Survival of Microorganisms on Filtering Respiratory Protective Devices Used at Agricultural Facilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Study
2.1.1. Filtering Nonwovens
2.1.2. Microorganisms
2.1.3. Thermal and Humidity Conditioning
2.1.4. Deposition of Organic and Inorganic Dust on Filtering Nonwovens
2.1.5. Acidic and Alkaline Sweat
2.1.6. Assessment of Microorganism Survival on Filtering Nonwovens
2.2. Study at Workplaces
2.2.1. Filtering Facepiece Respirators
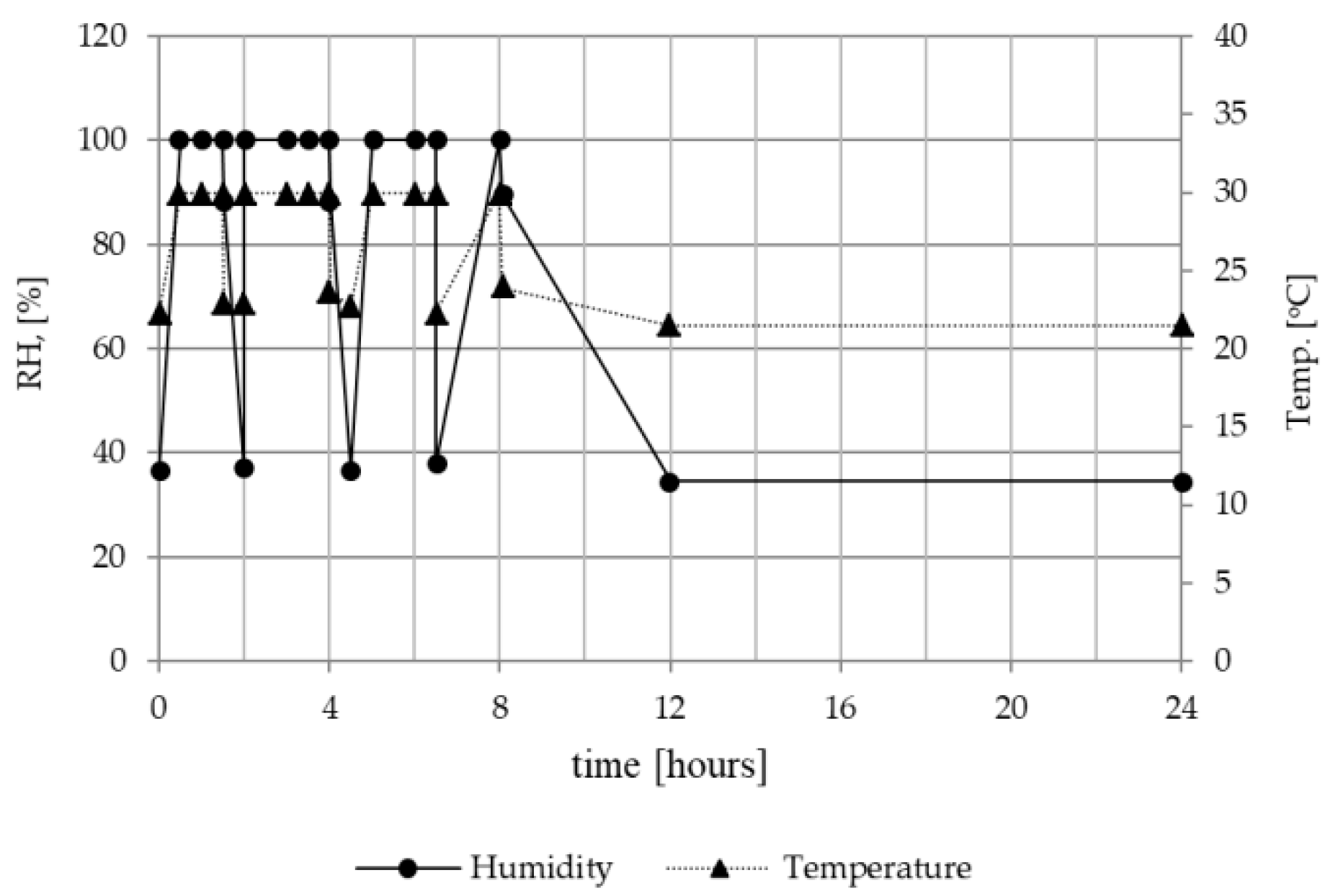
2.2.2. Ambient Conditions at the Workplace
2.2.3. Analysis of Dust Fractions Suspended in the Air
2.2.4. Microbiological Analysis of Air, Sedimented Dust and FFRs
2.2.5. Mathematical and Statistical Calculations
3. Results
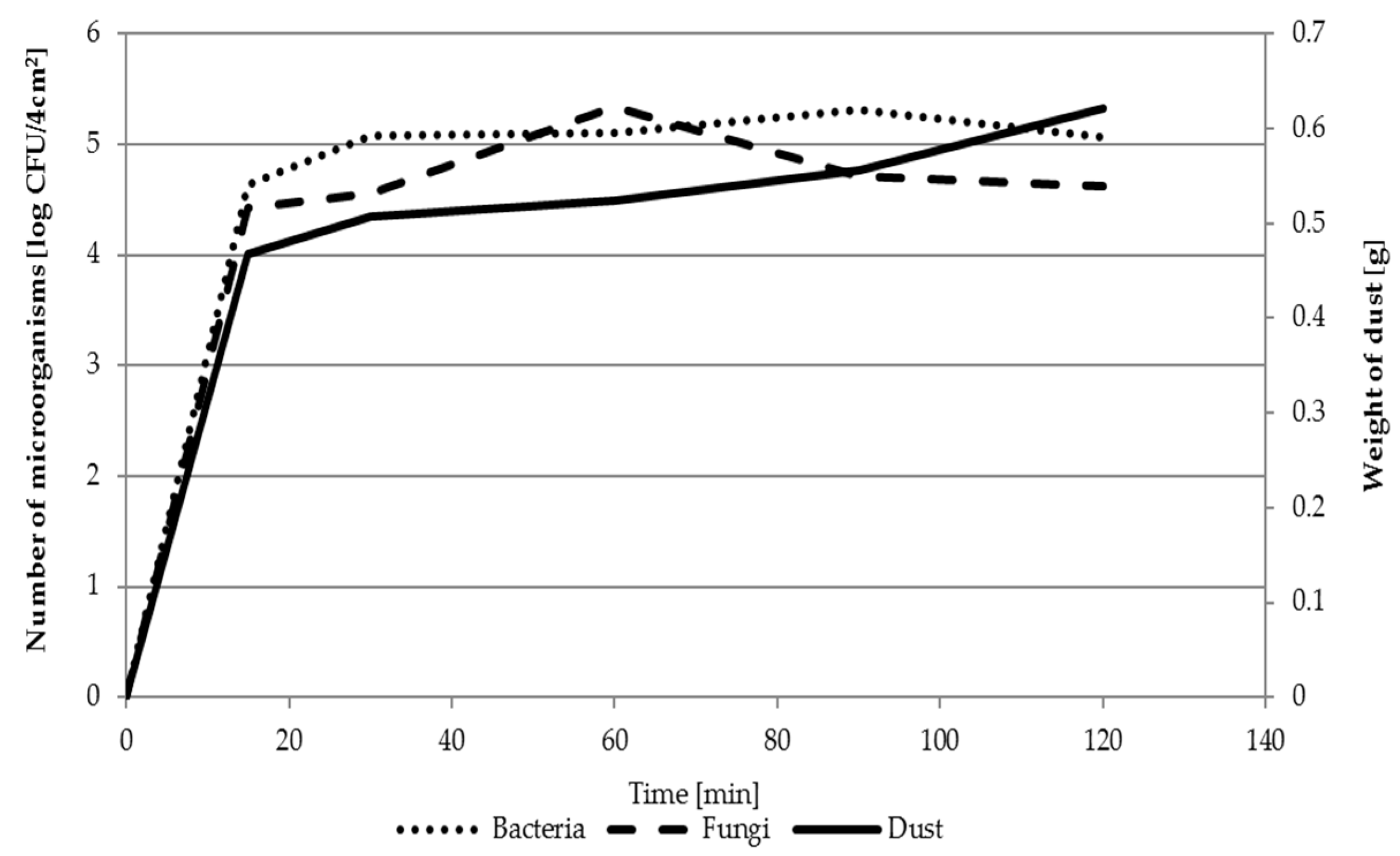
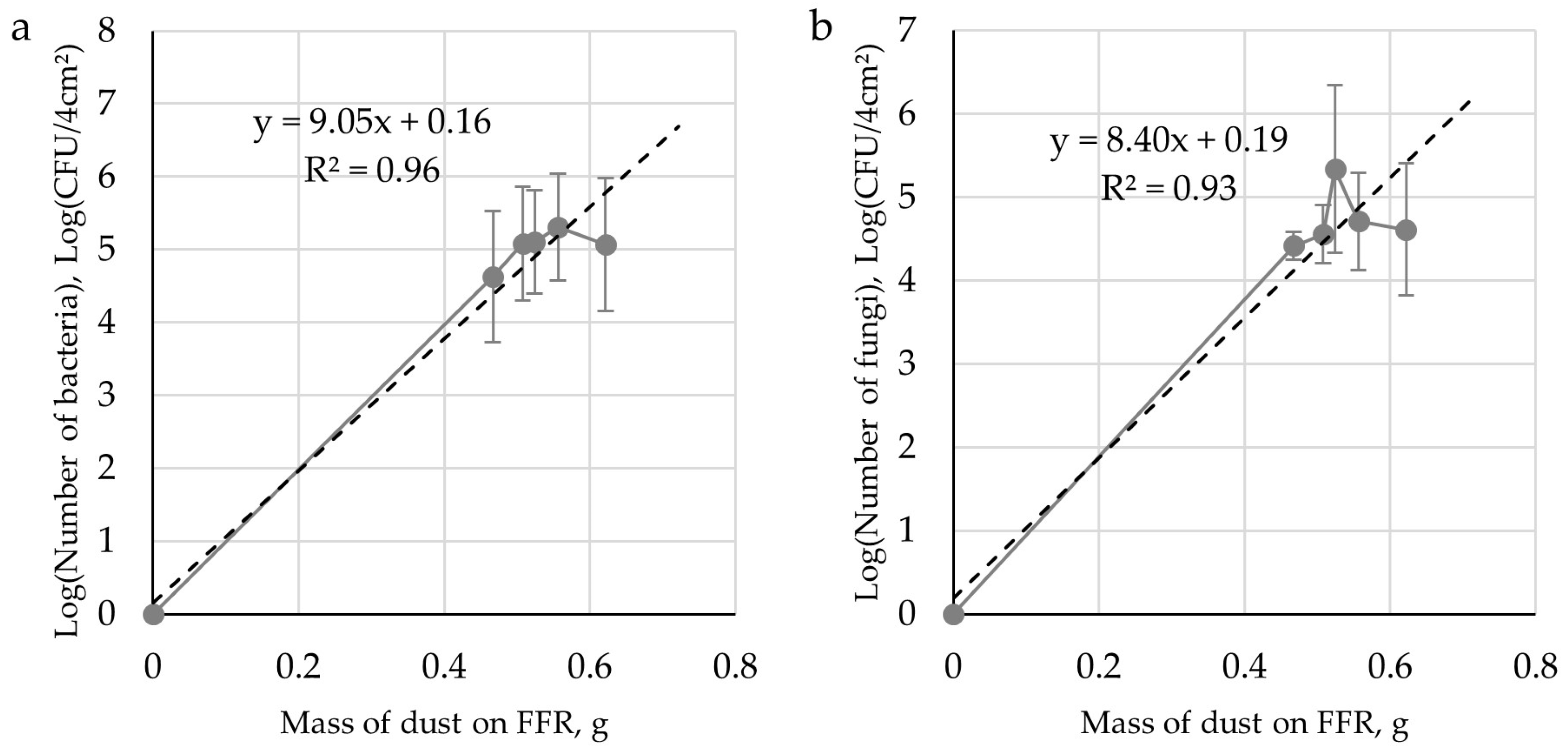
3.1. Model Study
3.2. Study at Workplaces
3.2.1. Analysis of Dust Fractions in the Air
3.2.2. Microbiological Analysis of Air, Sedimented Dust and FFRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ławniczek-Wałczyk, A.; Górny, R.L. Endotoxins and β-glucans as markers of microbiological contamination—Characteristics, detection, and environmental exposure. Ann. Agric. Environ. Med. 2010, 17, 193–208. [Google Scholar] [PubMed]
- Corrao, C.R.N.; Mazzotta, A.; La Torre, G.; De Giusti, M. Biological risk and occupational health. Ind. Health 2012, 50, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.T.; Lim, C.H. Biologically Hazardous Agents at Work and Efforts to Protect Workers’ Health: A Review of Recent Reports. Saf. Health Work. 2014, 5, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [PubMed] [Green Version]
- Rusca, S.; Charrière, N.; Droz, P.O.; Oppliger, A. Effects of bioaerosol exposure on work-related symptoms among Swiss sawmill workers. Int. Arch. Occup. Environ. Health 2008, 81, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, G.; Schaumburg, I.; Sigsgaard, T.; Schlunssen, V. Non-malignant respiratory diseases and occupational exposure to wood dust. Part I. Fresh wood and mixed wood industry. Ann. Agric. Environ. Med. 2010, 17, 15–28. [Google Scholar] [PubMed]
- Skóra, J.; Zduniak, K.; Gutarowska, B.; Rembisz, D. Harmful biological agents at museum workposts. Med. Pr. 2012, 63, 153–165. [Google Scholar]
- Jerez, S.B.; Cheng, Y.; Bray, J. Exposure of workers to dust and bioaerosol on a poultry farm. J. Appl. Poult. Res. 2014, 23, 7–14. [Google Scholar] [CrossRef]
- Gutarowska, B.; Skóra, J.; Stępień, Ł.; Szponar, B.; Otlewska, A.; Pielech-Przybylska, K. Assessment of microbial contamination within working environments of different types of composting plants. J. Air Waste Manag. Assoc. 2015, 65, 466–478. [Google Scholar] [CrossRef]
