Major Differences in the Diversity of Mycobiomes Associated with Wheat Processing and Domestic Environments: Significant Findings from High-Throughput Sequencing of Fungal Barcode ITS1
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection and Treatment
2.3. Statistical Analysis
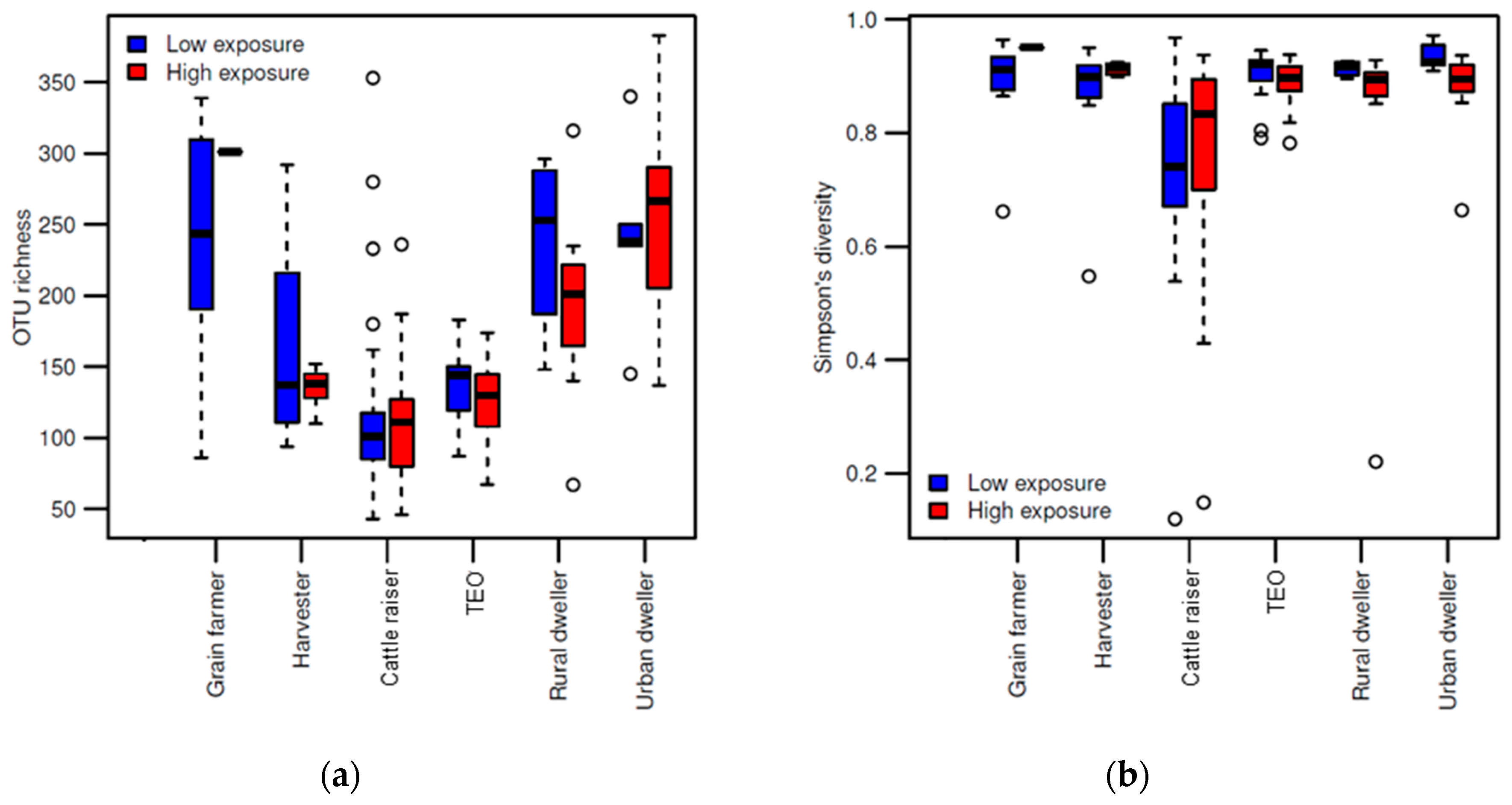
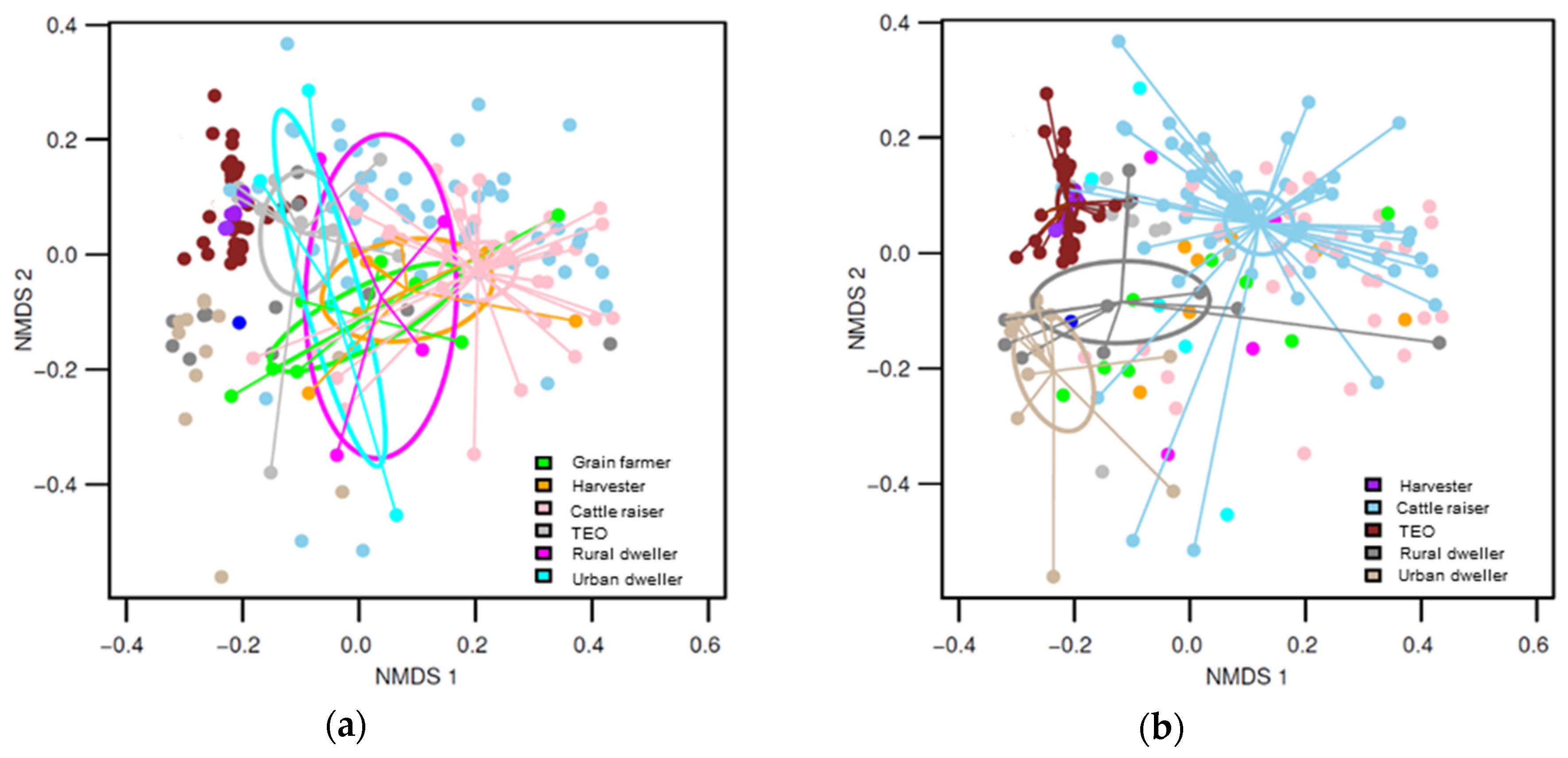
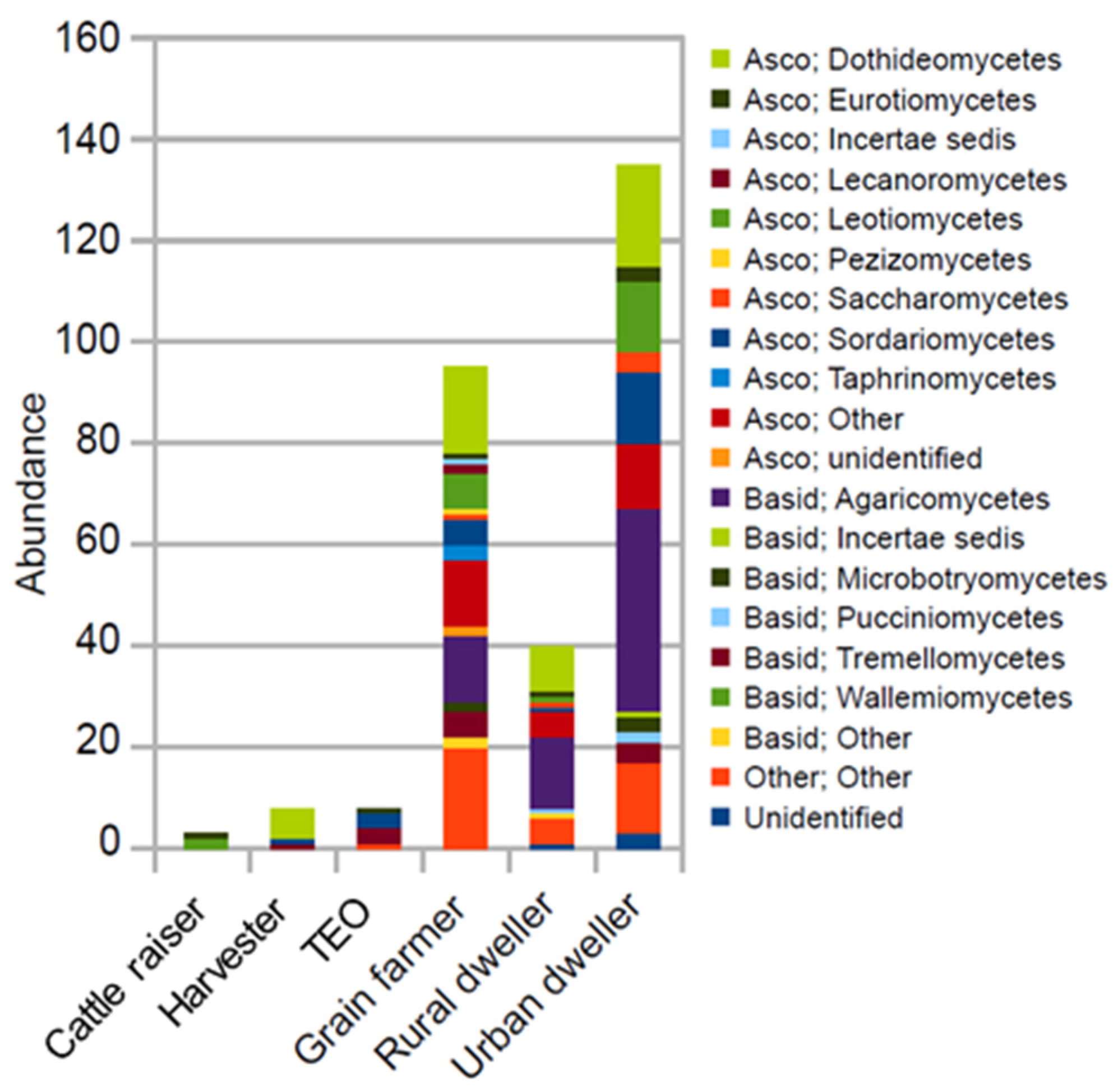
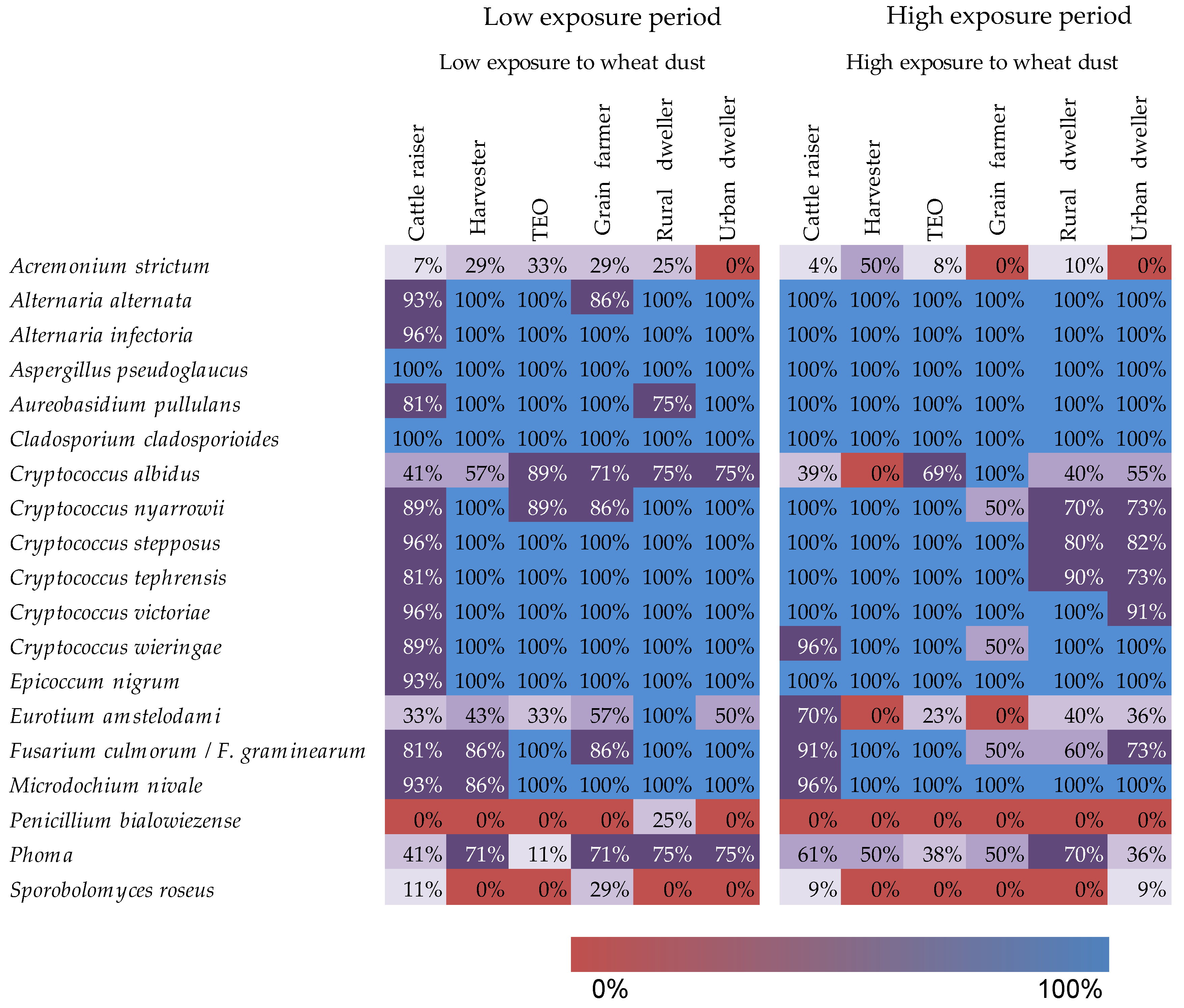
3. Results
3.1. Differences in Fungal Community Composition Between Environments
3.2. Indicator Species and Fungi of Clinical Interest
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barrera, C.; Wild, P.; Dorribo, V.; Savova-Bianchi, D.; Laboissiere, A.; Pralong, J.A.; Danuser, B.; Krief, P.; Millon, L.; Reboux, G.; et al. Exposure to field vs. storage