Abstract
Excessive ammonia is a common pollutant in the wastewater, which can cause eutrophication, poison aquatic life, reduce water quality and even threaten human health. Ammonia in aqueous solution was converted using various systems, i.e., ozonation (O3), ultrasound (US), catalyst (SrO-Al2O3), ultrasonic ozonation (US/O3), ultrasound-enhanced SrO-Al2O3 (SrO-Al2O3/US), SrO-Al2O3 ozonation (SrO-Al2O3/O3) and ultrasound-enhanced SrO-Al2O3 ozonation (SrO-Al2O3/US/O3) under the same experimental conditions. The results indicated that the combined SrO-Al2O3/US/O3 process achieved the highest NH4+ conversion rate due to the synergistic effect between US, SrO-Al2O3 and O3. Additionally, the effect of different operational parameters on ammonia oxidation in SrO-Al2O3/O3 and SrO-Al2O3/US/O3 systems was evaluated. It was found that the ammonia conversion increased with the increase of pH value in both systems. The NH3(aq) is oxidized by both O3 and ·OH at high pH, whereas the NH4+ oxidation is only carried out through ·OH at low pH. Compared with the SrO-Al2O3/O3 system, the ammonia conversion was significantly increased, the reaction time was shortened, and the consumption of catalyst dosage and ozone were reduced in the SrO-Al2O3/US/O3 system. Moreover, reasonable control of ultrasonic power and duty cycle can further improve the ammonia conversion rate. Under the optimal conditions, the ammonia conversion and gaseous nitrogen yield reached 83.2% and 51.8%, respectively. The presence of tert-butanol, CO32−, HCO3−, and SO42− inhibited the ammonia oxidation in the SrO-Al2O3/US/O3 system. During ammonia conversion, SrO-Al2O3 catalyst not only has a certain adsorption effect on NH4+ but accelerates the O3 decomposition to ·OH.
1. Introduction
Ammonia is a common contaminant. In particular, a large amount of ammonia wastewater is produced in the process of mining extraction and separation of rare-earth ore [1]. Once the discharge of ammonia exceeds the environmental capacity of the receiving waters, it causes several problems, including eutrophication, poisoning aquatic life, reducing water quality, and even threatening human health [2,3]. Hence, it is necessary to treat the ammonia in wastewater.
As an oxidant, ozone (O3) has been used in wastewater treatment and deep purification of drinking water [4,5]. Ozonation alone has low oxidation efficiency because of its unstable chemical properties (the half-life is approximately 15 min under neutral conditions at 298 K), which limits its oxidation ability [6,7]. To improve the oxidation efficiency of ozone, the synergy of catalyst has been investigated, namely catalytic ozonation, which can be classified into homogeneous and heterogeneous depending on the type of catalyst [8,9]. In homogeneous catalytic ozonation, liquid catalysts (mostly transition metal ions) are used to decompose the ozone, but it is difficult to separate from the effluent, thus causing secondary pollution in water bodies [9,10]. In heterogeneous catalytic ozonation, solid catalysts that are easily separated and maintain their catalytic activity for a long time are employed for decomposing ozone into hydroxyl radicals (·OH) [9,11]. ·OH is more oxidative than O3, because their oxidation potentials are 2.80 V and 2.07 V, respectively [12]. In addition, the solid catalyst in ozonation can directly convert ammonia into harmless substances (such as N2) under certain conditions [13,14]. Therefore, the suitable solid catalysts are very important for the ammonia conversion by heterogeneous catalytic ozonation.
Recently, the focus has been on alkaline earth metal oxides due to their lower solubility in the reaction process and the basic oxygen-containing groups (hydroxyl groups) on their surface [15]. The order of catalytic activity of alkaline earth metal oxides per unit surface area is SrO > CaO > MgO [16]. Furthermore, surface hydroxyl groups on the catalyst react easily with O3 to form ·OH [12]. However, the alkaline earth metal oxides are powdery, without mechanical strength, and are not easily separated from the effluent [17,18]. To overcome these limitations, attaching the alkaline earth metal oxide to some supports has been considered. Activated alumina, with acid-base active sites on their surface, high mechanical strength and good thermal stability, can be used as a suitable support [18]. Cotman et al. [19] prepared ruthenium metal supported on alumina catalyst (Ru-Al2O3) to catalyze ozonation of bisphenol A, and the catalyst Ru-Al2O3 showed excellent stability and reusability. Wang et al. [20] studied the manganese metal loading of 4% on Al2O3 (Mn-Al2O3) exhibited highest catalytic activity.
In the process of heterogeneous catalytic ozonation, catalyst deactivation is also an important issue affecting the catalytic efficiency due to the accumulation of by-products on the catalyst surface [21]. Currently, ultrasound (US) is combined with the heterogeneous catalytic ozonation process to treat organic pollutants in water [22,23,24]. When the ultrasound catalytic reaction is carried out, transient cavitation enhances the turbulent of the solution, thereby accelerating the mass transfer process of the reactants and by-products between the solution and the catalyst surfaces [25]. At the same time, the collapse of acoustic cavitation bubbles generates shock waves, thus creating a continuous cleaning effect on the surface of the catalyst [25,26,27]. To date, ultrasound-enhanced catalytic ozonation of ammonia in aqueous solution has rarely been studied.
