137–141. [Google Scholar]
- King, M.A.; Roberts, M.S. Multidisciplinary case conference reviews: Improving outcomes for nursing home residents, carers and health professionals. Pharm. World Sci. 2001, 23, 41–45. [Google Scholar] [CrossRef]
- Roberts, M.S.; Stokes, J.A.; King, M.A.; Lynne, T.A.; Purdie, D.M.; Glasziou, P.P.; Wilson, D.A.; McCarthy, S.T.; Brooks, G.E.; de Looze, F.J.; et al. Outcomes of a randomized controlled trial of a clinical pharmacy intervention in 52 nursing homes. Br. J. Clin. Pharmacol. 2001, 51, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.; Trygstad, T.; Sullivan, R.; Garmise, J.; Wegner, S.E. A pharmacy management intervention for optimizing drug therapy for nursing home patients. Am. J. Geriatr. Pharmacother. 2004, 2, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Crotty, M.; Halbert, J.; Rowett, D.; Giles, L.; Birks, R.; Williams, H.; Whitehead, C. An outreach geriatric medication advisory service in residential aged care: A randomised controlled trial of case conferencing. Age Ageing 2004, 33, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.W.; Wade, W.E.; Cook, C.L.; Burfield, A.H. Consultant pharmacist drug therapy recommendations acceptance and rejection from monthly drug regimen reviews in a geriatric nursing facility: Fourth year results and cost analysis. Hosp. Pharm. 2007, 42, 729–736. [Google Scholar] [CrossRef]
- Vu, T.; Harris, A.; Duncan, G.; Sussman, G. Cost-effectiveness of multidisciplinary wound care in nursing homes: A pseudo-randomized pragmatic cluster trial. Fam. Pract. 2007, 24, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Locca, J.F.; Ruggli, M.; Buchmann, M.; Huguenin, J.; Bugnon, O. Development of pharmaceutical care services in nursing homes: Practice and research in a Swiss canton. Pharm. World Sci. 2009, 31, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.M.; Hughes, C.M.; Cardwell, C.; Lapane, K.L.; Murray, A.M.; Crealey, G.E. A cluster randomized controlled trial of an adapted U.S. model of pharmaceutical care for nursing home residents in Northern Ireland (Fleetwood Northern Ireland study): A cost-effectiveness analysis. J. Am. Geriatr. Soc. 2011, 59, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Jõdar-Sánchez, F.; Martín, J.J.; Lõpez Del Amo, M.P.; García, L.; Araújo-Santos, J.M.; Epstein, D. Cost-utility analysis of a pharmacotherapy follow-up for elderly nursing home residents in Spain. J. Am. Geriatr. Soc. 2014, 62, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.S.; Ho, J.A.; Lim, B.D. Pharmacist review and its impact on Singapore nursing homes. Singap. Med. J. 2015, 56, 493–501. [Google Scholar] [CrossRef]
- World Health Organization. Global Health and Aging; National Institute of Aging: Bethesda, MD, USA, 2011; Available online: http://www.who.int/ageing/publications/global_health.pdf (accessed on 2 May 2019).