- Szulc, J.; Otlewska, A.; Okrasa, M.; Majchrzycka, K.; Sulyok, M.; Gutarowska, B. Microbiological Contamination at Workplaces in a Combined Heat and Power (CHP) Station Processing Plant Biomass. Int. J. Environ. Res. Public Health 2017, 14, 99. [Google Scholar] [CrossRef]
- Lee, S.A.; Adhikari, A.; Grinshpun, S.A.; McKay, R.; Shukla, R.; Reponen, T. Personal Exposure to Airborne Dust and Microorganisms in Agricultural Environments. J. Occup. Environ. Hyg. 2006, 3, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Simon, X.; Duquenne, P. Assessment of workers’ exposure to bioaerosols in a french cheese factory. Ann. Occup. Hyg. 2014, 58, 677–692. [Google Scholar] [PubMed]
- Degois, J.; Simon, X.; Bontemps, C.; Leblond, P.; Duquenne, P.; Clerc, F. First Metagenomic Survey of the Microbial Diversity in Bioaerosols Emitted in Waste Sorting Plants. Ann. Work Expo. Health 2017, 61, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.M.; Shaffer, R.E. Commentary Considerations for Recommending Extended Use and Limited Reuse of Filtering Facepiece Respirators in Health Care Settings. J. Occup. Environ. Hyg. 2014, 11, D115–D128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Adhikari, A.; Grinshpun, S.A.; McKay, R.; Shukla, R.; Zeigler, H.L.; Reponen, T. Respiratory protection provided by N95 filtering facepiece respirators against airborne dust and microorganisms in agricultural farms. J. Occup. Environ. Hyg. 2005, 2, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Grinshpun, S.A.; Adhikari, A.; Li, W.; Mckay, R.; Maynard, A.; Reponen, T. Laboratory and field evaluation of a new personal sampling system for assessing the protection provided by the N95 filtering facepiece respirators against particles. Ann. Occup. Hyg. 2005, 49, 245–257. [Google Scholar] [PubMed]
- Zheng, C.R.; Li, S.; Ye, C.; Li, X.; Zhang, C.; Yu, X. Particulate respirators functionalized with silver nanoparticles showed excellent real-time antimicrobial effects against pathogens. Environ. Sci. Technol. 2016, 50, 7144–7151. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Son, A.; Chua, B. Microorganism-ionizing respirator with reduced breathing resistance suitable for removing airborne bacteria. Sens. Actuators B Chem. 2018, 276, 437–446. [Google Scholar] [CrossRef]