Accordingly, (1) to enhance the ozone oxidation ability and its utilization rate, (2) to power the mechanical strength of alkaline earth metal oxides and easily separate from the solution, and (3) to delay the deactivation of the catalyst, this study combines an ultrasonic, supported alkaline earth metal oxide with ozone (SrO-Al2O3/US/O3) to oxidize the ammonia in aqueous solution. The effects of initial solution pH, reaction time, dosage of catalyst, ozone flow, ultrasonic power and duty cycle on ammonia conversion were investigated in the SrO-Al2O3/US/O3 system. For comparison, the effects of the same operating parameters on ammonia conversion were studied in SrO-Al2O3/O3 system to understand the role of US in the catalytic ozonation reaction. A set of experiments were also designed for assessing the effect of tert-butanol and inorganic ions (Na+; K+; Ca2+; Mg2+; SO42−; CO32−; HCO3−) on the conversion of ammonia in the SrO-Al2O3/US/O3 system. In addition, the mechanism for ultrasound-enhanced catalytic ozonation of ammonia in aqueous solution is discussed.
2. Experimental
2.1. Materials
Ammonium chloride (NH4Cl, analytical grade) was purchased from Tianjin Damao Chemical Reagent Factory (Tianjin, China), which was used to prepare simulated water containing NH4+. Both activated-alumina (Al2O3, analytical grade) and barium salt (Sr(NO3)2, analytical grade) were purchased from Shanghai Zhanyun Chemical Co. Ltd. (Shanghai, China).
2.2. Preparation of Supported Catalyst
Actived-Al2O3 (48–212 μm) was washed three times with deionized water, followed by ultrasonic cleaning for 15 min in an ultrasonic cleaner (KQ-100TDE, Kunshan Ultrasonic Instrument Co. Ltd., Kunshan, China) and dried at 110 °C for 5 h in an oven (101A-3, Shanghai Experimental Instrument Factory, Shanghai, China) to obtain pretreated activated-Al2O3 used as a support for the catalyst.
An impregnation method was used to prepare the SrO-Al2O3 catalyst. Briefly, the pretreated activated-Al2O3 and 0.1 mol/L Sr(NO3)2 solution were mixed in a solid-liquid ratio of 1 g/20 mL and shaken in a 60 °C water bath thermostat (SHA-C, Jintan Ronghua Instrument Manufacturing Co. Ltd., Jintan, China) for 20 h. The mixture was filtrated, dried at 80 °C for 3 h and heated to 110 °C for 2 h in an oven and then calcined at 700 °C for 4 h in a muffle furnace (SHF.M25/10, Shanghai Lingyi Industrial Co. Ltd., Shanghai, China).
2.3. Procedure
The experimental setup of the ultrasound-enhanced catalytic ozonation of ammonia process (SrO-Al2O3/US/O3) is shown in Figure 1. To begin with, 100 mL NH4Cl solution was prepared at an initial concentration of 50 mg/L and acidified or alkalized to a set pH value (4.5~11.5) with 1 mol/L HCl or NaOH before each experimental run. Then the resulting solution was placed in a 200 mL reactor and a predetermined amount of SrO-Al2O3 (0.5~3.0 g/L) was added. Subsequently the instruments were connected and run according to Figure 1.
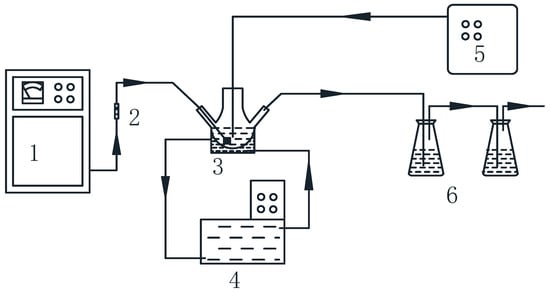
Figure 1.
Schematic of experimental apparatus. 1 Ozone generator, 2 Gas-flowmeter, 3 Reactor, 4 Intelligent cryostat, 5 Ultrasonic generator, 6 KI absorption liquid.
O3 was generated by an ozone generator (FL-815ET, FeiLi, Shengzhen, China) with an oxygen source and a control device for ozone flow. The ozone generator has a maximum ozone flow of 15 g/h and the ozone flow (0.4~3.8 g/h) was set by the control device. The ozone was continuously bubbled into NH4Cl solution and the remaining ozone was absorbed by KI solution.
Ultrasonic treatment was performed using an ultrasonic generator (XO-SM50N, Nanjing Xianou Instrument Manufacturing Co. Ltd., Nanjing, China) equipped with a 6 mm diameter titanium probe. The probe was inserted to a depth of about 10 mm in the solution and the ultrasound was performed in a pulse mode of 1 s (or 2 s) on and 1 s (or 2 s) off at a given ultrasonic power (90~450 W) and an ultrasonic frequency of 25 Hz. The temperature of the whole reaction system was maintained at 25 °C by an intelligent thermostat.
During the catalytic ozonation of ammonia experiment (SrO-Al2O3/O3), the ultrasonic generator was turned off while other conditions are consistent with SrO-Al2O3/US/O3.
2.4. Analysis
After the reaction, liquid samples were withdrawn from the reactor at pre-determined intervals and then the concentrations of ammonium (NH4+), nitrite (NO2−), and nitrate (NO3−) in solution were measured. Nessler’s reagent spectrophotometry method [28] was used for determining the concentration of NH4+ () and the spectrophotometry method [29] for determining the concentration of NO2− () in the liquid samples by a visible spectrophotometer (SP-756PC, Shanghai Spectrum Instrument Co. Ltd., Shanghai, China). Ultraviolet spectrophotometry method [30] was used for determining the nitrate () concentration using an ultraviolet spectrophotometer (722 N, Shanghai Spectrum Instrument Co. Ltd., Shanghai, China). In this study, according to the calculation of nitrogen balance, the products of oxidation of NH4+ were gaseous nitrogen in addition to residual NH4+, NO2−, and NO3− in liquid phase. Gaseous nitrogen may include N2, N2O, NO, NO2, etc. [13]. Percentages of NH4+ (), NO3− (), NO2− () and gaseous nitrogen () were calculated using Equations (1)–(4):
where , , are the concentration, of residual NH4+, formed NO3− and NO2− after the reaction, respectively, and is the initial ammonia concentration (mg/L).
