A Population Dynamic Model to Assess the Diabetes Screening and Reporting Programs and Project the Burden of Undiagnosed Diabetes in Thailand
Abstract
1. Introduction
2. Materials and Methods
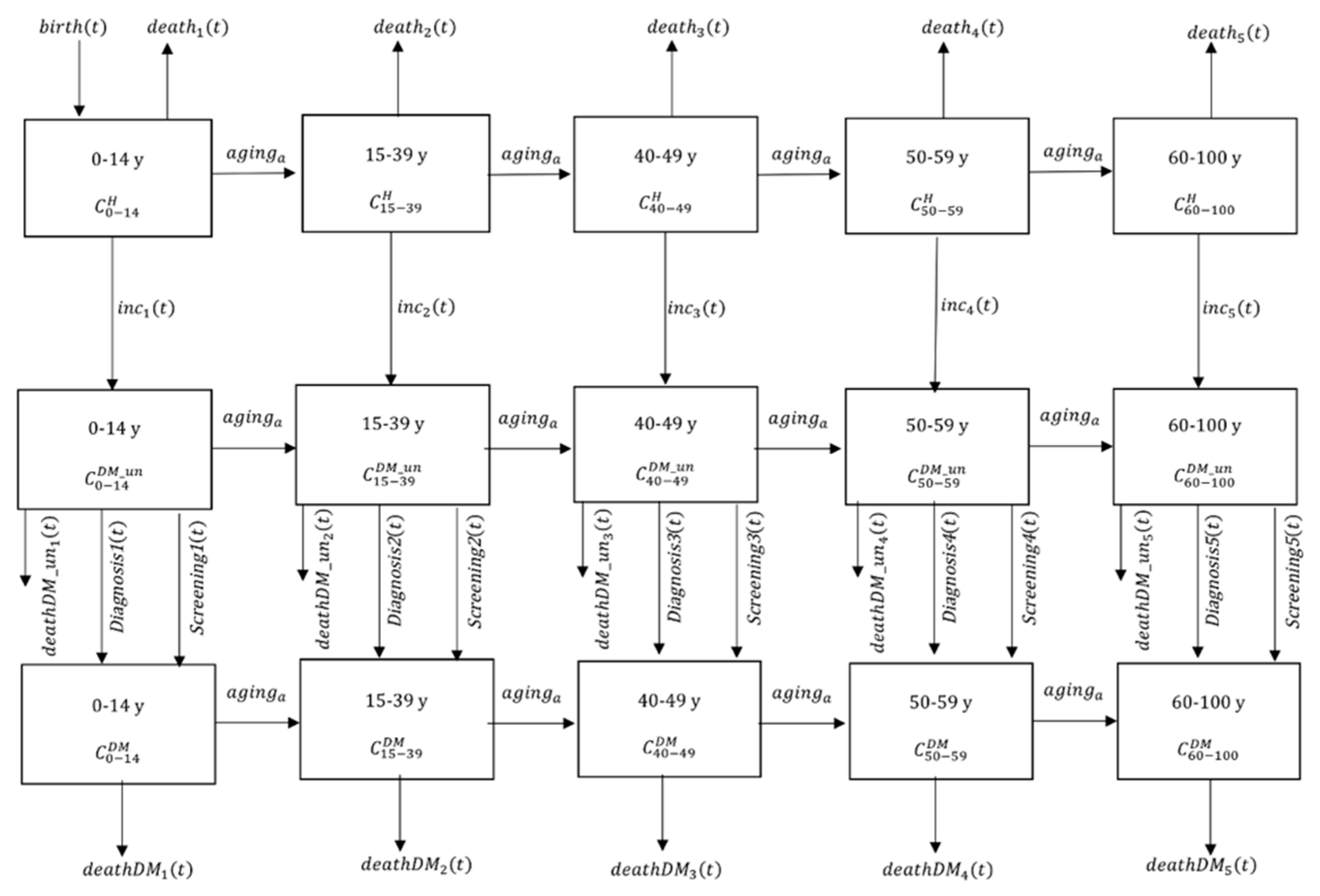
2.1. Demographic Sub-Model