- Lin, T.H.; Tang, F.C.; Chiang, C.H.; Chang, C.P.; Lai, C.Y. Recovery of bacteria in filtering Facepiece respirators and effects of artificial saliva/perspiration on bacterial survival and performance of respirators. Aerosol Air Qual. Res. 2017, 17, 187–197. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Okrasa, M.; Skora, J.; Gutarowska, B. Evaluation of the Survivability of Microorganisms Deposited on Filtering Respiratory Protective Devices under Varying Conditions of Humidity. Int. J. Environ. Res. Public Health 2016, 13, 98. [Google Scholar] [CrossRef]
- Brosseau, L.M.; McCullough, N.V.; Vesley, D. Bacterial Survival on Respirator Filters and Surgical Masks. J. Am. Biol. Saf. Assoc. 1997, 2, 32–43. [Google Scholar] [CrossRef]
- Reponen, T.A.; Wang, Z.; Willeke, K.; Grinshpun, S.A. Survival of Mycobacteria on N95 Personal Respirators. Infect. Control Hosp. Epidemiol. 1999, 20, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Survival of Bacteria on Respirator Filters. Aerosol Sci. Technol. 1999, 30, 300–308. [Google Scholar] [CrossRef]
- Jankowska, E.; Reponen, T.; Willeke, K.; Grinshpun, S.A.; Choi, K.J. Collection of fungal spores on air filters and spore re-entrainment from filters into air. J. Aerosol Sci. 2000, 31, 969–978. [Google Scholar] [CrossRef]
- Maus, R.; Goppelsröder, A.; Umhauer, H. Survival of bacterial and mold spores in air filter media. Atmos. Environ. 2001, 35, 105–113. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Okrasa, M.; Szulc, J.; Gutarowska, B. The impact of dust in filter materials of respiratory protective devices on the microorganisms viability. Int. J. Ind. Ergon. 2017, 58, 109–116. [Google Scholar] [CrossRef]
- Perrier, J.C.B.; Coq, L.L.; Andres, Y.; Cloirec, P.L. SFGP 2007-Microbial growth onto filter media used in air treatment devices. Int. J. Chem. React. Eng. 2019, 6, 1542–6580. [Google Scholar]
- Esser, D.S.; Leveau, J.H.J.; Meyer, K.M. Modeling microbial growth and dynamics. Appl. Microbiol. Biotechnol. 2015, 99, 8831–8846. [Google Scholar] [CrossRef] [PubMed]
- Brochocka, A.; Majchrzycka, K.; Makowski, K. Modified Melt-Blown Nonwovens for Respiratory Protective Devices Against Nanoparticles. Fibres Text. East. Eur. 2013, 21, 106–111. [Google Scholar]
- Majchrzycka, K.; Gutarowska, B.; Brochocka, A.; Brycki, B. New Filtering Antimicrobial Nonwovens With Various Carriers for Biocides as Respiratory Protective Materials Against Bioaerosol. Int. J. Occup. Saf. Ergon. 2012, 18, 375–385. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Okrasa, M.; Szulc, J.; Brycki, B.; Gutarowska, B. Time-dependent antimicrobial activity of filtering nonwovens with gemini surfactant-based biocides. Molecules 2017, 22, 1620. [Google Scholar] [CrossRef]
