Phthalate Ester Contamination in Intensively Managed Greenhouse Facilities and the Assessment of Carcinogenic and Non-Carcinogenic Risk: A Regional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Standards and Reagents
2.3. Sampling
2.4. Sample Analysis and Quality Control
2.5. Health Risk Assessment
2.6. Statistical Analysis
3. Results and Discussion
3.1. Soil Contamination with the Six Target PAEs
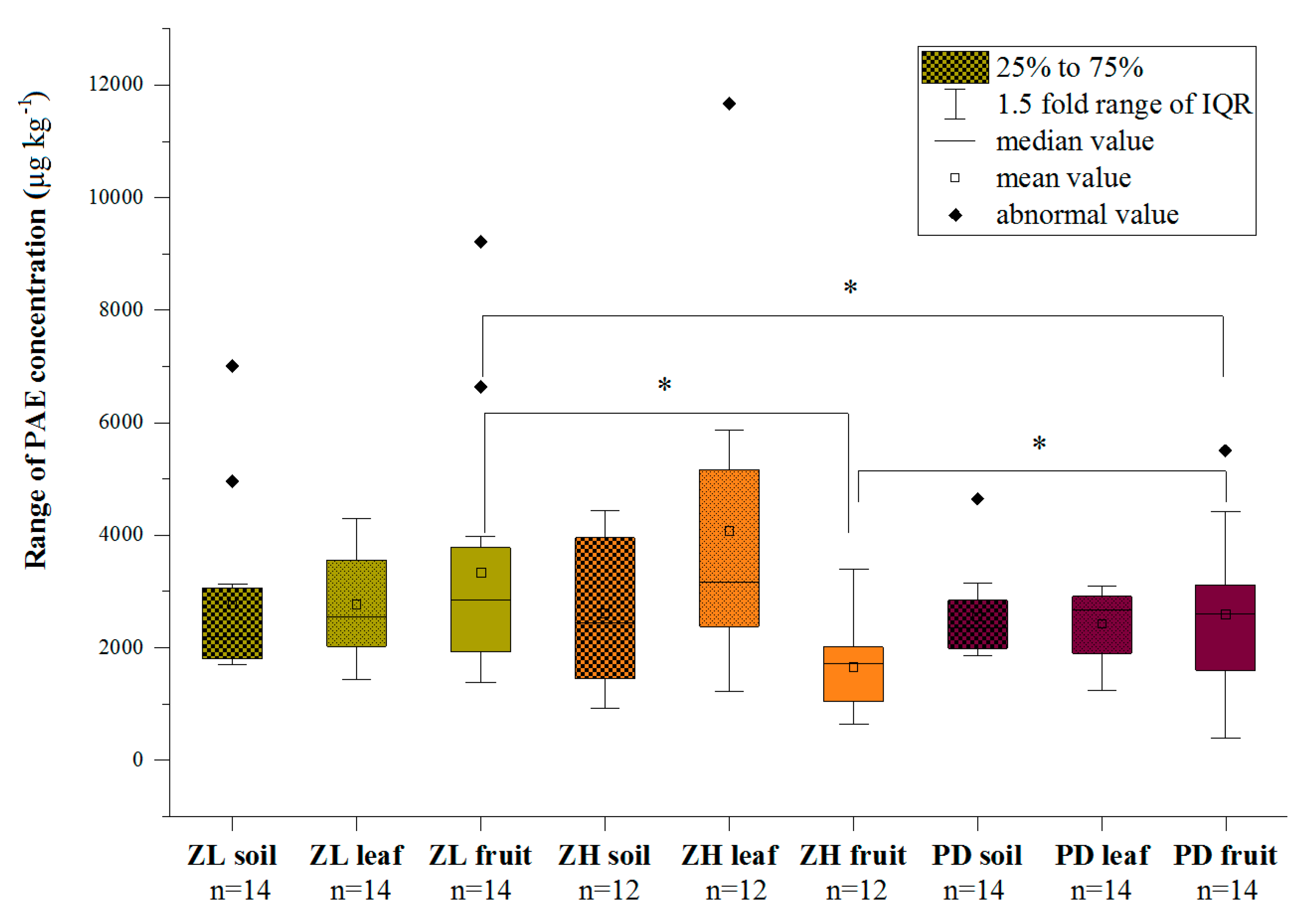
3.2. Plant Contamination Levels of the Six Target PAEs
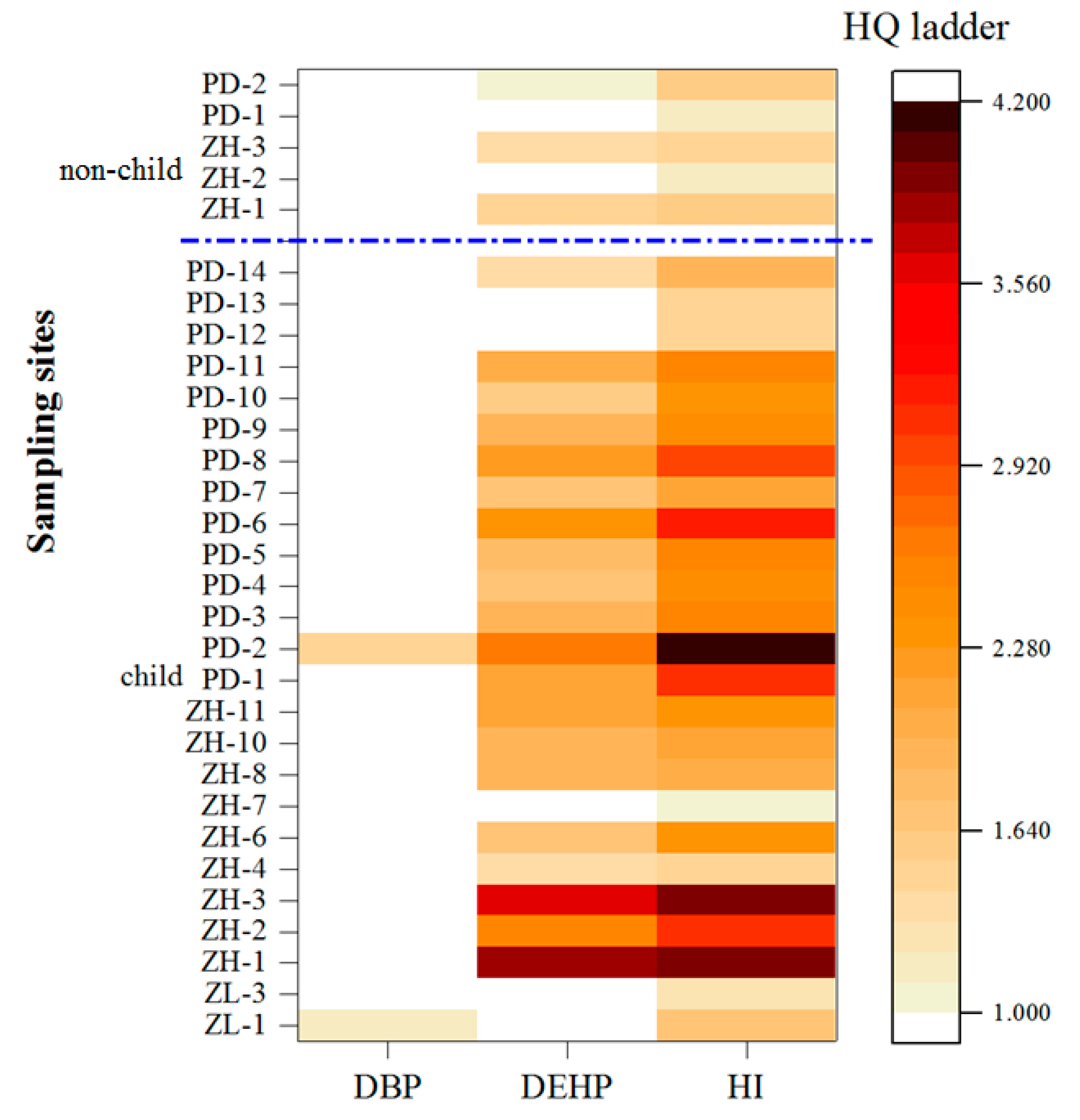
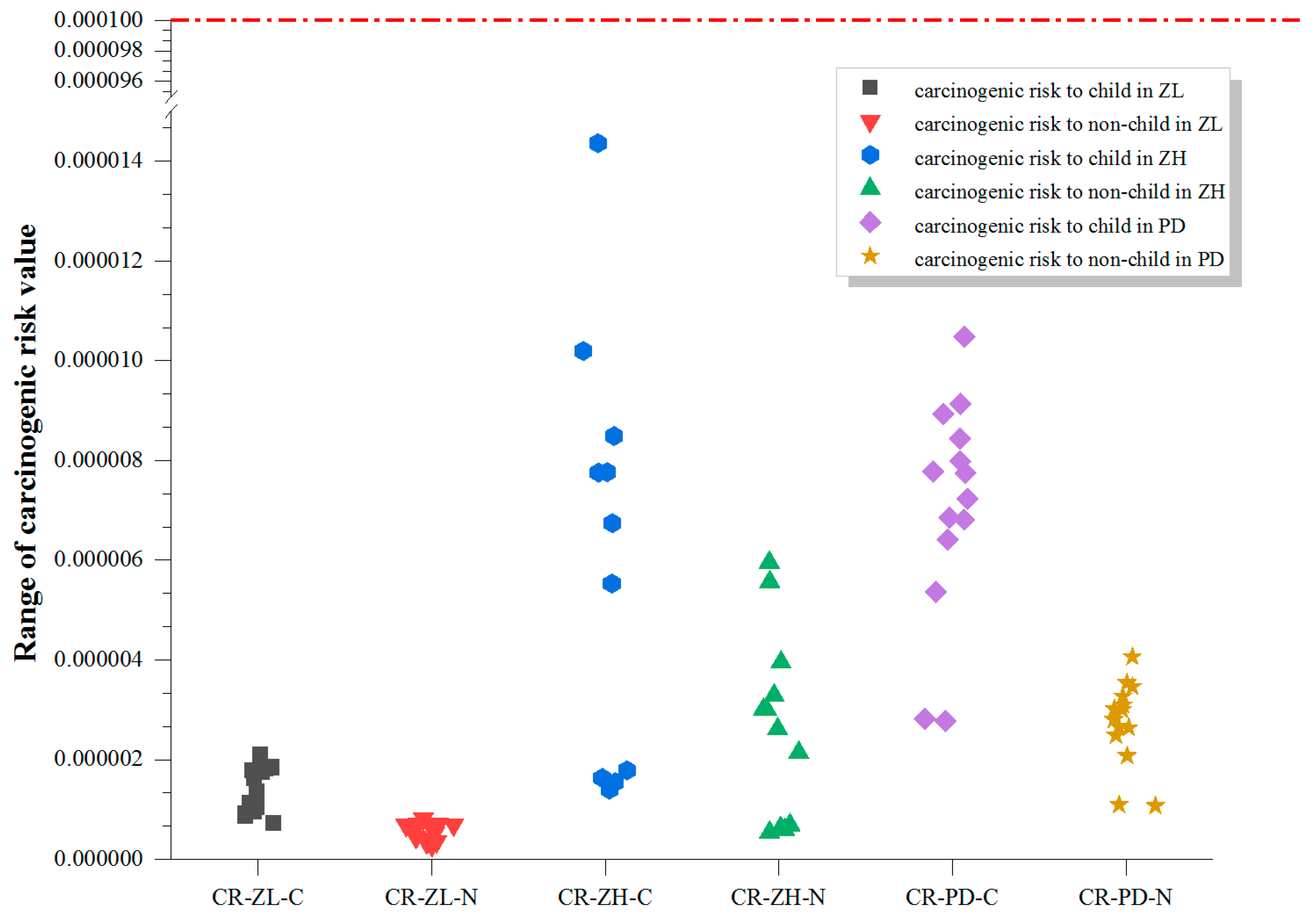
3.3. Non-Carcinogenic and Carcinogenic Risks from the Six Target PAEs
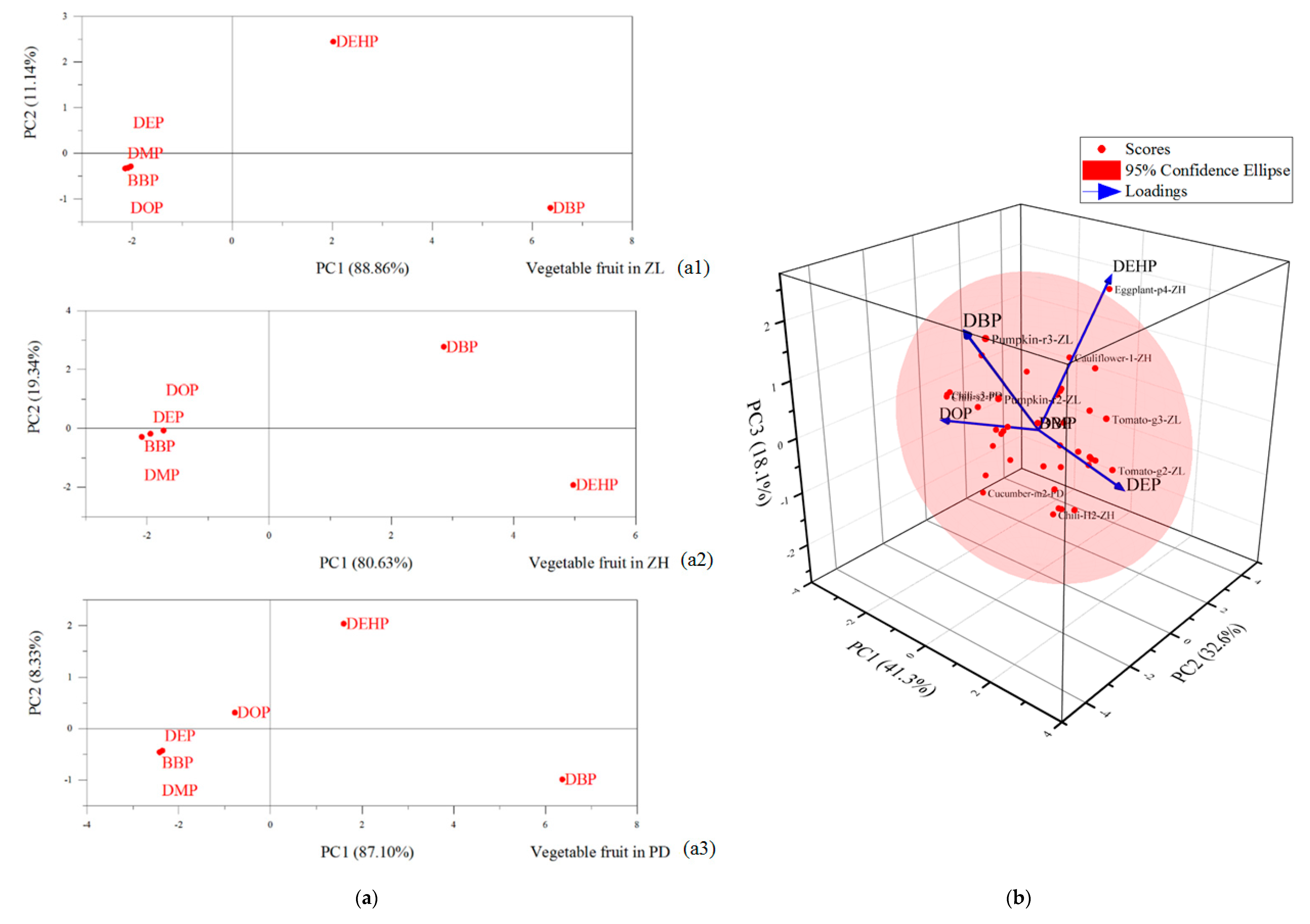
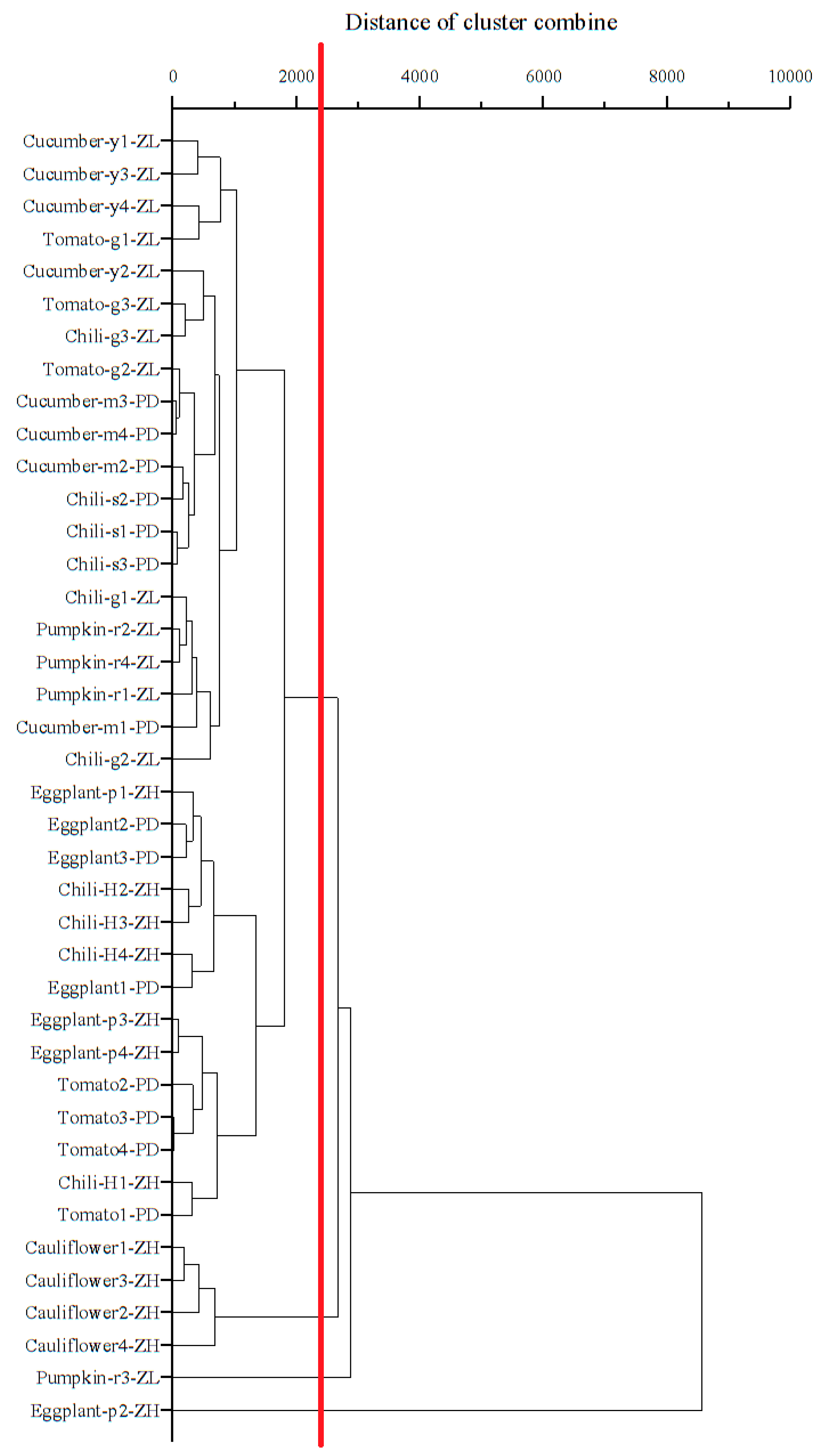
3.4. Source Identification of Target PAEs
3.5. Management Strategies to Control PAE Contamination in the Study Areas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- CCTV News. Available online: http://news.cctv.com/2017/09/18/ARTIYcw2vRCIknQh1V33oCc2170918.shtml (accessed on 1 July 2019).
- Sa, D.S.; Wang, J.L.; Liu, H.P. The harm of agricultural film residue and its solution. Anhui Agric. Sci. Bull. 2006, 3, 68. [Google Scholar]