2.2. Diabetes Dynamic Sub-Model
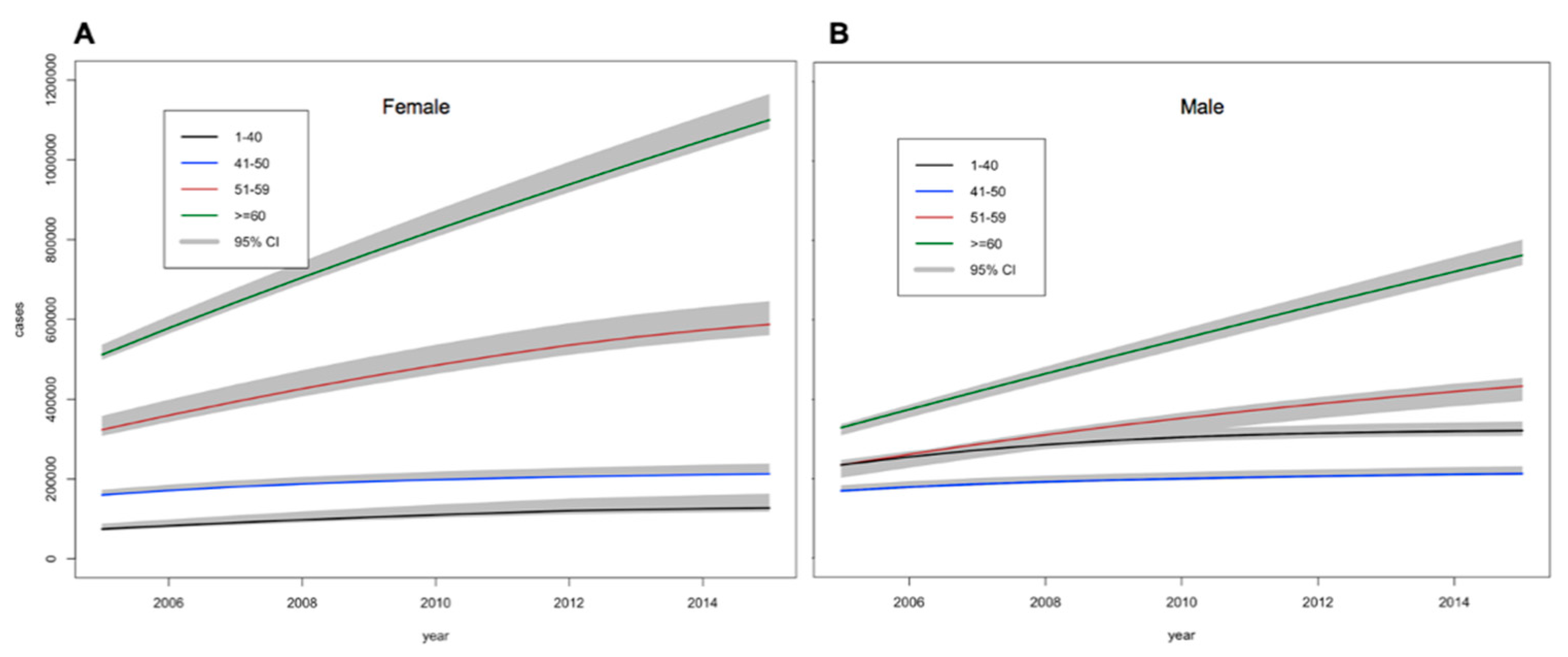
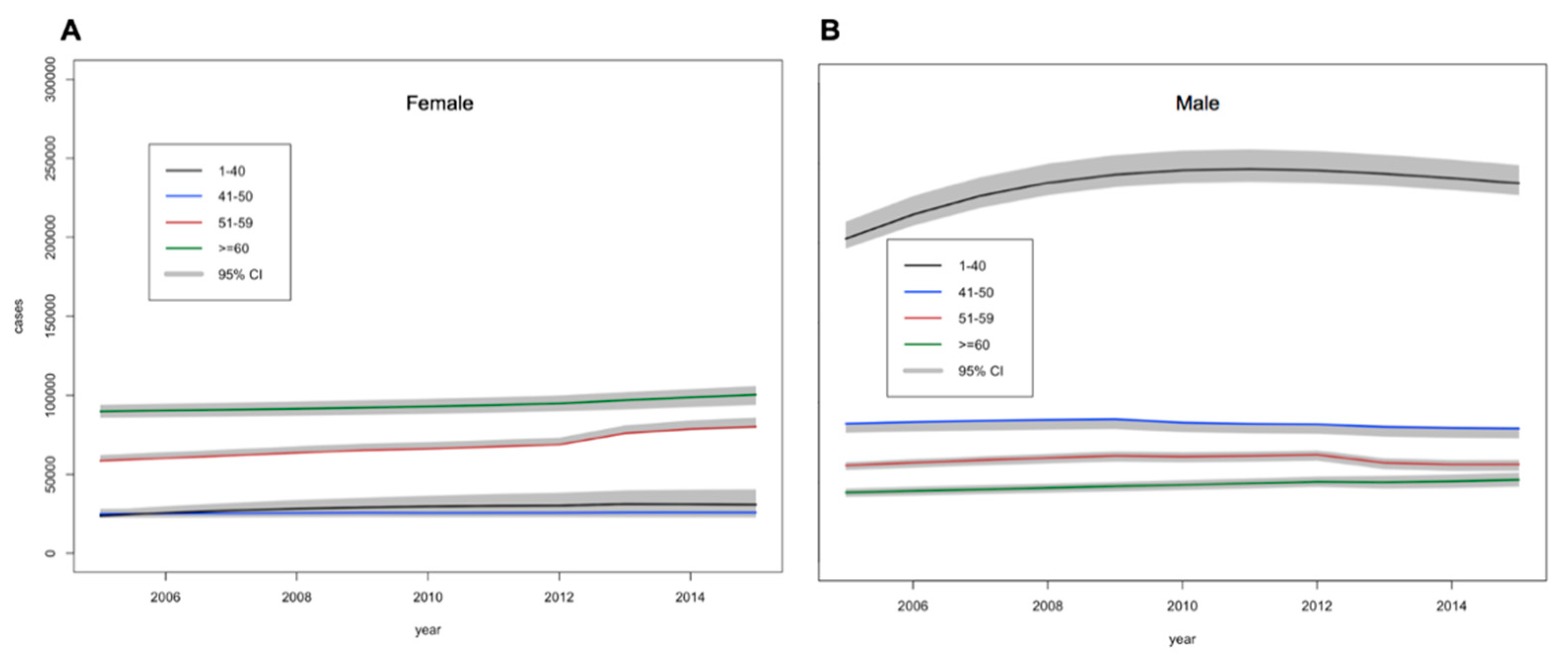
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IDF. IDF Diabetes Atlas. Available online: http://www.diabetesatlas.org (accessed on 27 May 2019).
- Thonghong, A. Annual Epidemilological Surveillance Report 2015; Bureau of Epidemiology, Ministry of Public Health: Nonthaburi, Thailand, 2015.
- HSRI. The Fifth National Health Examination Survey; Ministry of Public Health: Nonthaburi, Thailand, 2014.