- International Standard ISO 9073-2:1995. Textiles. Test Methods for Nonwovens. Part 2: Determination of Thickness; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- European Standard EN 29073-1:1992. Methods of Test for Nonwovens. Methods of Test for Nonwovens. Determination of Mass per Unit Area; European Committee for Standardization: Bruxelles, Belgium, 1992. [Google Scholar]
- European Standard EN 13274-7:2008. Respiratory Protective Devices. Methods of Test. Part 7: Determination of particle filter penetration; European Committee for Standardization: Bruxelles, Belgium, 2008. [Google Scholar]
- European Standard EN 13274-3:2001. Respiratory Protective Devices. Methods of Test. Part 3: Determination of Breathing Resistance; European Committee for Standardization: Bruxelles, Belgium, 2001. [Google Scholar]
- Gutarowska, B.; Szulc, J.; Nowak, A.; Otlewska, A.; Okrasa, M.; Jachowicz, A.; Majchrzycka, K. Dust at Various Workplaces—Microbiological and Toxicological Threats. Int. J. Environ. Res. Public Health 2018, 15, 877. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Okrasa, M.; Jachowicz, A.; Szulc, J.; Gutarowska, B. Microbial Growth on Dust-Loaded Filtering Materials Used for the Protection of Respiratory Tract as a Factor Affecting Filtration Efficiency. Int. J. Environ. Res. Public Health 2018, 15, 1902. [Google Scholar] [CrossRef]
- European Standard EN 149:2001+A1:2009. Respiratory Protective Devices. Filtering Half Masks to Protect against Particles. Requirements, Testing, Marking; European Committee for Standardization: Bruxelles, Belgium, 2009. [Google Scholar]
- Majchrzycka, K.; Gutarowska, B.; Brochocka, A. Aspects of tests assessment of filtering materials used for respiratory protection against bioaerosols. Part II—Sweat in environment, microorganisms in the form of bioaerosol. Int. J. Occup. Saf. Ergon. 2010, 16, 275–280. [Google Scholar] [CrossRef]
- Gutarowska, B.; Skóra, J.; Nowak, E.; Łysiak, I.; Wdówka, M. Antimicrobial activity and filtration effectiveness of nonwovens with Sanitized for respiratory protective equipment. Fibres Text. East. Eur. 2014, 22, 120–125. [Google Scholar]
- Gutarowska, B.; Michalski, A. Antimicrobial activity of filtrating meltblown nonwoven’s with addition of silver ions. Fibres Text. East. Eur. 2009, 17, 23–28. [Google Scholar]
- Gutarowska, B.; Brycki, B.; Majchrzycka, K.; Brochocka, A. New bioactive polymer filtering material composed of nonwoven polypropylene containing alkylammonium microbiocides on a perlite carrier. Polimery 2010, 55, 568–574. [Google Scholar] [CrossRef] [Green Version]
- Majchrzycka, K.; Gutarowska, B.; Brochocka, A. Aspects of tests assessment of filtering materials used for respiratory protection against bioaerosol. Part I—Type of active substance, contact time, microorganism species. Int. J. Occup. Saf. Ergon. 2010, 16, 263–273. [Google Scholar] [CrossRef]
