Dissipation Behavior of Three Pesticides in Prickly Pear (Opuntia ficus-indica (L.) Mill.) Pads in Morelos, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Calibration Graphs
2.3. Sampling Site
2.4. Field Experiments
2.5. Active Ingredients
2.6. Experimental Design
2.7. Sample Preparation and Analysis
2.8. Evaluation of Recovery Percentages
2.9. Liquid Chromatography Analysis
2.10. Dissipation Curves
3. Results and Discussion
3.1. Method Performance
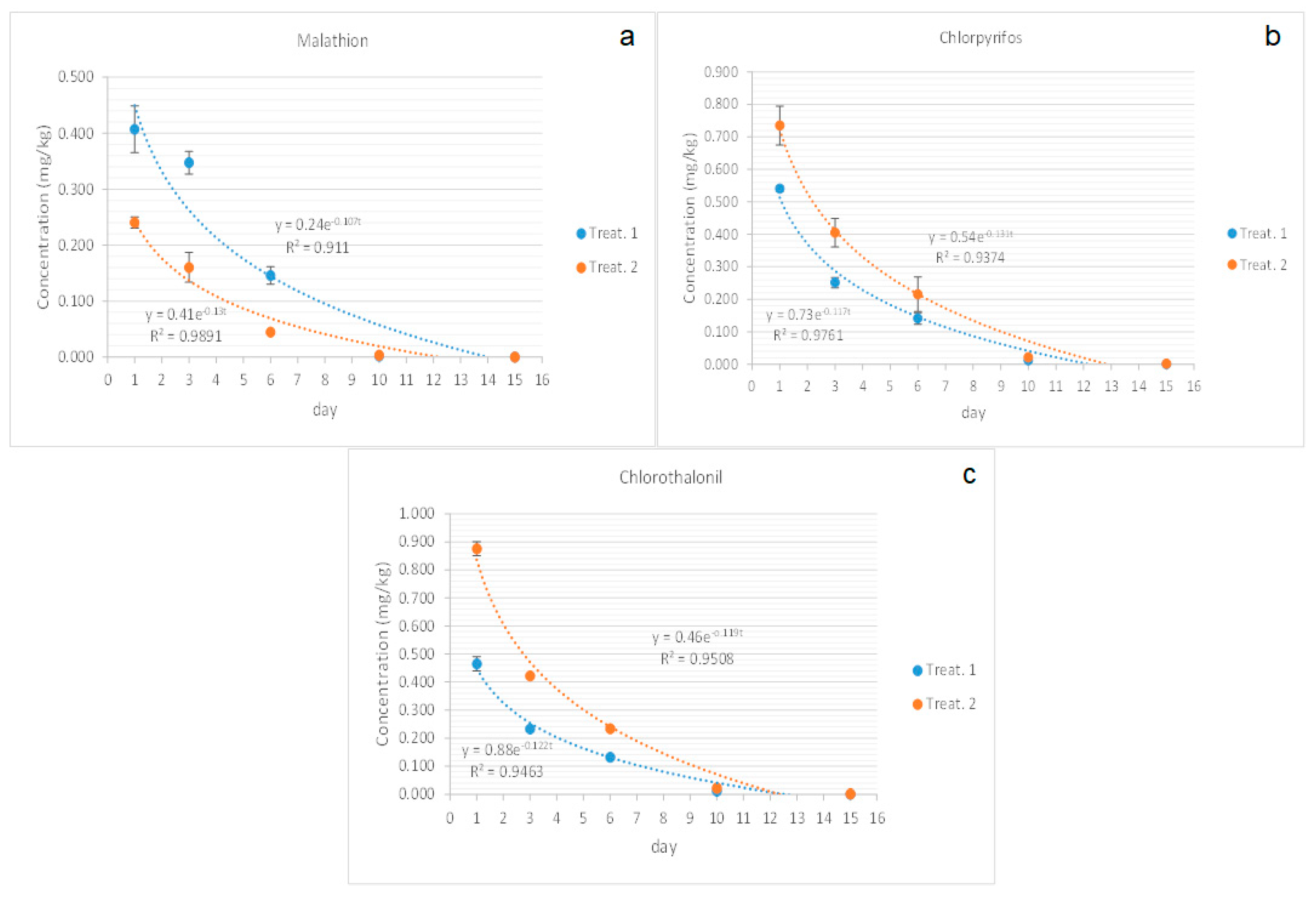
3.2. Dissipation Curves
3.2.1. Malathion
3.2.2. Chlorpyrifos
3.2.3. Chlorothalonil
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiesling, R. New synonyms of Opuntia ficus-indica (Cactaceae). Hickenia 1999, 2, 309–314. [Google Scholar]
- Atlas Agroalimentario 2018. Available online: https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2018/AtlasAgroalimentario-2018 (accessed on 16 March 2019).