- Han, J.; He, Y.X.; Zhong, P. How to break through the current situation of agricultural waste surrounding the countryside. Countrys. Agric. Farmers 2015, 12, 15–16. [Google Scholar]
- Fatoki, O.; Vernon, F. Phthalate esters in rivers of the greater Manchester area, UK. Sci. Total Environ. 1990, 95, 227–232. [Google Scholar] [CrossRef]
- Yu, L.H.; Wang, P.; Jiao, F.; Wang, M.X.; Yu, L.H. Pollution by phthalate esters and heavy metals in plastic films in soil-soybean system. Res. Soil Water Conserv. 2011, 18, 201–205. [Google Scholar]
- Bouhamidi, Y.; Kaouah, F.; Nouri, L.; Boumaza, S.; Trari, M. Adsorption of diethyl and dibutyl phthalates onto activated carbon produced from Albizia julibrissin pods: Kinetics and isotherms. Int. J. Environ. Sci. Technol. 2017, 14, 271–284. [Google Scholar] [CrossRef]
- He, L.; Gielen, G.; Bolan, N.S.; Zhang, X.; Wang, H. Contamination and remediation of phthalic acid esters in agricultural soils in China: A review. Agron. Sustain. Dev. 2015, 35, 519–534. [Google Scholar] [CrossRef]
- Gupta, S.; Gadi, R. Temporal variation of phthalic acid esters (PAEs) in ambient atmosphere of Delhi. Bull. Environ. Contam. Toxicol. 2018, 101, 1–7. [Google Scholar] [CrossRef]