- Manfreda, J.; Warren, C.P. The effect of grain dust on health. Rev. Environ. Health 1984, 4, 239–267. [Google Scholar]
- Dutkiewicz, J. Bacteria and fungi in organic dust as potential health hazard. Ann. Agric. Environ. Med. 1997, 4, 11–16. [Google Scholar]
- Eduar, W. Exposure to non-infectious microorganisms and endotoxins in agriculture. Ann. Agric. Environ. Med. 1997, 4, 179–186. [Google Scholar]
- Kulkarni, P.; Baron, P.A.; Willeke, K. Aerosol Measurement: Principles, Techniques, and Applications; John Wiley and Sons Inc. Publication: Hoboken, NJ, USA, 2011; p. 904. [Google Scholar]
- Chen, W.; Tong, D.Q.; Zhang, S.; Zhang, X.; Zhao, H. Local PM10 and PM2.5 emission inventories from agricultural tillage and harvest in northeastern China. J. Environ. Sci. 2017, 57, 15–23. [Google Scholar] [CrossRef]
- Krysińska-Traczyk, E.; Pande, B.N.; Skórska, C.; Sitkowska, J.; Prazmo, Z.; Cholewa, G.; Dutkiewicz, J. Exposure of Indian agricultural workers to airborne microorganisms, dust and endotoxin during handling of various plant products. Ann. Agric. Environ. Med. 2005, 12, 269–275. [Google Scholar]
- Góra, A.; Mackiewicz, B.; Krawczyk, P.; Golec, M.; Skórska, C.; Sitkowska, J.; Cholewa, G.; Larsson, L.; Jarosz, M.; Wójcik-Fatla, A.; et al. Occupational exposure to organic dust, microorganisms, endotoxin and peptidoglycan among plants processing workers in Poland. Ann. Agric. Environ. Med. 2009, 16, 143–150. [Google Scholar]
- Ławniczek-Wałczyk, A.; Górny, R.L.; Golofit-Szymczak, M.; Niesler, A.; Wlazło, A. Occupational exposure to airborne microorganisms, endotoxins and β-glucans in poultry houses at different stages of the production cycle. Ann. Agric. Environ. Med. 2013, 20, 259–268. [Google Scholar]
- Dutkiewicz, J.; Górny, R.L. Biologiczne czynniki szkodliwe dla zdrowia—Klasyfikacja i kryteria oceny narażenia. Med. Pr. 2002, 53, 29–39. [Google Scholar]
- Skowroń, J.; Górny, R.L. Harmful Biological Agents. In The Interdepart-mental Commission for Maximum Admissible Concen—Trations and Intensities for Agents Harmful to Health in the Working Environment: Limit Values 2012; Augustyńska, D., Pośniak, M., Eds.; Centralny Instytut Ochrony Pracy—Państwowy Instytut Badawczy: Warsaw, Poland, 2012. (In Polish) [Google Scholar]
- Majchrzycka, K.; Małgorzata, O.; Brochocka, A.; Irzmańska, E. Application of superabsorbent polymers in textile materials. Przem Chem. 2017, 96, 2124–2127. [Google Scholar]
- Brochocka, A. Filtration Properties of Nonwoven Structures with Superabsorbents for Respiratory Protective Devices. Fibres Text. East. Eur. 2017, 25, 62–67. [Google Scholar] [CrossRef]