- Papier, K.; Jordan, S.; D’Este, C.; Bain, C.; Peungson, J.; Banwell, C.; Yiengprugsawan, V.; Seubsman, S.A.; Sleigh, A. Incidence and risk factors for type 2 diabetes mellitus in transitional Thailand: Results from the Thai cohort study. BMJ Open 2016, 6, 014102. [Google Scholar] [CrossRef] [PubMed]
- Pratipanawatr, T.; Rawdaree, P.; Chetthakul, T.; Bunnag, P.; Ngarmukos, C.; Benjasuratwong, Y.; Leelawatana, R.; Kosachunhanun, N.; Plengvidhya, N.; Deerochanawong, C.; et al. Thailand Diabetic Registry cohort: Predicting death in Thai diabetic patients and causes of death. J. Med. Assoc. Thai. 2010, 93, 12–20. [Google Scholar]
- Rawla, P.; Vellipuram, A.R.; Bandaru, S.S.; Pradeep Raj, J. Euglycemic diabetic ketoacidosis: A diagnostic and therapeutic dilemma. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017. Available online: https://edm.bioscientifica.com/view/journals/edm/2017/1/EDM17-0081.xml. [CrossRef] [PubMed]
- Gregg, E.W.; Sattar, N.; Ali, M.K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016, 4, 537–547. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Liu, W.; Wang, Q.; Qian, Y.; Lu, P. Correlations among Diabetic Microvascular Complications: A Systematic Review and Meta-analysis. Sci. Rep. 2019, 9, 3137. [Google Scholar] [CrossRef] [PubMed]
- Geard, N.; Glass, K.; McCaw, J.M.; McBryde, E.S.; Korb, K.B.; Keeling, M.J.; McVernon, J. The effects of demographic change on disease transmission and vaccine impact in a household structured population. Epidemics 2015, 13, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.M. Adding Life to Years Report of the Expert Group on Healthcare of Older People. Available online: http://www.sehd.scot.nhs.uk/publications/alty/alty-00.htm (accessed on 1 June 2018).
- Boutayeb, A.; Twizell, E.H. An age structured model for complications of diabetes mellitus in Morocco. Simul. Model. Pract. Theory 2004, 12, 77–87. [Google Scholar] [CrossRef]
- Boyle, J.P.; Honeycutt, A.A.; Narayan, K.M.; Hoerger, T.J.; Geiss, L.S.; Chen, H.; Thompson, T.J. Projection of diabetes burden through 2050: Impact of changing demography and disease prevalence in the U.S. Diabetes Care 2001, 24, 1936–1940. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Brown, J.; Vistisen, D.; Sicree, R.; Shaw, J.; Nichols, G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res. Clin. Pr. 2010, 87, 293–301. [Google Scholar] [CrossRef]
- Deerochanawong, C.; Ferrario, A. Diabetes management in Thailand: A literature review of the burden, costs, and outcomes. Glob. Health 2013, 9, 11. [Google Scholar] [CrossRef]
- Panket, P. Annual Report 2013; Bureau of Non Communicable Diseases, Ministry of Public Health: Nonthaburi, Thailand, 2013.
- MoPH. Key Performance Indicator. Available online: http://healthdata.moph.go.th/kpi/ (accessed on 11 June 2018 ).