wheat dust: Different consequences on respiratory symptoms and immune response among grain workers. Int. Arch. Occup. Environ. Health 2018, 91, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Dorribo, V.; Wild, P.; Pralong, J.A.; Danuser, B.; Reboux, G.; Krief, P.; Niculita-Hirzel, H. Respiratory health effects of fifteen years of improved collective protection in a wheat-processing worker population. Ann. Agric. Environ. Med. 2015, 22, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Roussel, S.; Sudre, B.; Reboux, G.; Waser, M.; Buchele, G.; Vacheyrou, M.; Dalphin, J.C.; Millon, L.; Braun-Fahrlander, C.; von Mutius, E.; et al. Exposure to moulds and actinomycetes in Alpine farms: A nested environmental study of the PASTURE cohort. Environ. Res. 2011, 111, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Niculita-Hirzel, H.; Hantier, G.; Storti, F.; Plateel, G.; Roger, T. Frequent occupational exposure to Fusarium mycotoxins of workers in the Swiss grain industry. Toxins 2016, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, L.; Oppliger, A.; Hirzel, A.H.; Savova-Bianchi, D.; Mbayo, G.; Mascher, F.; Kellenberger, S.; Niculita-Hirzel, H. Airborne and grain dust fungal community compositions are shaped regionally by plant genotypes and farming practices. Appl. Environ. Microbiol. 2016, 82, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Noss, I.; Wouters, I.M.; Visser, M.; Heederik, D.J.; Thorne, P.S.; Brunekreef, B.; Doekes, G. Evaluation of a low-cost electrostatic dust fall collector for indoor air endotoxin exposure assessment. Appl. Environ. Microbiol. 2008, 74, 5621–5627. [Google Scholar] [CrossRef] [PubMed]
- Frankel, M.; Timm, M.; Hansen, E.W.; Madsen, A.M. Comparison of sampling methods for the assessment of indoor microbial exposure. Indoor Air 2012, 22, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Scherer, E.; Rocchi, S.; Reboux, G.; Vandentorren, S.; Roussel, S.; Vacheyrou, M.; Raherison, C.; Millon, L. qPCR standard operating procedure for measuring microorganisms in dust from dwellings in large cohort studies. Sci. Total Environ. 2014, 466–467, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Fungal Barcoding, Consortium Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D. Vegan: Community Ecology Package. R package version 2.0-2. 2017. Available online: https://www.researchgate.net/publication/282247686 (accessed on 6 June 2017).