- Jokanovic, N.; Tan, E.C.; Sudhakaran, S.; Kirkpatrick, C.M.; Dooley, M.J.; Ryan-Atwood, T.E.; Bell, J.S. Pharmacist-led medication review in community settings: An overview of systematic reviews. Res. Social Adm. Pharm. 2017, 13, 661–685. [Google Scholar] [CrossRef]
- Loh, Z.W.; Cheen, M.H.; Wee, H.L. Humanistic and economic outcomes of pharmacist-provided medication review in the community-dwelling elderly: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2016, 41, 621–633. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S.S.; Thiruchelvam, K.; Kow, C.S.; Ghori, M.U.; Babar, Z.U. Economic evaluation of pharmacist-led medication reviews in residential aged care facilities. Expert Rev. Pharmacoecon. Outcomes Res. 2017, 17, 431–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desborough, J.; Houghton, J.; Wood, J.; Wright, D.; Holland, R.; Sach, T.; Ashwell, S.; Shaw, V. Multi-professional clinical medication reviews in care homes for the elderly: Study protocol for a randomised controlled trial with cost effectiveness analysis. Trials 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Nakayama, K.; Togari, T.; Suzuki, K.; Hayashi, N.; Murakami, Y.; Iioka, Y.; Osaka, W.; Yagasaki, K.; Nakamura, S.; et al. Information sharing and case conference among the multidisciplinary team improve patients’ perceptions of care. Open Nurs. J. 2011, 5, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kane, B.; Luz, S. Information sharing at multidisciplinary medical team meetings. Group Decis. Negot. 2011, 20, 437–464. [Google Scholar] [CrossRef]
- Devitt, B.; Philip, J.; McLachlan, S.A. Team dynamics, decision making, and attitudes toward multidisciplinary cancer meetings: Health professionals’ perspectives. J. Oncol. Pract. 2010, 6, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.; Markle-Reid, M. An interprofessional team approach to fall prevention for older home care clients ‘at risk’ of falling: Health care providers share their experiences. Int. J. Integr. Care 2009, 9, e15. [Google Scholar] [CrossRef]
- Liedtka, J.M.; Whitten, E. Enhancing care delivery through cross-disciplinary collaboration: A case study. J. Healthc. Manag. 1998, 43, 185–203. [Google Scholar] [CrossRef]
- Davidsson, M.; Vibe, O.E.; Ruths, S.; Blix, H.S. A multidisciplinary approach to improve drug therapy in nursing homes. J. Multidiscip. Healthc. 2011, 4, 9–13. [Google Scholar] [Green Version]
- King, N.; Ross, A. Professional identities and interprofessional relations: Evaluation of collaborative community schemes. Soc. Work Health Care 2003, 38, 51–72. [Google Scholar] [CrossRef]
- Holland, R.; Desborough, J.; Goodyer, L.; Hall, S.; Wright, D.; Loke, Y.K. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2008, 65, 303–316. [Google Scholar] [CrossRef] [Green Version]
- McDonough, R.P.; Doucette, W.R. Building working relationships with providers. J. Am. Pharm. Assoc. 2003, 43, 44–45. [Google Scholar]
- Zermansky, A.G.; Silcock, J. Is medication review by primary-care pharmacists for older people cost effective? A narrative review of the literature, focusing on costs and benefits. Pharmacoeconomics 2009, 27, 11–24. [Google Scholar] [CrossRef] [PubMed]
| Study (Year) | Selection Bias | Study Design | Confounders | Blinding | Data Collection Methods | Withdrawals and Drop-Outs | Intervention Integrity | Analyses | Global Rating | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of Participants Receiving the Allocated Intervention | Intervention Consistency Measured | Unintended Intervention | Indication of the Allocation Unit | Indication of the Analysis Unit | Appropriate Statistical Methods | Analysis by Intervention Allocation Status | ||||||||
| Cooper et al. (1997) [23] | Moderate | Moderate | Weak | Moderate | Strong | Weak | Uncertain | Yes | No | Organization/institution | Individual | Yes | Uncertain | Weak |
| Burns et al. (2000) [24] | Moderate | Strong | Weak | Moderate | Strong | Strong | 80–100 | Yes | No | Organization/institution | Individual | Yes | No | Moderate |
| King et al. (2001) [25] | Strong | Strong | Strong | Moderate | Strong | Strong | <60 | No | No | Individual | Individual | Yes | Yes | Strong |
| Roberts et al. (2001) [26] | Weak | Strong | Strong | Moderate | Strong | Strong | 80–100 | Yes | No | Organization/institution | Organization/institution | Yes | Yes | Moderate |