- Vargas, A.; Flores, A.; Basaldua, J.F. Dinámica poblacional de las principales plagas de nopal Opuntia spp. en la zona semiárida de Querétaro. Rev. Chapingo Ser. Zonas. Árid. 2008, 7, 21–27. Available online: https://www.redalyc.org/articulo.oa?id=455545066004 (accessed on 22 November 2018).
- Mann, J. Cactus-feeding insects and mites. Bull. United States Nat. Mus. 1969, 256, 1–158. Available online: https://repository.si.edu/handle/10088/10142 (accessed on 22 November 2018). [CrossRef]
- Badii, M.H.; Flores, A.E. Prickly pear cacti pests and their control in México. Fla. Entomol. 2001, 84, 503–505. Available online: http://journals.fcla.edu/flaent/article/view/74995 (accessed on 24 November 2018). [CrossRef]
- Chávez-Moreno, C.K.; Tecante, A.; Casas, A.; Claps, L.E. Distribution and habitat in Mexico of Dactylopius Costa (Hemiptera: Dactylopiidae) and their cacti hosts (Cactaceae: Upontioideae). Neotrop. Entomol. 2011, 40, 62–71. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1519-566X2011000100009&lng=en&nrm=iso&tlng=en (accessed on 27 November 2018). [CrossRef] [PubMed]
- Cerón-González, C.; Rodríguez-Leyva, E.; Lomeli-Flores, J.R.; Hernández-Olmos, C.E.; Peña-Martínez, R.; Mora-Aguilera, G. Evaluación de insecticidas sintéticos sobre adultos de Metamasius spinolae (Coleóptera: Curculionidae) procedentes de Tlalnepantla, Morelos. Rev. Mex. Cienc. Agríc. 2012, 3, 217–229. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-09342012000200001 (accessed on 22 November 2018).
- Angeles-Núñez, J.G.; Anaya-López, J.L.; Arévalo-Galarza, M.L.; Leyva-Ruela, G.; Anaya, S.; Martínez-Martínez, T.O. Análisis de la calidad sanitaria de nopal verdura en Otumba, Estado de México. Rev. Mex. Cienc. Agric. 2014, 5, 129–141. [Google Scholar] [CrossRef]
- Guerrero, J.; Valencia, E. Limpieza por cromatografía de permeación por gel en la determinación de residuos de n-metilcarbamatos en fresa. Rev. Colomb. Quím. 2008, 37, 161–172. Available online: https://revistas.unal.edu.co/index.php/rcolquim/article/view/9445 (accessed on 15 December 2018).
- Narváez, J.F.; Palacio, J.A.; Molina, F.J. Persistencia de plaguicidas en el ambiente y su ecotoxicidad: Una revisión de los procesos de degradación natural. Gestión y Ambiente 2012, 15, 27–37. Available online: https://www.redalyc.org/articulo.oa?id=169424893002 (accessed on 15 November 2018).