- Jia, S.L.; Sankaran, G.; Wang, B.; Shang, H.T.; Fang, M.L. Exposure and risk assessment of volatile organic compounds and airborne phthalates in Singapore’s Child Care Centers. Chemosphere 2019, 224, 85–92. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, J.E.; Choe, W.; Kim, T.; Lee, J.Y.; Kho, Y.L.; Choi, K.; Zoh, K.D. Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ. Int. 2019, 126, 635–643. [Google Scholar] [CrossRef]
- Gao, X.Y.; Li, J.; Wang, X.N.; Zhou, J.L.; Fan, B.; Li, W.W.; Liu, Z.T. Exposure and ecological risk of phthalate esters in the Taihu Lake basin, China. Ecotoxicol. Environ. Saf. 2019, 171, 564–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Sun, X.; Wang, L.; Du, N.; Tao, Y.; Sun, G.Q.; Erinle, K.O.; Wang, P.J.; Zhou, C.J.; et al. Effect of dimethyl phthalate (DMP) on germination, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. Environ. Sci. Pollut. Res. 2016, 23, 1183–1192. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Chang, Q.; Tao, Y.; Wang, L.; Dong, J.; Lin, Y.L.; Zhang, Y. Effect of di-n-butyl phthalate (DBP) on the fruit quality of cucumber and the health risk. Environ. Sci. Pollut. Res. 2016, 23, 24298–24304. [Google Scholar] [CrossRef]