- Roberts, D. Labdsv: Ordination and Multivariate Analysis for Ecology. R package version 1.7-0. 2015. Available online: https://github.com/cran/labdsv/commit/a56af17c747fd1480a0cac60818928776fc07edf (accessed on 5 May 2015).
- de Ana, S.G.; Torres-Rodriguez, J.M.; Ramirez, E.A.; Garcia, S.M.; Belmonte-Soler, J. Seasonal distribution of Alternaria, Aspergillus, Cladosporium and Penicillium species isolated in homes of fungal allergic patients. J. Investig. Allergol. Clin. Immunol. 2006, 16, 357–563. [Google Scholar] [PubMed]
- Poursafar, A.; Ghosta, Y.; Orina, A.S.; Gannibal, P.B.; Javan-Nikkhah, M.; Lawrence, D.P. Taxonomic study on Alternaria sections Infectoriae and Pseudoalternaria associated with black (sooty) head mold of wheat and barley in Iran. Mycol. Prog. 2018, 17, 343–356. [Google Scholar] [CrossRef]
- Ferreira Lopes, S.; Vacher, G.; Ciarlo, E.; Savova-Bianchi, D.; Roger, T.; Niculita-Hirzel, H. Primary and immortalized human respiratory cells display different patterns of cytotoxicity and cytokine release upon exposure to Deoxynivalenol, Nivalenol and Fusarenon-X. Toxins 2017, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Olstorpe, M.; Borling, J.; Schnurer, J.; Passoth, V. Pichia anomala yeast improves feed hygiene during storage of moist crimped barley grain under Swedish farm conditions. Anim. Feed Sci. Technol. 2010, 156, 47–56. [Google Scholar] [CrossRef]
- Olstorpe, M.; Schnurer, J.; Passoth, V. Microbial changes during storage of moist crimped cereal barley grain under Swedish farm conditions. Anim. Feed Sci. Technol. 2010, 156, 37–46. [Google Scholar] [CrossRef]
- Segvic Klaric, M.; Jaksic Despot, D.; Kopjar, N.; Rasic, D.; Kocsube, S.; Varga, J.; Peraica, M. Cytotoxic and genotoxic potencies of single and combined spore extracts of airborne OTA-producing and OTA-non-producing Aspergilli in Human lung A549 cells. Ecotoxicol. Environ. Saf. 2015, 120, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.; Rocchi, S.; Degano, B.; Soumagne, T.; Laurent, L.; Bellanger, A.P.; Laplante, J.J.; Millon, L.; Dalphin, J.C.; Reboux, G. Microbial exposure to dairy farmers’ dwellings and COPD occurrence. Int. J. Environ. Health Res. 2019, 29, 387–399. [Google Scholar] [CrossRef] [PubMed]

| Worker Population | High Exposure | Low Exposure | ||||
|---|---|---|---|---|---|---|
| N Samples | N Individuals | N Sites | N Samples | N Individuals | N Sites | |
| Terminal elevator operator | 25 | 18 | 7 | 11 | 11 | 8 |
| Harvester | 2 | 2 | 2 | 7 | 7 | 7 |
| Grain farmer | 2 | 2 | 2 | 8 | 8 | 8 |
| Cattle raiser | 56 | 24 | 24 | 39 | 28 | 28 |
| Rural dweller | 15 | 15 | 15 | 4 | 4 | 4 |
| Urban dweller | 13 | 13 | 13 | 5 | 5 | 5 |
| Total | 113 | 74 | 63 | 74 | 63 | 60 |
| PERMANOVA Pairs | F.Model | R2 | p-Value | p-Adjusted | ||
|---|---|---|---|---|---|---|
| Low exposure period | vs. | High exposure period | ||||
| Cattle raiser | vs. | Cattle raiser | 3.76 | 0.04 | 0.022 | 0.052 |
| Harvester | vs. | Harvester | 15.444 | 0.58 | 0.002 | 0.007 |
| TEO 1 | vs. | TEO | 6.325 | 0.14 | 0.001 | 0.004 |