- Belfroid, A.C.; van Drunen, M.; Beek, M.A.; Schrap, S.M.; van Gestel, C.A.M.; van Hattum, B. Relative risks of transformation products of pesticides for aquatic ecosystems. Sci. Total Environ. 1998, 222, 167–183. [Google Scholar] [CrossRef]
- Raymond, J.W.; Rogers, T.N.; Shonnard, D.R.; Kline, A.A. A review of structure-based biodegradation estimation methods. J. Hazard. Mater. 2001, 84, 189–215. [Google Scholar] [CrossRef]
- Reemtsma, T.; Jekel, M. Organic Pollutants in the Water Cycle: Properties, Occurrence, Analysis and Enviromental Relevance of Polar Compounds, 1st ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 1–336. [Google Scholar]
- Paschal, D.C.; Neville, M.E. Chemical and microbial degradation of malaoxon in an Illinois soil. J. Environ. Qual. 1976, 5, 441–443. [Google Scholar] [CrossRef]
- Chapman, R.A.; Chapman, P.C. Persistence of granular and EC formulation of chlorpyrifos in a mineral and an organic soil incubated in open and closed containers. J. Environ. Sci. Health Part B 1986, 21, 47–456. [Google Scholar] [CrossRef]
- Getzin, L.W. Degradation of chlorpyrifos in soil: Influence of autoclaving, soil moisture, and temperature. J. Econ. Entomol. 1981, 74, 158–162. [Google Scholar] [CrossRef]
- Racke, K.D.; Steele, K.P.; Yoder, R.N.; Dick, W.A.; Avidov, E. Factors effecting the hydrolytic degradation of chlorpyrifos in soil. J. Agric. Food Chem. 1996, 44, 1582–1592. [Google Scholar] [CrossRef]
- Putnam, R.A.; Nelson, J.O.; Clark, J.M. The persistence and degradation of chlorothalonil and chlorpyrifos in a cranberry bog. J. Agric. Food Chem. 2003, 51, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Tzilivakis, J. Development of a data set of pesticide dissipation rates in/on various plant matrices for the Pesticide Properties Database (PPDB). Data 2017, 2, 28. [Google Scholar] [CrossRef]
- Sivakumar, S.; Anitha, P.; Ramesh, B.; Suresh, G. Analysis of EAWAG-BBD pathway prediction system for the identification of malathion degrading microbes. Bioinformation 2017, 13, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Kaur, J.; Singh, K. Microbial degradation of an organophophate pesticide, malathion. Crit. Rev. Microbiol. 2014, 40, 146–154. [Google Scholar] [CrossRef]
- Fantke, F.; Gillespie, B.W.; Juraske, R.; Jolliet, O. Estimating Half-Lives for Pesticide Dissipation from Plants. Environ. Sci. Technol. 2014, 48, 8588–8602. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Rahnemaie, R.; Soufizadeh, S.; Malakouti, M.J.; Eshaghi, A. Residual effect of thiobencarb and oxadiargyl on spinach and lettuce in rotation with rice. J. Agric. Sci. Technol. 2011, 13, 785–794. Available online: http://jast-old.modares.ac.ir/article_4759.html (accessed on 5 November 2018).