| Christensen et al. (2004) [27] | Strong | Moderate | Strong | Moderate | Strong | Moderate | 80–100 | Yes | No | Organization/institution | Individual | Yes | No | Strong |
| Crotty et al. (2004) [28] | Moderate | Strong | Strong | Strong | Strong | Moderate | 80–100 | Yes | No | Organization/institution | Organization/institution | Yes | Yes | Strong |
| Cooper et al. (2007) [29] | Moderate | Moderate | Weak | Moderate | Strong | Weak | Uncertain | Yes | No | Organization/institution | Individual | Yes | Uncertain | Weak |
| Vu et al. (2007) [30] | Moderate | Strong | Moderate | Moderate | Strong | Weak | 80–100 | Yes | No | Organization/institution | Individual | Yes | Yes | Moderate |
| Locca et al. (2009) [31] | Moderate | Moderate | Strong | Moderate | Strong | Strong | 80–100 | Yes | No | Organization/institution | Organization/institution | Yes | Yes | Strong |
| Patterson et al. (2011) [32] | Moderate | Strong | Strong | Strong | Strong | Moderate | 80–100 | Yes | No | Organization/institution | Individual | Yes | Yes | Strong |
| Jodar-Sanchez et al. (2014) [33] | Strong | Strong | Weak | Moderate | Strong | Strong | 80–100 | Uncertain | No | Organization/institution | Individual | Yes | Uncertain | Moderate |
| Chia et al. (2015) [34] | Moderate | Moderate | Weak | Moderate | Strong | Weak | 80–100 | Uncertain | No | Organization/institution | Individual | Yes | Uncertain | Weak |
| Study (Year, Country) | Type of Study | No. of NHs (Subjects) | Subject Mean Age (Years) | Duration of Intervention (Months) | Type of Economic Analysis | Main Interventions | Economic Outcomes |
|---|---|---|---|---|---|---|---|
| King et al. (2001, Australia) [25] | Controlled clinical trial | 3 (245) | 81.0 | 9 | CMA |
|
|
| Crotty et al. (2004, Australia) [28] | Cluster RCT | 10 (154) | 85.0 | 3 | CMA |
|
|
| Study (Year, Country) | Type of Study | No. of NHs (Subjects) | Subject Mean Age (Years) | Duration of Intervention (Months) | Type of Economic Analysis | Main Interventions | Economic Outcomes |
|---|---|---|---|---|---|---|---|
| Cooper Jr (1997, USA) [23] | Prospective cohort study | 1 (204) | 83.2 | 24 | CBA |
|
|
| Burns et al. (2000, UK) [24] | Cluster RCT | 14 (335) | 83.5 | 4 | CMA |
|
|
| Roberts et al. (2001, Australia) [26] | Cluster RCT | 52 (3230) | N/A | 12 | CMA |
|
|
| Cooper Jr JW. (2007, USA) [29] | Prospective cohort study | 1 (184) | 84.3 | 12 | CBA |
|
|
| Jõdar-Sánchez F, et al. (2014, Spain) [33] | Controlled clinical trial | 15 (332) | 81.6 | 12 | CUA |
|
|
| Chia HS, et al. (2015, Singapore) [34] | Retrospective before-and-after study | 3 (480) | N/A | 6 | CMA |
|
|
| Study (Year, Country) | Type of Study | No. of NHs (Subjects) | Subject Mean Age (Years) | Duration of Intervention (Months) | Type of Economic Analysis | Main Interventions | Economic Outcomes |
|---|---|---|---|---|---|---|---|
| Christensen et al. (2004, USA) [27] | Retrospective, before-and-after study | 235 (9280) | 76.8 | 6 | CMA |
|
|
| Vu et al. (2007, Australia) [30] | Cluster RCT | 44 (176) | 83.3 | 6 | CEA |
|
|
| Locca et al. (2009, Switzerland) [31] | Prospective cohort study | 42 (2214) | 83.2 | 48 | CMA |
|
|
| Patterson et al. (2011, UK) [32] | Cluster RCT | 22 (253) | 82.4 | 12 | CEA |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, A.; Moon, Y.J.; Song, Y.-K.; Yun, H.-Y.; Kim, K. Economic Impact of Pharmacist-Participated Medication Management for Elderly Patients in Nursing Homes: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2955. https://doi.org/10.3390/ijerph16162955
Kwak A, Moon YJ, Song Y-K, Yun H-Y, Kim K. Economic Impact of Pharmacist-Participated Medication Management for Elderly Patients in Nursing Homes: A Systematic Review. International Journal of Environmental Research and Public Health. 2019; 16(16):2955. https://doi.org/10.3390/ijerph16162955
Chicago/Turabian StyleKwak, Arim, Yoo Jin Moon, Yun-Kyoung Song, Hwi-Yeol Yun, and Kyungim Kim. 2019. "Economic Impact of Pharmacist-Participated Medication Management for Elderly Patients in Nursing Homes: A Systematic Review" International Journal of Environmental Research and Public Health 16, no. 16: 2955. https://doi.org/10.3390/ijerph16162955
APA StyleKwak, A., Moon, Y. J., Song, Y.-K., Yun, H.-Y., & Kim, K. (2019). Economic Impact of Pharmacist-Participated Medication Management for Elderly Patients in Nursing Homes: A Systematic Review. International Journal of Environmental Research and Public Health, 16(16), 2955. https://doi.org/10.3390/ijerph16162955