- Pan, J.; Jin, D.; Jiang, H.; Leng, X.; Zhang, A.; Bai, Z. The phyllosphere indigenous microbiota of Brassica campestris L. Change its diversity in responding to di-n-butyl phthalate pollution. World J. Microbiol. Biotechnol. 2019, 35, 21. [Google Scholar] [CrossRef]
- Rashtian, J.; Chavkin, D.E.; Merhi, Z. Water and soil pollution as determinant of water and food quality/contamination and its impact on female fertility. Reprod. Biol. Endocrinol. 2019, 17, 5. [Google Scholar] [CrossRef]
- Ma, T.; Yin, X.Q.; Han, R.T.; Ding, J.; Zhang, H.; Han, X.D.; Li, D.M. Effects of in utero exposure to di-n-butyl phthalate on testicular development in rat. Int. J. Environ. Res. Public Health 2017, 14, 1284. [Google Scholar] [CrossRef]
- Praveena, S.M.; Teh, S.W.; Rajendran, R.K.; Kannan, N.; Lin, C.C.; Abdullah, R.; Kumar, S. Recent updates on phthalate exposure and human health: A special focus on liver toxicity and stem cell regeneration. Environ. Sci. Pollut. Res. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Lu, X.L.; Xu, X.J.; Lin, Y.C.; Zhang, Y.; Huo, X. Phthalate exposure as a risk factor for hypertension. Environ. Sci. Pollut. Res. 2018, 25, 20550–20561. [Google Scholar] [CrossRef]