| No. | Nonwoven Type | Thickness * (mm) | Surface Mass ** (g/m2) | Paraffin Oil Mist Penetration *** (%) | Air Flow Resistance **** (Pa) |
|---|---|---|---|---|---|
| 1. | electret Polypropylene melt-blown nonwovens (PPQ) | 1.73 ± 0.13 | 75.8 ± 9.9 | 7.33 ± 1.91 | 200 ± 31 |
| 2. | composite electret Polypropylene melt-blown nonwovens (CPPQ) | 2.97 ± 0.18 | 135.3 ± 6.5 | 1.50 ± 0.17 | 361 ± 16 |
| Mass of Dust Deposited in the Nonwoven (mg/sample) | |||
|---|---|---|---|
| Dust from the Composting Plant | Dust from the Cement Plant | ||
| 2 min (deposition time) | 4 min (deposition time) | 2 min (deposition time) | 4 min (deposition time) |
| X: 24.6 | X: 51.2 | X: 33.6 | X: 60.6 |
| SD: 10.4 | SD:24.7 | SD: 10,9 | SD:15.9 |
| No. | Study Location | Type of Work Performed at Workstation | Type of Sample Taken at Workstation | Microclimate Conditions |
|---|---|---|---|---|
| 1. | Cultivated field (2.7 ha), indoor working premises, Odolin, Lodz voivodeship, Poland (DMS: 52°10′44.271′′ N 19°36′27.669′′ E) | A—combine harvester operator—open cab without glazing (wheat harvesting and pouring grain onto a trailer with the use of a feeder) | Air: X prior, Y during working the fields | T: 385 RH: 27.8 W: 0.87 |
| Dust: I dust sedimented on the combine, II dust gathered behind the combine (from the rear end of the machine) | ||||
| B—tractor driver (grain transport to the site of collection and storage on the farm premises) | Air: X prior, Y during working inside the premises | T: 35.6 RH: 30.9 W: 0.1 | ||
| Dust: III sedimented on the premises | ||||
| 2. | Cultivated field (5.2 ha), indoor working premises, Wola Miłkowska, Lodz voivodeship, Poland (DMS: 51°48′42.452′′ N 18°36′15.331′′ E) | A—combine harvester operator—closed cab with glazing (wheat harvesting and pouring grain onto a trailer with the use of a feeder) | Air: X prior, Y during working the fields | T: 33.2 RH: 34.9 W: 0.22 |
| Dust: I dust sedimented on the combine, II dust gathered behind the combine (from the rear end of the machine) | ||||
| B—tractor driver (grain transport to the site of collection and storage on the working premises, straw baling) | Air: X prior, Y during working inside the premises | T: 28.8 RH: 42 W: 0.15 | ||
| Dust: III sedimented on the premises |
| Microorganism | Growth Parameters | Factors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | ||
| B. subtilis | λstat (hours) | 22.28 | 18.17 | 32.64 | 24.53 | 19.01 | 10.86 | 14.57 | 18.37 | 2.61 |
| tstat (hours) | 5.93 | 7.15 | 8.08 | 2.95 | 5.71 | 6.22 | 6.10 | 7.43 | 3.98 | |