3. Results and Discussions
3.1. Performance Comparison of Different Treatment Systems
Tests were carried out in the absence and presence of SrO-Al2O3 to evaluate the adsorption capacity of SrO-Al2O3 on ammonia, the results is presented in Figure 2a. As shown in Figure 2a, when aqueous solution pH 8.5, 3.5% of NH4+ was converted to NH3 when no catalyst was present. In the SrO-Al2O3 alone system, the conversion rate of NH4+ was 10.3% and no nitrate and nitrite nitrogen were produced, since the yield of NH3 was 3.5%, indicating that 6.8% of NH4+ was adsorbed by the SrO-Al2O3 catalyst.
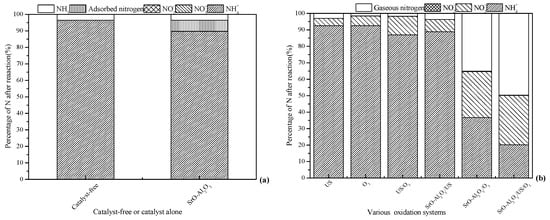
Figure 2.
Performance comparison in the various treatment systems on ammonia conversion. Reaction conditions: initial NH4+ concentration 50 mg/L, initial pH 8.5, ozone flow 1.5 g/h, catalytic dosage 2.0 g/L, reaction temperature 25 °C, reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W, ultrasonic operation 1 s and interval 2 s. (a) Catalyst-free or catalyst alone; (b) various oxidation systems.
To evaluate the ammonia conversion rate in various systems, experiments were carried out with US, O3, US/O3, SrO-Al2O3/US, SrO-Al2O3/O3 and SrO-Al2O3/US/O3 systems, respectively, and the results are given in Figure 2b. Figure 2b shows that the US alone and O3 alone system have a small effect on the oxidation of ammonia in aqueous solution, with ammonia conversion and gaseous nitrogen yield of less than 7.4% and 3.0%, respectively. Simultaneously, the yields of NO3− were 4.4% and 5.8%, respectively.
In the US/O3 system, less than 13% of NH4+ was oxidized and the NO3− yield was 11.2%, which were higher than those of O3 alone and US alone. When US was introduced into the O3 system, the propagation of ultrasonic waves in solution produced transient cavitation, causing turbulence and reducing the liquid film thickness of the ozone-containing gas bubbles, which facilitates the mass transfer of ozone [31]. On the other hand, the decomposition of ozone and H2O would produce ·OH in the cavitation bubbles (Equations (6)–(8)) [32]. Where the “)))” indicate ultrasound. Subsequently, the cavitation bubbles are ruptured, and the free radicals formed diffuse from the internal into the solution to oxidize the ammonia in aqueous solution [32,33].
When US was introduced into the SrO-Al2O3 system, both the ammonia conversion and gaseous nitrogen yield were low (11.2% and 3.6%, respectively). In the SrO-Al2O3/O3 system, the ammonia conversion and production of gaseous nitrogen were 63.2% and 35.1%, respectively. One possible reason for this is that the active sites of SrO-Al2O3 can stimulate the decomposition of ozone to produce ·OH. Compared to the SrO-Al2O3/US system, the oxidation effect of ammonia is obviously enhanced, which can be explained by co-oxidation of O3 and ·OH in the SrO-Al2O3/O3 system.
As for the SrO-Al2O3/US/O3 system, both ammonia conversion and gaseous nitrogen yield were higher than that of SrO-Al2O3/O3 processes. The reason may be that the active sites of SrO-Al2O3 were gradually occupied by the NH4+ and NO3− during the catalytic ozonation process in the SrO-Al2O3/O3 system. The active sites of SrO-Al2O3 were opened by transient cavitation when US was introduced in the SrO-Al2O3/US/O3 system. The experimental results indicated that the ammonia conversion is dominated by the oxidation of O3 and ·OH, which comes from the synergistic effect of US, SrO-Al2O3 and O3 in the SrO-Al2O3/US/O3 system.
Comparison with the production of gaseous nitrogen, being 3.5% for the catalyst-free system and 6.8% NH4+ adsorbed by the SrO-Al2O3 catalyst in the catalyst alone system in Figure 2a, the NO3− can be considered to come from the simultaneous oxidation of NH4+ and NH3 in the O3, US, US/O3, SrO-Al2O3/US, SrO-Al2O3/O3 and SrO-Al2O3/US/O3 systems.
3.2. Effect of Operating Parameters on Ammonia Conversion
3.2.1. Initial Solution pH
Figure 3 depicts the results showing that the pH of the initial solution is positively correlated with ammonia conversion and gaseous nitrogen selectivity. With the initial pH increasing from 4.5 to 11.5, the percentage of NH4+ decreased, and the production of NO3− and gaseous nitrogen increased in the SrO-Al2O3/O3 and SrO-Al2O3/US/O3 system. On the one hand, the form of ammonia in aqueous solution depends on pH (Equation (9)), NH4+ is predominant at pH lower than pKa, and free ammonia (NH3(aq)) increases at pH higher than pKa [34].