- Li, A.J.; Martinez-Moral, M.P.; Al-Malki, A.L.; Al-Ghamdi, M.A.; Al-Bazi, M.M.; Kumosani, T.A.; Kurunthachalam Kannan, K. Mediation analysis for the relationship between urinary phthalate metabolites and type 2 diabetes via oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Int. 2019, 126, 153–161. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Proposed Guidelines for Carcinogen Risk Assessment; USEPA: Washington, DC, USA, 1996; pp. 17960–18011.
- Krais, A.M.; Andersen, C.; Eriksson, A.C.; Johnsson, E.; Nielsen, J.; Pagels, J.; Gudmundsson, A.; Lindh, C.H.; Wierzbicka, A. Excretion of urinary metabolites of the phthalate esters DEP and DEHP in 16 volunteers after inhalation and dermal exposure. Int. J. Environ. Res. Public Health 2018, 15, 2514. [Google Scholar] [CrossRef]
- The State Council of People’s Republic of China. Outline of the Medium—And Long-Term Plan for Scientific and Technological Development in China (2006 to 2020); The State Council of People’s Republic of China: Beijing, China, 2005; p. 44.
- Hubei Provincial Department of Agriculture and Rural Affairs (HDAR). 2017. Available online: http://nyt.hubei.gov.cn/zdzl/sswgg/200021244.htm (accessed on 1 July 2019).