- Zhu, J.W.; Wang, J.; Di Tommaso, A.; Zhang, C.; Zheng, G.; Liang, W.; Faisal, I.; Yang, C.; Chen, X.; Zhou, W. Weed research status, challenges, and opportunities in China. Crop Prot. 2018, in press. [Google Scholar] [CrossRef]
- Coscollá, R. Residuos de Plaguicidas en Alimentos Vegetales, 1st ed.; Mundiprensa: Madrid, Spain, 1993; pp. 1–205. [Google Scholar]
- Chen, H.; Yin, P.; Wang, Q.; Jiang, Y.; Liu, X. A modified QuEChERS sample preparation method for the analysis of 70 pesticide residues in tea using gas chromatography-tandem mass spectrometry. Food Anal. Methods 2014, 7, 1577–1587. [Google Scholar] [CrossRef]
- Hou, R.Y.; Jiao, W.T.; Xiao, Y.; Guo, J.G.; Lv, Y.N.; Tan, H.R.; Hu, J.; Wan, X. Novel use of PVPP in a modified QuEChERS extraction for UPLC–MS/MS analysis of neonicotinoid insecticides in tea. Anal. Methods 2015, 7, 5521–5529. [Google Scholar] [CrossRef]
- Ramírez-Bustos, I.I.; López-Martínez, V.; Juarez-Lopez, P.; Alía-Tejacal, I.; Guillén-Sánchez, D.; Saldarriaga-Noreña, H.; León-Rivera, I. Monitoring of pesticides in the cultivation of nopal vegetable (Opuntia ficus-indica (L.)) Mill, Morelos, México. Agriculture 2018, 8, 174. Available online: https://www.mdpi.com/2077-0472/8/11/174 (accessed on 22 November 2018). [CrossRef]
- Instituto Nacional de Estadísticas y Geografía (INEGI). Características Principales del Cultivo de Nopal en el Distrito Federal: Caso Milpa Alta, 1st ed.; Instituto Nacional de Estadísticas y Geografía: D.F., Mexico, 2007; pp. 1–68. [Google Scholar]
- Ramírez-Bustos, I.I.; López-Martínez, V.; Juárez-López, P.; Guillén-Sánchez, D.; Alia-Tejacal, I.; Rivera-León, I.; Saldarriaga-Noreña, H.A.; Jiménez-García, D. Identificación de envases vacíos de plaguicidas en plantaciones de nopal verdura, [Opuntia ficus-indica (L.) Mill]. (Cactaceae), en Morelos, México. Acta Agric. y Pecu. 2018, 4, 18–25. Available online: http://aap.uaem.mx/index.php/agricolaypecuaria/article/view/265 (accessed on 2 September 2018). [CrossRef]
- EURL-DataPool. Available online: http://www.eurl-pesticides-datapool.eu (accessed on 1 December 2018).
- Norma Mexicana NMX-FF-068-1988. Food Products Fresh Vegetable. Prickly Pear (Opuntia spp.) Specifications. Available online: http://www.colpos.mx/bancodenormas/nmexicanas/NMX-FF-068-1968.PDF (accessed on 1 December 2018).
- World Health Organization, United Nations Organization for Food and Agriculture. Recommended. Methods of Sampling for the Determination of Pesticide Residues for Compliance with MRLs CAC/GL.33-1999. Available online: http://www.fao.org/input/download/standards/361/CXG_033e.pdf (accessed on 16 December 2017).
- Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed; European Commission: Brussel, Belgium, 2015.
- Prodhan, M.D.H.; Akon, M.W.; Alam, S.N. Determination of pre-harvest interval for quinalphos, malathion, diazinon and cypermethrin in major vegetables. J. Environ. Anal. Toxicol. 2018, 8, 1. [Google Scholar]
- Kulczycki, C.; Navarro, R.; Turaglio, E.; Becerra, V.; Sosa, A. Cinética de degradación y persistencia de clorpirifos en mandarinas y naranjas del Noreste argentino (NEA). Rev. Investig. Agropec. 2012, 38, 282–288. [Google Scholar]
- Alister, C.; Araya, M.; Morandé, J.; Volosky, C.; Kogan, M. Disipación de plaguicidas utilizados en uva vinífera y traspaso de sus residuos al vino. Rev. Redagrícola 2014, 61, 56–57. Available online: Sidal.cl/assets/pdf-13.pdf (accessed on 18 October 2018).
- Lu, M.-X.; Jiang, W.W.; Wang, J.-L.; Jian, Q.; Shen, Y.; Liu, X.-J.; Yu, X.-Y. Persistence and dissipation of chlorpyrifos in brassica chinensis, lettuce, celery, asparagus lettuce, eggplant, and pepper in a greenhouse. PLoS ONE 2014, 9, e100556. [Google Scholar] [CrossRef]
- Hoja de Datos de Seguridad. Pugil 75 WG. Available online: http://www.inquiport.net/files/msds/fungicidas/Pugil.pdf (accessed on 15 November 2018).