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pr. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.M.; Boyle, J.P.; Geiss, L.S.; Saaddine, J.B.; Thompson, T.J. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care 2006, 29, 2114–2116. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.P.; Thompson, T.J.; Gregg, E.W.; Barker, L.E.; Williamson, D.F. Projection of the year 2050 burden of diabetes in the US adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul. Health Metr. 2010, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.P.; Alkema, L.; Tai, E.S.; Tan, K.H.; Yang, Q.; Lim, W.Y.; Teo, Y.Y.; Cheng, C.Y.; Wang, X.; Wong, T.Y.; et al. Forecasting the burden of type 2 diabetes in Singapore using a demographic epidemiological model of Singapore. BMJ Open Diabetes Res. Care 2014, 2, 000012. [Google Scholar] [CrossRef] [PubMed]
- Boutayeb, A.; Twizell, E.H.; Achouayb, K.; Chetouani, A. A mathematical model for the burden of diabetes and its complications. Biomed. Eng. Online 2004, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Al-Quwaidhi, A.J.; Pearce, M.S.; Sobngwi, E.; Critchley, J.A.; O’Flaherty, M. Comparison of type 2 diabetes prevalence estimates in Saudi Arabia from a validated Markov model against the International Diabetes Federation and other modelling studies. Diabetes Res. Clin. Pr. 2014, 103, 496–503. [Google Scholar] [CrossRef]
- Appuhamy, J.A.; Kebreab, E.; France, J. A mathematical model for determining age-specific diabetes incidence and prevalence using body mass index. Ann. Epidemiol. 2013, 23, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Boutayeb, A.; Chetouani, A. A critical review of mathematical models and data used in diabetology. Biomed. Eng. Online 2006, 5, 43. [Google Scholar] [CrossRef]
- Chang, H.J.; Kuo, H.S.; Tung, T.H.; Chou, P.; Chen, T.H. Evaluation of a population-based screening for type 2 diabetes: A community-based screening project in Puli, Taiwan. Prev. Med. 2000, 31, 396–402. [Google Scholar] [CrossRef]
- Carral, F.; Olveira, G.; Aguilar, M.; Ortego, J.; Gavilan, I.; Domenech, I.; Escobar, L. Hospital discharge records under-report the prevalence of diabetes in inpatients. Diabetes Res. Clin. Pr. 2003, 59, 145–151. [Google Scholar] [CrossRef]
- USCB. International Data Base. Available online: https://www.census.gov/population/international/data/idb/informationGateway.php (accessed on 21 August 2018).
- NSO. The 2010 Population and Housing Census; National Statistical Office, Office of the Prime Minister: Bangkok, Thailand, 2010.
- MoPH. Public Health Statistics A.D.2010; Ministry of Public Health: Nonthaburi, Thailand, 2010.
- Leoprapai, B. Thailand’s Population; Mahidol University: Nakhon Pathom, Thailand, 2014. [Google Scholar]
- Huguet, J.W. Thailand Migration Report 2011; International Organization for Migration: Bangkok, Thailand, 2011. [Google Scholar]
- Feldman, A.L.; Long, G.H.; Johansson, I.; Weinehall, L.; Fharm, E.; Wennberg, P.; Norberg, M.; Griffin, S.J.; Rolandsson, O. Change in lifestyle behaviors and diabetes risk: Evidence from a population-based cohort study with 10 year follow-up. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Bazata, D.D.; Fox, K.M.; Grandy, S.; Group, S.S. Health-related behaviours of people with diabetes and those with cardiometabolic risk factors: Results from SHIELD. Int. J. Clin. Pr. 2007, 61, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, K.; Petzoldt, T.; Setzer, R.W. Solving Differential Equations in R: Package deSolve. J. Stat. Softw. 2010, 33, 1–25. [Google Scholar] [CrossRef]
- Hartig, F.; Minunno, F.; Paul, S. BayesianTools: General-Purpose MCMC and SMC Samplers and Tools for Bayesian Statistics. R package version. R package version 0.1.3. Available online: https://cran.r-project.org/web/packages/BayesianTools/ndex.html (accessed on 1 June 2019).