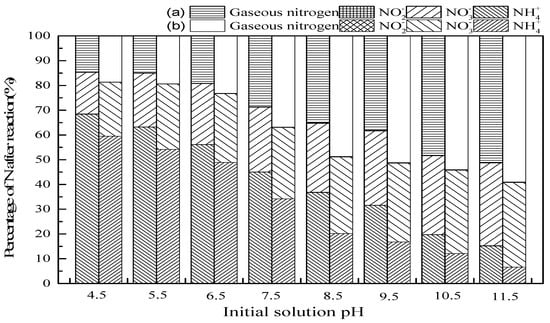
Figure 3.
The effect of initial solution pH on ammonia conversion. Reaction conditions: initial NH4+ concentration 50 mg/L, initial pH 4.5 to 11.5, ozone flow 1.5 g/h, catalytic dosage 2.0 g/L, temperature 25 °C reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W, ultrasonic operation 1 s and interval 2 s. (a) SrO-Al2O3/O3, (b) SrO-Al2O3/US/O3.
The fraction of NH3 (aq) at a given pH can be calculated according to Equation (10), where Kb is the ionization constant, and its value is 1.774 × 10−5 at 298 K [3]. Thus, NH4+ is gradually turned into NH3 (aq) with the increase of initial solution pH. For instance, the fraction of NH3 (aq) at pH = 4.5 is only 1.8 × 10−5, which increases to 0.99 at pH = 11.5.
On the other hand, as shown in Equations (11) and (12), ·OH would be generated from the ozone dissociation at alkaline conditions which contributed to the oxidation NH3(aq) [33]. Hence, according to Figure 3, the oxidation of NH3(aq) occurs by both O3 and ·OH at high pH. As more NH3(aq) is continuously oxidized, the reaction equilibrium (9) shifts to the formation of NH3(aq), resulting in an increase of ammonia conversion rate. However, since O3 cannot oxidize NH4+ directly [3,32,34], the oxidation of NH4+ can be carried out through ·OH at low pH, which was derived from ultrasound-enhanced decomposition of O3 and H2O (Equations (6)–(8)).
Comparing the two system SrO-Al2O3/O3 and SrO-Al2O3/US/O3, when the initial pH was 9.5, the introduction of ultrasound increased the ammonia conversion from 68.4% to 83.2%, and the proportion of gaseous nitrogen increased from 38.2% to 51.2%. It is confirmed that the combination of US, SrO-Al2O3 and O3 can synergistically enhance the ammonia conversion.
3.2.2. Reaction Time
Figure 4 shows that the effect of reaction time on ammonia conversion in SrO-Al2O3/O3 and SrO-Al2O3/US/O3 system. According to Figure 4, the ammonia conversion and the gaseous nitrogen yield increased with the prolongation of the reaction time in the two systems. The reaction time was 120 min in the SrO-Al2O3/O3 system, the ammonia conversion and gaseous nitrogen yield reached 81.6% and 49.6%, respectively, while the reaction time was 60 min in the SrO-Al2O3/US/O3 system, the ammonia conversion and gaseous nitrogen yield reached 83.4% and 51.4%, respectively. In SrO-Al2O3/O3 system, the reaction time for reaching the maximum ammonia conversion rate and gaseous nitrogen yield was twice that of the SrO-Al2O3/US/O3 system, indicating that the introduction of US can greatly shorten the reaction time.
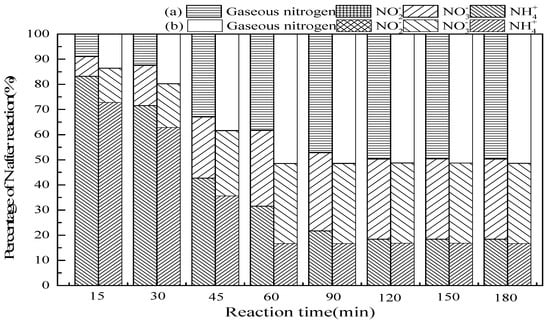
Figure 4.
The effect of reaction time on ammonia conversion. Reaction conditions: initial NH4+ concentration 50 mg/L, initial pH 9.5, ozone flow 1.5 g/h, catalytic dosage 2.0 g/L, temperature 25 °C, reaction time from 15 to 180 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W, ultrasonic operation 1 s and interval 2 s. (a) SrO-Al2O3/O3, (b) SrO-Al2O3/US/O3.
3.2.3. Catalyst Dosage
In general, increased catalyst dosages provide more surface-active sites, thus facilitating O3 decomposition into ·OH [35]. As seen in Figure 5, the ammonia conversion and gaseous nitrogen production improved with an increase of SrO-Al2O3 dosage. The dosage of SrO-Al2O3 was 2.5 g/L in the SrO-Al2O3/O3 system, the ammonia conversion and gaseous nitrogen yield reached 81.0% and 50.0%, respectively, while the dosage was 2.0 g/L in the SrO-Al2O3/US/O3 system, the ammonia conversion and gaseous nitrogen yield reached 83.2% and 51.2%, respectively. It is illustrated that the introduction of US cannot only enhance the ammonia conversion but reduce the catalyst dosage.