| Urban dweller | vs. | Urban dweller | 4.075 | 0.21 | 0.011 | 0.029 |
| High exposure period | vs. | High exposure period | ||||
| Cattle raiser | vs. | Rural dweller | 8.967 | 0.13 | 0.001 | 0.004 |
| Harvester | vs. | Cattle raiser | 16.741 | 0.23 | 0.001 | 0.004 |
| Harvester | vs. | Rural dweller | 8.659 | 0.35 | 0.001 | 0.004 |
| TEO | vs. | Urban dweller | 37.728 | 0.48 | 0.001 | 0.004 |
| TEO | vs. | Cattle raiser | 65.139 | 0.45 | 0.001 | 0.004 |
| TEO | vs. | Rural dweller | 19.793 | 0.33 | 0.001 | 0.004 |
| TEO | vs. | Grain farmer 2 | 2.403 | 0.07 | 0.078 | 0.142 |
| Urban dweller | vs. | Harvester | 22.252 | 0.58 | 0.001 | 0.004 |
| Urban dweller | vs. | Cattle raiser | 17.217 | 0.22 | 0.001 | 0.004 |
| Urban dweller | vs. | Rural dweller | 2.71 | 0.11 | 0.034 | 0.073 |
| Low exposure period | vs. | Low exposure period | ||||
| Cattle raiser | vs. | Urban dweller | 7.328 | 0.17 | 0.002 | 0.007 |
| Cattle raiser | vs. | Grain farmer | 4.291 | 0.1 | 0.008 | 0.022 |
| Cattle raiser | vs. | Rural dweller | 2.477 | 0.06 | 0.069 | 0.129 |
| Harvester | vs. | Cattle raiser | 3.453 | 0.08 | 0.027 | 0.061 |
| Harvester | vs. | Urban dweller | 2.466 | 0.2 | 0.089 | 0.154 |
| TEO | vs. | Harvester | 5.469 | 0.26 | 0.006 | 0.017 |
| TEO | vs. | Cattle raiser | 25.821 | 0.38 | 0.001 | 0.004 |
| TEO | vs. | Urban dweller | 2.59 | 0.16 | 0.056 | 0.11 |
| TEO | vs. | Grain farmer | 5.699 | 0.25 | 0.004 | 0.012 |
| TEO | vs. | Rural dweller | 3.674 | 0.22 | 0.023 | 0.053 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yashiro, E.; Savova-Bianchi, D.; Niculita-Hirzel, H. Major Differences in the Diversity of Mycobiomes Associated with Wheat Processing and Domestic Environments: Significant Findings from High-Throughput Sequencing of Fungal Barcode ITS1. Int. J. Environ. Res. Public Health 2019, 16, 2335. https://doi.org/10.3390/ijerph16132335
Yashiro E, Savova-Bianchi D, Niculita-Hirzel H. Major Differences in the Diversity of Mycobiomes Associated with Wheat Processing and Domestic Environments: Significant Findings from High-Throughput Sequencing of Fungal Barcode ITS1. International Journal of Environmental Research and Public Health. 2019; 16(13):2335. https://doi.org/10.3390/ijerph16132335
Chicago/Turabian StyleYashiro, Erika, Dessislava Savova-Bianchi, and Hélène Niculita-Hirzel. 2019. "Major Differences in the Diversity of Mycobiomes Associated with Wheat Processing and Domestic Environments: Significant Findings from High-Throughput Sequencing of Fungal Barcode ITS1" International Journal of Environmental Research and Public Health 16, no. 13: 2335. https://doi.org/10.3390/ijerph16132335
APA StyleYashiro, E., Savova-Bianchi, D., & Niculita-Hirzel, H. (2019). Major Differences in the Diversity of Mycobiomes Associated with Wheat Processing and Domestic Environments: Significant Findings from High-Throughput Sequencing of Fungal Barcode ITS1. International Journal of Environmental Research and Public Health, 16(13), 2335. https://doi.org/10.3390/ijerph16132335