- Wang, B.W.; Tang, F.; Shi, M.F. NOCs analysis in water of Hanjiang River and East Lake. Environ. Sci. Technol. 2004, 27, 55–57. [Google Scholar]
- Zhang, D.; Liu, H.; Liang, Y.; Wang, C.; Liang, H.; Cai, H. Distribution of phthalate esters in the groundwater of Jianghan Plain, Hubei, China. Front. Earth Sci. 2009, 3, 73–79. [Google Scholar] [CrossRef]
- PGJM: People’s Government of Jingmen Municipality. 2017. Available online: http://www.jingmen.gov.cn/govinfo/szbmdw_4799/snyj/201704/t20170411_207867.shtml (accessed on 1 July 2019).
- Ma, T.T.; Teng, Y.; Christie, P.; Luo, Y.M.; Chen, Y.S.; Ye, M.; Huang, Y.J. A new procedure combining GC-MS with accelerated solvent extraction for the analysis of phthalic acid esters in contaminated soils. Front. Environ. Sci. Eng. 2013, 7, 31–42. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Science Society of China. In Analysis Methods of Soil Agricultural Chemicals; Science and Technology of China Press: Beijing, China, 1999. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Mid Atlantic Risk Assessment; Regional Screening Level (RSL) Summary Table; USEPA: Washington, DC, USA, 2013.