- Jankowska, M.; Kaczynski, P.; Hrynko, I.; Lozowicka, B. Dissipation of six fungicides in greenhouse-grown tomatoes with processing and health risk. Environ. Sci. Pollut. Res. 2016, 23, 11885–11900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
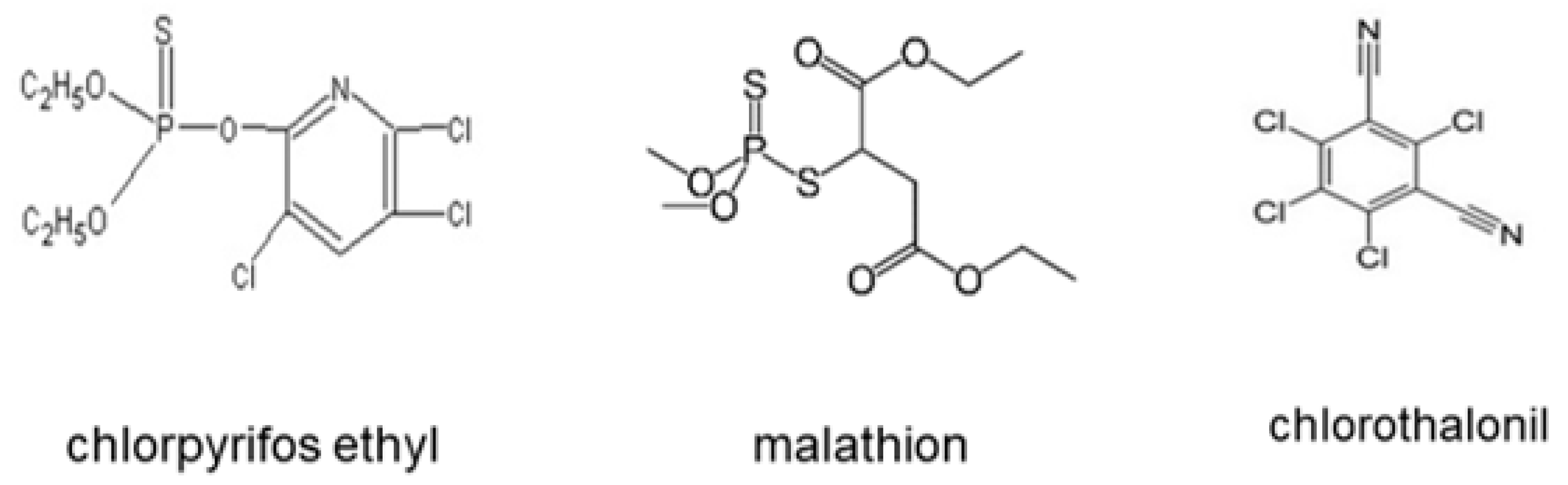
| Commercial Name | Active Ingredient | Action Mode | Chemical Group | Molecular Formula |
|---|---|---|---|---|
| Disparo® | Chlorpyrifos ethyl | Insecticide/acaricide | Organophosphate | C7H7CI3NO3PS C22H19CI2NO3 |
| Malathion® 1000 | Malathion | Insecticide/acaricide | Organophosphate | C10H19O6PS2 |
| Thalonil® 75 | Chlorothalonil | Fungicide | Chloronitrile | C8CI4N2 |
| Treatment | Active Ingredient | Dose |
|---|---|---|
| (n = 4, treatment) | mg/kg | |
| T11 | Malathion | 0.240 |
| T2 | Malathion | 0.407 |
| T3 | Chlorpyrifos ethyl | 0.540 |
| T4 | Chlorpyrifos ethyl | 0.730 |
| T5 | Chlorothalonil | 0.460 |
| T6 | Chlorothalonil | 0.870 |
| T7 | Untreated (negative) control |
| Analyte | RT (min) | First transition | Collision energy | Second transition | Collision energy | Quantifier ion |
|---|---|---|---|---|---|---|
| (m/z) | (V) | (m/z) | (V) | |||
| Malathion | 6.3 | 173→99 | 12 | 173→127 | 5 | 173 |
| Chlorpyrifos ethyl | 7.1 | 314→286 | 20 | 314→258 | 10 | 324 |
| Chlorothalonil | 8.5 | 244.9→174.9 | 28 | 244.9→181.9 | 20 | 263 |
| Analyte | LOD | % Recovery | % RSD |
|---|---|---|---|
| mg/Kg | (n = 3) | (n = 3) | |
| Chlorpyrifos ethyl | 0.00004 | 84.5 | 9.54 |
| Malathion | 0.00006 | 87.8 | 6.54 |
| Chlorothalonil | 0.00005 | 86.5 | 7.35 |
| Analyte | Minimal Dose | Maximum Dose | ||||
|---|---|---|---|---|---|---|
| Kinetic Model a | R2 | t1/2 | Kinetic Model a | R2 | t1/2 | |
| Malahion | C= 0.24e−0.107t | 0.911 | 5.77 | C = 0.41e−0.13t | 0.9891 | 5.33 |