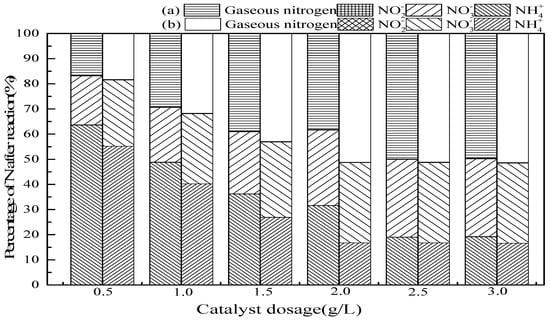
Figure 5.
The effect of catalytic dosage on ammonia conversion. Reaction conditions: initial NH4+ concentration 50 mg/L, initial pH 9.5, ozone flow 1.5 g/h, catalytic dosage from 0.5 g/L to 3.0 g/L, temperature 25 °C, reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W, ultrasonic operation 1 s and interval 2 s. (a) SrO-Al2O3/O3, (b) SrO-Al2O3/US/O3.
3.2.4. Ozone Flow
Figure 6 shows the effect of the ozone flow on ammonia conversion. The results indicated that when ozone flow increased from 0.4 mg/min to 3.0 mg/min in SrO-Al2O3/O3 system, the ammonia conversion and gaseous nitrogen yield increased from 58.6% to 79.2% and 31.8% to 47.8%, respectively. While in the SrO-Al2O3/US/O3 system, ozone flow from 0.4 mg/min to 0.8 mg/min, the ammonia conversion and gaseous nitrogen yield increased from 75.8% to 83.1% and 46.2% to 51.7%, respectively. The increase in ozone flow means an increase in ozone concentration, which activates the ·OH generation and ultimately enhances the ammonia oxidation in the reaction system [36]. While continuing to increase the ozone flow, it was found that the ammonia conversion, gaseous nitrogen yield and NO3− production in the solution have a little bit increase. The reason for this is that with a certain amount of SrO-Al2O3 catalyst, the number of active sites is fixed, and cannot completely decompose excessive ozone to ·OH. Comparing the two system of SrO-Al2O3/O3 and SrO-Al2O3/US/O3, it can be concluded that the introduction of US can reduce the consumption of ozone to a large extent.
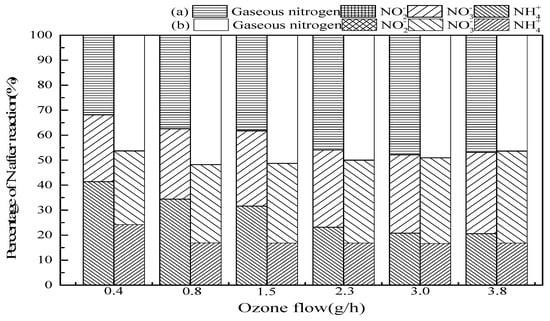
Figure 6.
The effect of ozone flow on ammonia conversion. Reaction conditions: initial NH4+ concentration 50 mg/L, initial pH 9.5, ozone flow from 0.4 to 3.8 g/h, catalytic dosage 2.0 g/L, temperature 25 °C, reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W, ultrasonic operation 1 s and interval 2 s. (a) SrO-Al2O3/O3, (b) SrO-Al2O3/US/O3.
3.2.5. Ultrasonic Power
Figure 7 shows the effect of ultrasonic power on ammonia conversion in SrO-Al2O3/US/O3 system. Presence of US improved the ammonia oxidation. With the increase of ultrasonic power from 0 to 450 W, residual NH4+ concentration and gaseous nitrogen yield after the reaction decrease first and then rises. Increasing ultrasonic power from 0 to 270 W can enhance the ultrasonic cavitation, but exceeding 270 W, acoustic shielding appears to reduce the ultrasonic cavitation and the utilization of acoustic energy [31,37]. When the ultrasonic power is 270 W, the ammonia conversion and gaseous nitrogen yield both maximize 83.2% and 51.8%.
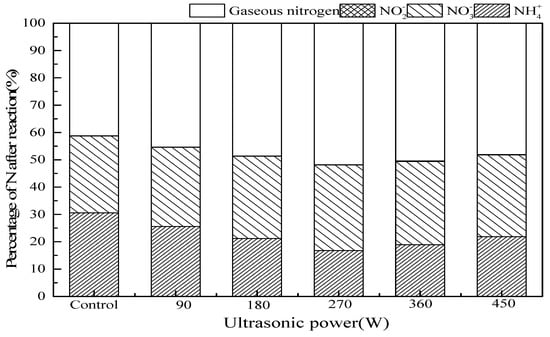
Figure 7.
The effect of ultrasonic power on ammonia conversion. Reaction conditions: initial NH4+ concentration 50 mg/L, initial pH 9.5, ozone flow 0.8 g/h, catalytic dosage 2.0 g/L, temperature 25 °C, reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power from 0 to 450 W, ultrasonic operation 1 s and interval 2 s.
3.2.6. Duty Cycle
The US can be introduced either continuously or intermittently in the experiments. Since the continuous addition consumes a large amount energy, the intermittent method is adopted. To explore the effect of intermittent ultrasonic operation mode on ammonia conversion in the SrO-Al2O3/US/O3 process, five intermittent operation modes were set up, which were (a) no ultrasound, (b) ultrasonic operation 1 s and interval 1 s (duty cycle 1:2), (c) ultrasonic operation 1 s and interval 2 s (duty cycle 1:3), (d) ultrasonic operation 2 s and interval 1 s (duty cycle 2:3), and (e) ultrasonic operation 2 s and interval 2 s (duty cycle 2:4), as observed in Figure 8. When the duty cycle was 1:3, the ammonia conversion was 83.2%, gaseous nitrogen reached the maximum of 51.8% and the production of NO3− 31.28%. When the duty cycle was 2:3, the ammonia conversion reached a maximum of 86.4%, gaseous nitrogen 41.3% and NO3− production 45.0%.