| Ymax (log CFU/4 cm2) | 3.03 | 3.24 | 1.28 | 1.01 | 2.40 | 3.03 | 2.93 | 2.50 | 0.57 | |
| E. coli | λstat (hours) | 4.69 | 1,00 | 5.72 | 3.42 | 1.00 | 8.00 | 19.99 | 20.22 | 3.38 |
| tstat (hours) | 8.19 | 8.53 | 6.49 | 3.01 | 11.89 | 4.18 | 4.19 | 4.31 | 5.83 | |
| Ymax (log CFU/4 cm2) | 0.95 | 2.68 | 0.66 | 0.22 | 0.99 | 0.63 | 0.96 | 1.01 | 0.20 | |
| S. aureus | λstat (hours) | 4.21 | 10.34 | 2.45 | 0.34 | 7.21 | 30.62 | 1.36 | 3.77 | nb |
| tstat (hours) | 8.54 | 17.87 | 9.80 | 11.76 | 6.66 | 9.16 | 18.51 | 8.88 | nb | |
| Ymax (log CFU/4 cm2) | 0.92 | 0.52 | 0.16 | 1.39 | 0.61 | 0.54 | 0.72 | 0.44 | 0.21 | |
| A. niger | λstat (hours) | 43.79 | 1.94 | 2.22 | 10.69 | 3.17 | 1.18 | 19.56 | 17.52 | 3.29 |
| tstat (hours) | 4.81 | 5.31 | 7.44 | 4.51 | 4.92 | 12.98 | 9.15 | 9.89 | 9.13 | |
| Ymax (log CFU/4 cm2) | 0.29 | 0.21 | 0.31 | 0.16 | 0.19 | 0.60 | 0.43 | 0.48 | 0.40 | |
| C. albicans | λstat (hours) | 46.77 | 7.81 | 1.18 | 1.91 | 3.56 | 0.59 | 16.76 | 4.92 | 4.13 |
| tstat (hours) | 4.18 | 12.41 | 11.35 | 8.37 | 4.66 | 8.68 | 9.24 | 10.63 | 4.57 | |
| Ymax (log CFU/4 cm2) | 0.72 | 0.70 | 0.19 | 0.21 | 0.18 | 0.61 | 0.58 | 0.49 | 0.07 | |
| Workplace | Sample Type | Airborne Dust Mass Concentrations Corresponding to Particle Size Fractions (mg/m3) | ||||
|---|---|---|---|---|---|---|
| PM1 | PM2.5 | PM4 | PM10 | PMtotal | ||
| 1A | X | M: 0.09 a | M: 0.09 a | M: 0.09 a | M: 0.13 b | M: 0.23 b |
| SD: 0.15 | SD: 0.16 | SD: 0.16 | SD: 0.27 | SD: 0.60 | ||
| Y | M: 2.47 a,* | M: 2.50 a,* | M: 2.63 a,* | M: 4.79 a,* | M: 10.62 a,* | |
| SD: 3.72 | SD: 3.75 | SD: 3.92 | SD: 6.98 | SD: 15.90 | ||
| 1B | X | M: 0.07 a | M: 0.07 a | M: 0.07 a | M: 0.08 a | M: 0.10 a |
| SD: 0.02 | SD: 0.02 | SD: 0.02 | SD: 0.04 | SD: 0.09 | ||
| Y | M: 2.53 a,* | M: 2.56 a,* | M: 2.70 a,* | M: 4.76 a,* | M: 10.25 a,* | |
| SD: 1.98 | SD: 2.00 | SD: 2.10 | SD: 3.65 | SD: 7.87 | ||
| 2A | X | M: 0.79 c | M: 0.80 c | M: 0.84 c | M: 1.57 d | M: 3.29 d |
| SD: 0.32 | SD: 0.32 | SD: 0.34 | SD: 0.68 | SD: 1.41 | ||
| Y | M: 1.93 a,* | M: 1.94 a,* | M: 2.03 a,* | M: 3.52 a,* | M: 7.22 a,* | |
| SD: 1.65 | SD: 1.66 | SD: 1.73 | SD: 2.83 | SD: 5.88 | ||
| 2B | X | M: 0.16 b | M: 0.16 b | M: 0.18 b | M: 0.31 c | M: 0.52 c |
| SD: 0.17 | SD: 0.18 | SD: 0.20 | SD: 0.40 | SD: 0.74 | ||
| Y | M: 2.01 a,* | M: 2.02 a,* | M: 2.05 a,* | M: 2.83 a,* | M: 6.05 a,* | |
| SD: 3.73 | SD: 3.74 | SD: 3.77 | SD: 4.76 | SD: 9.77 | ||
| Workplace | Sample Type | Microorganism Number (CFU/m3) | |
|---|---|---|---|
| Bacteria | Fungi | ||
| 1A | X | M: 6.3 × 103 | M: 1.7 × 104 |
| SD: 1.2 × 103 | SD: 6.3 × 103 | ||