- Ma, T.T.; Wu, L.H.; Chen, L.; Zhang, H.B.; Luo, Y.M. Phthalate esters contamination in soils and vegetables of plastic film greenhouses of suburb Nanjing, China and the potential human health risk. Environ. Sci. Pollut. Res. 2015, 22, 12018–12028. [Google Scholar] [CrossRef] [Green Version]
- USEPA (United States Environmental Protection Agency). Supplemental Guidance for Developing Soil Screening Level for Superfund Sites; OSWER 9355.4-24; Office of Solid Waste and Emergency Response; USEPA: Washington, DC, USA, 2001.
- Wang, H.; Liang, H.; Gao, D.W. Occurrence and risk assessment of phthalate esters (PAEs) in agricultural soils of the Sanjiang Plain, Northeast China. Environ. Sci. Pollut. Res. 2017, 24, 1–10. [Google Scholar] [CrossRef]
- Chinese Ministry of Health (MoH). Summary of China’s Health Statistics in 2005. Available online: http://www.moh.gov.cn/ (accessed on 1 July 2019).
- USEPA (United States Environmental Protection Agency). Risk Assessment Guidance for Superfund, Vol. I: Human Health Evaluation Manual; EPA/540/1-89/002; Office of Solid Waste and Emergency Response; USEPA: Washington, DC, USA, 1989.
- Wang, L.; Zhang, W.; Tao, W.; Wang, L.; Shi, X.; Lu, X. Investigating into composition, distribution, sources and health risk of phthalic acid esters in street dust of Xi’an City, Northwest China. Environ. Geochem. Health 2017, 39, 865–877. [Google Scholar] [CrossRef]
- Niu, L.; Xu, Y.; Xu, C.; Yun, L.X.; Liu, W.P. Status of phthalate esters contamination in agricultural soils across China and associated health risks. Environ. Pollut. 2014, 195, 16–23. [Google Scholar] [CrossRef]
- NMED (New Mexico Environment Department). Technical Background Document for Development of Soil Screening Levels; NMED: Santa Fe, NM, USA, 2009.
- Hu, W.; Zhang, Y.; Huang, B.; Teng, Y. Soil environmental quality in greenhouse vegetable production systems in eastern China: Current status and management strategies. Chemosphere 2017, 170, 183–195. [Google Scholar] [CrossRef]
- Lv, H.X.; Mo, C.H.; Zhao, H.M.; Xiang, L.; Katsoyiannis, A.; Li, Y.W.; Cai, Q.Y.; Wong, M.H. Soil contamination and sources of phthalates and its health risk in China: A review. Environ. Res. 2018, 164, 417–429. [Google Scholar]
- Wang, L.X. Studies on Phthalate Esters Pollution in Protected Fields. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2007. [Google Scholar]
- Zhang, Y.; Wang, P.; Wang, L.; Sun, G.; Zhao, J.; Zhang, H.; Du, N. The influence of facility agriculture production on phthalate esters distribution in black soils of northeast China. Sci. Total Environ. 2015, 506–507, 118–125. [Google Scholar] [CrossRef]
- Li, K.; Ma, D.; Wu, J.; Chai, C.; Shi, Y. Distribution of phthalate esters in agricultural soil with plastic film mulching in Shandong Peninsula, East China. Chemosphere 2016, 164, 314–321. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Christie, P.; Zhang, M.; Luo, Y.; Teng, Y. Occurrence and risk assessment of phthalate esters (PAEs) in vegetables and soils of suburban plastic film greenhouses. Sci. Total Environ. 2015, 523, 129–137. [Google Scholar] [CrossRef]
- Chen, N.; Shuai, W.; Hao, X.; Zhang, H.; Zhou, D.; Gao, J. Contamination of phthalate esters in vegetable agriculture and human cumulative risk assessment. Pedosphere 2017, 27, 439–451. [Google Scholar] [CrossRef]
- Yin, R.; Lin, X.G.; Wang, S.G.; Zhang, H.Y. Influence of phthalic acid esters in vegetable garden soil on quality of capsicum fruit. Agro-Environ. Prot. 2002, 21, 1–4. [Google Scholar]
- Zhang, M.; Ouyang, Y. Response evaluation of seven crops to phthalate esters contamination. Acta Agric. Univ. Jiangxiensis 2017, 39, 1032–1036. [Google Scholar]