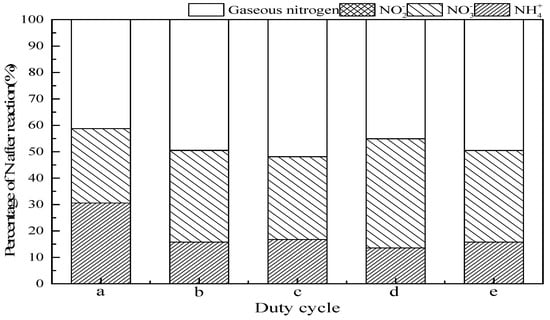
Figure 8.
Effect of duty cycle on ammonia conversion. Reaction conditions: initial NH4+ concentration 50 mg/L, initial pH 9.5, ozone flow 0.8 g/h, catalytic dosage 2.0 g/L, temperature 25 °C, reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W. (a) No ultrasound, (b) duty cycle 1:2, (c) duty cycle 1:3, (d) duty cycle 2:3, (e) duty cycle 2:4.
3.3. Effect of Tert-Butanol and Inorganic Ions on Ammonia Conversion
3.3.1. Effect of Tert-Butanol
Tert-butanol, a common ·OH scavengers’ agent, is difficult to directly react with O3 (reaction rate constant is K = 3.0 × 10−2 M−1 S−1) but reacts quickly with ·OH (K = 6.0 × 108 M−1 S−1) [38]. In addition, tert-butanol would react with ·OH in bulk solution to form inert substances, which inhibited the chain reaction of ·OH [39,40]. The effect of tert-butanol on ammonia conversion in the SrO-Al2O3/US/O3 system is shown in Figure 9. With tert-butanol concentration increasing from 0 to 18.0 mg/L, the residual NH4+ increased from 16.8% to 49.2%, and the percentage of NO3− and gaseous nitrogen decreased from 31.3% to 20.5%, and 51.8% to 30.2%, respectively. That is to say, the addition of tert-butanol largely inhibits the ammonia oxidation in the SrO-Al2O3/US/O3 system, indicating that the intermediate product (·OH) plays an important role.
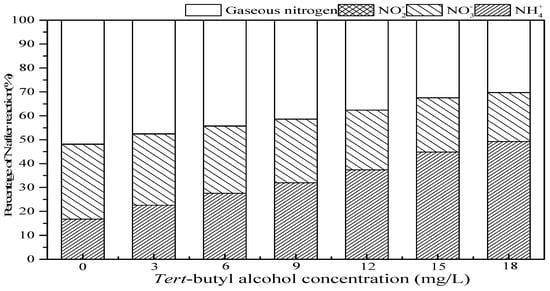
Figure 9.
Effect of tert-butanol on ammonia conversion in SrO-Al2O3/US/O3 system. Reaction conditions: initial NH4+ concentration 50 mg/L, initial pH 9.5, ozone flow 0.8 g/h, catalytic dosage 2.0 g/L, temperature 25 °C, reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W, duty cycle 1:3.
3.3.2. Effect of Inorganic Ions
Large amounts of cations and anions often appear in the actual water body, the influence of main cations (Na+, K+, Ca2+, Mg2+) and anions (CO32−, HCO3−, SO42−) should be evaluated on ammonia conversion in SrO-Al2O3/US/O3 system. As observed in Figure 10, the addition of Na+, K+, Ca2+ and Mg2+ had no significant effect on ammonia conversion, while CO32−, HCO3− and SO42− suppressed the ammonia conversion. The anions’ radical-scavenging properties, as shown in Equations (13)–(15) [40,41,42], reduced the amount of ·OH produced, thereby causing a decrease in ammonia conversion.
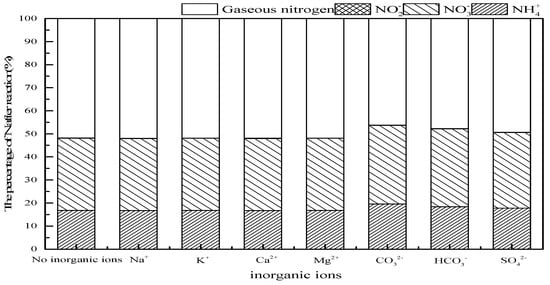
Figure 10.
Effect of inorganic ions on ammonia conversion in SrO-Al2O3/US/O3 system. Reaction conditions: initial NH4+ concentration 50 mg/L, initial pH 9.5, ozone flow 0.8 g/h, catalytic dosage 2.0 g/L, temperature 25 °C, reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W, duty cycle 1:3.
3.4. Actual Ammonium-Containing Wastewater Oxidized in the SrO-Al2O3/US/O3 System
The actual wastewater was collected from a rare earth metallurgical plant located in Longnan County, Jiangxi province, China and transferred into clean plastic bottles, and immediately transported to the laboratory for proper storage for future use. The characteristics of the actual wastewater used in this study are shown in Table 1. Actual wastewater with an initial NH4+ concentration of 50 mg/L was obtained by diluting the actual wastewater with an initial NH4+ concentration of 302 mg/L with deionized water.

Table 1.
Characteristics of actual wastewater.