- Aekplakorn, W.; Chariyalertsak, S.; Kessomboon, P.; Sangthong, R.; Inthawong, R.; Putwatana, P.; Taneepanichskul, S. Thai National Health Examination Survey IV Study Group, Prevalence and management of diabetes and metabolic risk factors in Thai adults: The Thai National Health Examination Survey IV, 2009. Diabetes Care 2011, 34, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Flores-Le Roux, J.A.; Comin, J.; Pedro-Botet, J.; Benaiges, D.; Puig-de Dou, J.; Chillaron, J.J.; Goday, A.; Bruguera, J.; Cano-Perez, J.F. Seven-year mortality in heart failure patients with undiagnosed diabetes: An observational study. Cardiovasc. Diabetol. 2011, 10, 39. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Loss of follow-up of diabetic patients: What are the reasons? Indian J. Endocrinol. Metab. 2011, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, W.H. Behavioral Factors Associated with Disease, Injury, and Death among Men: Evidence and Implications for Prevention. J. Men’s Stud. 2000, 9, 81–142. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Mahasakpan, P.; Chaovakiratipong, C.; Nguntra, P.; Tatip, Y.; Koonchote, S.; Boonmak, C.; Tharmaphornpilas, P. Screening assessment of persons 40–59 years of age in rural Thailand by a mobile health unit. J. Med. Assoc. Thai. 1999, 82, 131–139. [Google Scholar]
- WHO. Screening for Type 2 Diabetes; World Health Organization, Department of Noncommunicable Disease Management: Geneva, Switzerland, 2002. [Google Scholar]
- WHO. Global Report on Diabetes; World Health Organization: Paris, France, 2016. [Google Scholar]
- Johnson, J.A.; Majumdar, S.R.; Simpson, S.H.; Toth, E.L. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 2002, 25, 2244–2248. [Google Scholar] [CrossRef]
- Monesi, L.; Baviera, M.; Marzona, I.; Avanzini, F.; Monesi, G.; Nobili, A.; Tettamanti, M.; Cortesi, L.; Riva, E.; Fortino, I.; et al. Prevalence, incidence and mortality of diagnosed diabetes: Evidence from an Italian population-based study. Diabet Med. 2012, 29, 385–392. [Google Scholar] [CrossRef]
| Year | Age-Group (Years) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–39 | 40–49 | 50–59 | ≥60 | ||||||
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| 2005 | 240 | 40 | 80 | 110 | 120 | 210 | 210 | 340 | 1350 |
| 2010 | 230 | 70 | 180 | 160 | 210 | 320 | 300 | 510 | 1980 |
| 2015 | 320 | 130 | 240 | 210 | 420 | 590 | 710 | 1100 | 3720 |
| 2035 | 230 | 100 | 210 | 210 | 460 | 620 | 1300 | 1800 | 4930 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahikul, W.; J White, L.; Poovorawan, K.; Soonthornworasiri, N.; Sukontamarn, P.; Chanthavilay, P.; Pan-ngum, W.; F Medley, G. A Population Dynamic Model to Assess the Diabetes Screening and Reporting Programs and Project the Burden of Undiagnosed Diabetes in Thailand. Int. J. Environ. Res. Public Health 2019, 16, 2207. https://doi.org/10.3390/ijerph16122207
Mahikul W, J White L, Poovorawan K, Soonthornworasiri N, Sukontamarn P, Chanthavilay P, Pan-ngum W, F Medley G. A Population Dynamic Model to Assess the Diabetes Screening and Reporting Programs and Project the Burden of Undiagnosed Diabetes in Thailand. International Journal of Environmental Research and Public Health. 2019; 16(12):2207. https://doi.org/10.3390/ijerph16122207
Chicago/Turabian StyleMahikul, Wiriya, Lisa J White, Kittiyod Poovorawan, Ngamphol Soonthornworasiri, Pataporn Sukontamarn, Phetsavanh Chanthavilay, Wirichada Pan-ngum, and Graham F Medley. 2019. "A Population Dynamic Model to Assess the Diabetes Screening and Reporting Programs and Project the Burden of Undiagnosed Diabetes in Thailand" International Journal of Environmental Research and Public Health 16, no. 12: 2207. https://doi.org/10.3390/ijerph16122207
APA StyleMahikul, W., J White, L., Poovorawan, K., Soonthornworasiri, N., Sukontamarn, P., Chanthavilay, P., Pan-ngum, W., & F Medley, G. (2019). A Population Dynamic Model to Assess the Diabetes Screening and Reporting Programs and Project the Burden of Undiagnosed Diabetes in Thailand. International Journal of Environmental Research and Public Health, 16(12), 2207. https://doi.org/10.3390/ijerph16122207