| Y | M: 2.5 × 104 * | M: 6.9 × 103 | |
| SD: 9.2 × 103 | SD: 2.5 × 103 | ||
| 1B | X | M: 1.6 × 104 | M: 4.5 × 103 |
| SD: 4.9 × 103 | SD: 7.4 × 102 | ||
| Y | M: 7.2 × 104 | M: 7.2 × 104 | |
| SD: 5.9 × 104 | SD: 5.9 × 104 | ||
| 2A | X | M: 5.4 × 103 | M: 6.6 × 103 |
| SD: 3.2 × 103 | SD: 5.8 × 103 | ||
| Y | M: 7.5 × 103 | M: 5.7 × 103 | |
| SD: 1.8 × 103 | SD: 2.0 × 103 | ||
| 2B | X | M: 8.0 × 103 | M: 3.9 × 103 |
| SD: 3.4 × 103 | SD: 1.3 × 103 | ||
| Y | M: 3.4 × 104 | M: 6.7 × 103 | |
| SD: 9.9 × 103 | SD: 3.8 × 103 | ||
| Marked Microorganisms | Microorganism Number (CFU/g) | ||
|---|---|---|---|
| A/I | A/II | B/III | |
| Bacteria | M: 3.1 × 106 a | M: 3.3 × 106 a | M: 3.1 × 106 a |
| SD: 1.8 × 106 | SD: 5.7 × 105 | SD: 5.4 × 105 | |
| Actinomycetes | M: 2.7 × 106 a | M: 2.7 × 106 a | M: 2.0 × 106 a |
| SD: 1.3 × 106 | SD: 2.0 × 106 | SD: 5.2 × 105 | |
| Staphylococci spp.* | M: 7.4 × 104 a | M: 3.8 × 104 b | M: 6.7 × 104 a,b |
| SD: 1.3 × 104 | SD: 1.9 × 104 | SD: 3.5 × 104 | |
| Pseudomonas fluorescens | M: 4.4 × 106 a | M: 4.5 × 106 a | M: 4.1 × 106 a |
| SD: 1.4 × 106 | SD: 1.3 × 106 | SD: 4.7 × 105 | |
| Fungi | M: 1.2 × 105 a | M: 2.8 × 104 b | M: 8.1 × 104 b |
| SD: 3.7 × 104 | SD: 1.5 × 104 | SD: 3.5 × 104 | |
| Xerophilic Fungi | M: 2.0 × 105 a | M: 3.4 × 104 b | M: 1.1 × 105 a |
| SD: 8.1 × 104 | SD: 1.4 × 104 | SD: 4.7 × 104 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jachowicz, A.; Majchrzycka, K.; Szulc, J.; Okrasa, M.; Gutarowska, B. Survival of Microorganisms on Filtering Respiratory Protective Devices Used at Agricultural Facilities. Int. J. Environ. Res. Public Health 2019, 16, 2819. https://doi.org/10.3390/ijerph16162819
Jachowicz A, Majchrzycka K, Szulc J, Okrasa M, Gutarowska B. Survival of Microorganisms on Filtering Respiratory Protective Devices Used at Agricultural Facilities. International Journal of Environmental Research and Public Health. 2019; 16(16):2819. https://doi.org/10.3390/ijerph16162819
Chicago/Turabian StyleJachowicz, Anita, Katarzyna Majchrzycka, Justyna Szulc, Małgorzata Okrasa, and Beata Gutarowska. 2019. "Survival of Microorganisms on Filtering Respiratory Protective Devices Used at Agricultural Facilities" International Journal of Environmental Research and Public Health 16, no. 16: 2819. https://doi.org/10.3390/ijerph16162819
APA StyleJachowicz, A., Majchrzycka, K., Szulc, J., Okrasa, M., & Gutarowska, B. (2019). Survival of Microorganisms on Filtering Respiratory Protective Devices Used at Agricultural Facilities. International Journal of Environmental Research and Public Health, 16(16), 2819. https://doi.org/10.3390/ijerph16162819