As shown in Figure 11, there were some slight differences in the treatment between simulated water and actual wastewater with an initial NH4+ concentration of 50 mg/L. Among them, the ammonia conversion was 83.2% and 79.6%, respectively, and the gaseous nitrogen yield was 51.8% and 53.9%, respectively. However, when the actual wastewater with an initial NH4+ concentration of 302 mg/L was treated in SrO-Al2O3/US/O3 system, the ammonia conversion and gaseous nitrogen yield were 58.1% and 40.7%, respectively. This indicates that the treatment efficiency of the SrO-Al2O3/US/O3 process is limited and is currently only suitable for treating low concentrations of ammonia-containing wastewater.
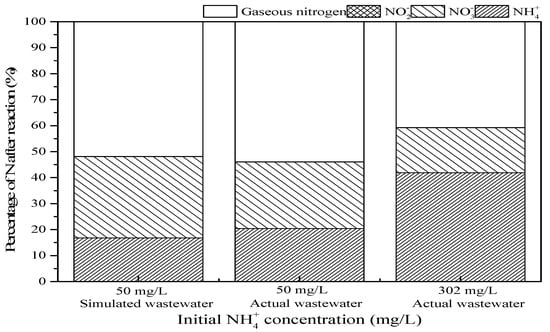
Figure 11.
Comparison of oxidation of simulated wastewater and actual NH4+-containing wastewater in SrO-Al2O3/US/O3 system. Reaction conditions: initial pH 9.5, ozone flow 0.8 g/h, catalytic dosage 2.0 g/L, temperature 25 °C, reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W, duty cycle 1:3.
3.5. Morphology Analysis of Catalyst
Surface topographies of Al2O3 and SrO-Al2O3 were observed by scanning electron microscopy (JSM-6360LV, Japanese electronics company, Japan) and the results are shown in Figure 12. Figure 12a shows that the surface of Al2O3 is rough and has obvious channels, which will facilitate the loading of SrO. Figure 12b shows that a large amount of irregular small particles is evenly distributed on the surface of SrO-Al2O3 and these small particles are closely joined.
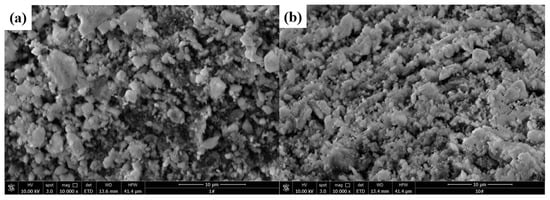
Figure 12.
Morphology analysis of Al2O3 (a) and SrO-Al2O3 (b)
3.6. The Pathway for Ammonia in Water Oxidized in the SrO-Al2O3/US/O3 System
According to the above experimental results, the pathway for ammonia conversion in the SrO-Al2O3/US/O3 system can be inferred, as shown in Figure 13. Under alkaline conditions, ammonia mainly exists in a free state (NH3(aq)). The NH3(aq) oxidation occurs by both O3 and ·OH, whereas NH4+ oxidation is only carried out through ·OH. During ammonia conversion, the SrO-Al2O3 catalyst not only has a certain adsorption effect on NH4+, but accelerates the O3 decomposition to ·OH. US enhances the turbulence degree of liquid phase to promote the ammonia conversion to NH3(aq), while the cavitation effect of US causes H2O to crack to ·OH. In addition, US improves the mass transfer rate of O3, strengthens the O3 decomposition and produces more ·OH. Moreover, the ultrasonic wave cleans the surface of catalyst and slows down the deactivation of the catalyst, thus prolonging the service life of the catalyst. Therefore, the ammonia conversion has been greatly improved due to the synergistic effect of US, SrO-Al2O3 and O3 in the SrO-Al2O3/US/O3 system.
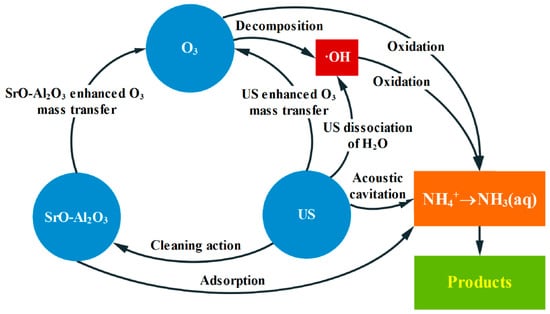
Figure 13.
The pathway of ammonia in water oxidized by SrO-Al2O3/US/O3 system.
In this study, the products of oxidation of ammonia in aqueous solution are gaseous nitrogen in the gas phase and the residual NH4+, NO2−, and NO3− in the liquid phase. Among them, gaseous nitrogen may include N2, N2O, NO, NO2, NH3 [13,43,44]. The production of NO2− and NO3− was derived from the oxidation of NH4+ and NH3 (aq).
4. Conclusions
The experimental results indicated that, compared with US, O3, US/O3, SrO-Al2O3/US and SrO-Al2O3/O3 systems, the highest ammonia conversion rate was obtained by the SrO-Al2O3/US/O3 system. The free ammonia (NH3 (aq)) was oxidized by both O3 and ·OH at high pH, while the NH4+ was oxidized through ·OH generated by the decomposition of O3 and H2O at low pH. Compared to the SrO-Al2O3/O3 system, the reaction time required in the SrO-Al2O3/US/O3 system is shortened, and the consumption of catalyst dosage and ozone was reduced. Moreover, reasonable control of ultrasonic power and duty cycle can further improve the ammonia conversion and gaseous nitrogen. Under the conditions of initial NH4+ concentration 50 mg/L, initial pH 9.5, ozone flow 0.8 g/h, catalytic dosage 2.0 g/L, temperature 25 °C, reaction time 60 min, ultrasonic frequency 25 kHz, ultrasonic power 270 W, and duty cycle 1:3, the ammonia conversion and gaseous nitrogen yield were 83.2% and 51.8%, respectively. The oxidation of ammonia in the SrO-Al2O3/US/O3 system was affected by tert-butanol, CO32−, HCO3−, SO42−, indicating that ·OH is an important oxidizer involved in the ammonia ozonation.