- Yang, X.; Chen, D.; Lv, B.; Miao, H.; Wu, Y.; Zhao, Y. Dietary exposure of the Chinese population to phthalate esters by a total diet study. Food Control 2018, 89, 314–321. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.Y.; Wang, J.H.; Han, P.; Lu, A.X. Phthalate esters in soil, plastic film, and vegetables from greenhouse vegetable production bases in Beijing, China: Concentrations, sources, and risk assessment. Sci. Total Environ. 2016, 568, 1037–1043. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Zhang, M.; Chen, G.; Zhu, T.; Zhang, S.; Teng, Y.; Christie, P.; Luo, Y. Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere 2016, 151, 171–177. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Li, L.; Chen, B.; Zhang, Y. Exposure assessment of phthalates in non-occupational populations in China. Sci. Total Environ. 2012, 427–428, 60–69. [Google Scholar] [CrossRef]
- Nizzetto, L.; Langaas, S.; Futter, M. Pollution: Do microplastics spill on to farm soils? Nature 2016, 537, 488. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]

| Parameter | Young Children | Older Children and Adults | Unit | References | ||
|---|---|---|---|---|---|---|
| Age | 0 to 6 | 7 to 70 | Ma et al. [30] | |||
| Body weight (BW) | 13.6 | Female 57.3 | Male 66.2 | kg | Chinese MoH [33] | |
| 61.75 | ||||||
| IRF (daily vegetable intake) | Carrot (not leafy) | 0.125 | 0.054 | g DW (kg BW day−1) | Ma et al. [30] | |
| EF (exposure frequency) | Production frequency of plastic greenhouses (calculated from Table S1) | days year−1 | Ma et al. [30] | |||
| 1/3 of adults | 273 ZL, 303 ZH, 334 PD | |||||
| ED (Exposure duration) | Production duration of plastic greenhouses (see Table S1) | year | Ma et al. [30] | |||
| CF (conversion factor) | 10−6 | kg mg−1 | USEPA [34] | |||
| AT (average time) | 365×ED for hazard quotient (HQ), lifetime (25,550) for carcinogenic risk (CR) | days | Wang et al. [35] | |||
| IRS (soil ingestion rate) | 200 | 100 | mg day−1 | USEPA [31] | ||
| SA (soil surface area) | 5700 | cm2 day−1 | Wang et al. [35] | |||
| AF (soil adherence factor) | 0.07 | mg cm−2 | Wang et al. [35] | |||
| ABS (fraction of contaminant absorbed dermally from the soil) | 0.1 | unitless | Wang et al. [35] | |||
| PEF (particles emission factor) | 1.36 × 109 | m3 kg−1 | Wang et al. [35] | |||
| SFO (oral slope factor of the carcinogen) | BBP | 1.90 × 10−3 | (mg kg−1 day−1)−1 | Niu et al. [36] | ||
| DEHP | 1.40 × 10−2 | |||||
| RfD (New Mexico Environment Department, NMED) | DMP | 10 | 103 µg (kg day)−1 | NMED [37] | ||
| DEP | 0.8 | |||||
| BBP | 0.2 | |||||
| DBP | 0.1 | |||||
| DEHP | 0.02 | |||||
| DOP | 0.04 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Zhou, W.; Chen, L.; Christie, P.; Luo, Y.; Wu, P. Phthalate Ester Contamination in Intensively Managed Greenhouse Facilities and the Assessment of Carcinogenic and Non-Carcinogenic Risk: A Regional Study. Int. J. Environ. Res. Public Health 2019, 16, 2818. https://doi.org/10.3390/ijerph16162818
Ma T, Zhou W, Chen L, Christie P, Luo Y, Wu P. Phthalate Ester Contamination in Intensively Managed Greenhouse Facilities and the Assessment of Carcinogenic and Non-Carcinogenic Risk: A Regional Study. International Journal of Environmental Research and Public Health. 2019; 16(16):2818. https://doi.org/10.3390/ijerph16162818
Chicago/Turabian StyleMa, Tingting, Wei Zhou, Like Chen, Peter Christie, Yongming Luo, and Peng Wu. 2019. "Phthalate Ester Contamination in Intensively Managed Greenhouse Facilities and the Assessment of Carcinogenic and Non-Carcinogenic Risk: A Regional Study" International Journal of Environmental Research and Public Health 16, no. 16: 2818. https://doi.org/10.3390/ijerph16162818
APA StyleMa, T., Zhou, W., Chen, L., Christie, P., Luo, Y., & Wu, P. (2019). Phthalate Ester Contamination in Intensively Managed Greenhouse Facilities and the Assessment of Carcinogenic and Non-Carcinogenic Risk: A Regional Study. International Journal of Environmental Research and Public Health, 16(16), 2818. https://doi.org/10.3390/ijerph16162818