| Chlorpyrifos | C= 0.54e−0.131t | 0.9374 | 5.29 | C = 0.73e−0.117t | 0.9761 | 5.89 |
| Chlotothalonil | C= 0.46e−0.119t | 0.9508 | 5.8 | C = 0.88e−0.122t | 0.9463 | 5.68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Bustos, I.I.; Saldarriaga-Noreña, H.; Fernández-Herrera, E.; Juárez-López, P.; Alia-Tejacal, I.; Guillén-Sánchez, D.; Rivera-León, I.; López-Martínez, V. Dissipation Behavior of Three Pesticides in Prickly Pear (Opuntia ficus-indica (L.) Mill.) Pads in Morelos, Mexico. Int. J. Environ. Res. Public Health 2019, 16, 2922. https://doi.org/10.3390/ijerph16162922
Ramírez-Bustos II, Saldarriaga-Noreña H, Fernández-Herrera E, Juárez-López P, Alia-Tejacal I, Guillén-Sánchez D, Rivera-León I, López-Martínez V. Dissipation Behavior of Three Pesticides in Prickly Pear (Opuntia ficus-indica (L.) Mill.) Pads in Morelos, Mexico. International Journal of Environmental Research and Public Health. 2019; 16(16):2922. https://doi.org/10.3390/ijerph16162922
Chicago/Turabian StyleRamírez-Bustos, Irene Iliana, Hugo Saldarriaga-Noreña, Ernesto Fernández-Herrera, Porfirio Juárez-López, Iran Alia-Tejacal, Dagoberto Guillén-Sánchez, Ismael Rivera-León, and Víctor López-Martínez. 2019. "Dissipation Behavior of Three Pesticides in Prickly Pear (Opuntia ficus-indica (L.) Mill.) Pads in Morelos, Mexico" International Journal of Environmental Research and Public Health 16, no. 16: 2922. https://doi.org/10.3390/ijerph16162922
APA StyleRamírez-Bustos, I. I., Saldarriaga-Noreña, H., Fernández-Herrera, E., Juárez-López, P., Alia-Tejacal, I., Guillén-Sánchez, D., Rivera-León, I., & López-Martínez, V. (2019). Dissipation Behavior of Three Pesticides in Prickly Pear (Opuntia ficus-indica (L.) Mill.) Pads in Morelos, Mexico. International Journal of Environmental Research and Public Health, 16(16), 2922. https://doi.org/10.3390/ijerph16162922