To better clarify the mechanism of oxidizing ammonia, it is necessary to further determine the components of gaseous nitrogen qualitatively and quantitatively.
Author Contributions
Conceptualization, Y.C.; Formal analysis, C.L.; Investigation, C.H. and R.Y.; Writing—original draft, C.L.; Writing—review & editing, Y.C., J.L. and T.Q.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2018YFC1903401) and the National Natural Science Foundation of China (grant number 51568023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luo, X.P.; Yan, Q.; Wang, C.Y.; Luo, C.G.; Zhou, N.N.; Jian, C.S. Treatment of Ammonia Nitrogen Wastewater in Low Concentration by Two-Stage Ozonation. Int. J. Environ. Res. Public Health 2015, 12, 11975–11987. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Xiao, X.M.; Yan, B. Complex treatment of the ammonium nitrogen wastewater from rare-earth separation plant. Desalin. Water Treat. 2009, 8, 109–117. [Google Scholar] [CrossRef]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Removal of ammonia from water by ozone microbubbles. Ind. Eng. Chem. Res. 2013, 52, 318–326. [Google Scholar] [CrossRef]
- Evelin, P.N.; Iiho, K.T.; Hiroaki, T.; Chikashim, S. Ozone treatment process for the removal of pharmaceuticals and personal care products in wastewater. Ozone Sci. Eng. 2019, 41, 3–16. [Google Scholar] [CrossRef]
- Gunten, U.V. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Rakness, K.L. Ozone in Drinking Water Treatment: Process Design, Operation and Optimization; American Water Works Association: Denver, CO, USA, 2011; ISBN 1613000227. [Google Scholar]
- Haag, W.R.; Hoigne, J.; Bader, H. Improved ammonia oxidation by ozone in the presence of bromide ion during water treatment. Water Res. 1984, 18, 1125–1128. [Google Scholar] [CrossRef]
- Mahardiani, L.; Kamiya, Y. Enhancement of catalytic activity of cobalt oxide for catalytic ozonation of ammonium ion in water with repeated use. J. Jpn. Petrol. Inst. 2016, 59, 31–34. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziolek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Beltran, F.J.; Rivas, F.J.; Montero-de-Espinosa, R. Iron type catalysts for the ozonation of oxalic acid in water. Water Res. 2005, 39, 3553–3564. [Google Scholar] [CrossRef]
- Sanchez-Polo, M.; Rivera-Utrilla, J. Ozonation of 1,3,6-naphthalenetrisulfonic acid in presence of heavy metals. J. Chem. Technol. Biotechnol. 2004, 79, 902–909. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.P.; Wu, D.L.; Zhang, Y.L. Heterogeneous catalytic ozonation reaction mechanism. Prog. Chem. 2016, 28, 1374–1379. [Google Scholar] [CrossRef]
- Ichikawa, S.; Mahardiani, L.; Kamiya, Y. Catalytic oxidation of ammonium ion in water with ozone over metal oxide catalysts. Catal. Today 2014, 232, 192–197. [Google Scholar] [CrossRef]
- Chen, Y.N.; Wu, Y.; Liu, C.; Guo, L.; Nie, J.X.; Chen, Y.; Qiu, T.S. Low-temperature conversion of ammonia to nitrogen in water with ozone over composite metal oxide catalyst. J. Environ. Sci. 2018, 66, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, C.V.; Rao, K.V.; Sriniva, T.; Sumana, V.S.; Prasad, K.M.M.K. Review on heterogeneous catalysis for biodiesel production. Asian J. Res. Chem. 2011, 4, 524–536. [Google Scholar]
- Zhang, G.; Hattori, H.; Tanabe, K. Aldol addition of acetone catalyzed by solid base catalysts: Magnesium oxide, calcium oxide, strontium oxide, barium oxide, lanthanum (III) oxide, and zirconium oxide. Appl. Catal. 1988, 36, 189–197. [Google Scholar] [CrossRef]
- Kabashima, H.; Tsuji, H.; Shibuya, T.; Hattori, H. Michael addition of nitromethane to α, β-unsaturated carbonyl compounds over solid base catalysts. J. Mol. Catal. A Chem. 2000, 155, 23–29. [Google Scholar] [CrossRef]
- Wei, T.; Wang, M.H.; Wei, W.; Sun, H.Y.; Zhong, B. Solid base catalysts. Chem. Bull. 2002, 65, 594–600. [Google Scholar] [CrossRef]
- Cotman, M.; Erjavec, B.; Djinovic, P.; Pintar, A. Catalyst support materials for prominent mineralization of bisphenol A in catalytic ozonation process. Environ. Sci. Pollut. Res. 2016, 23, 10223–10233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, W.Z.; Yin, X.S.; Liu, Y. The role of Mn-doping for catalytic ozonation of phenol using Mn/γ-Al2O3 nano catalyst: Performance and mechanism. J. Environ. Chem. Eng. 2016, 4, 3415–3425. [Google Scholar] [CrossRef]
- Einage, H.; Futamura, S. Effect of water vapor on catalytic oxidation of benzene with ozone on alumina-supported manganese oxides. J. Catal. 2016, 243, 446–450. [Google Scholar] [CrossRef]
- Ziylan, A.; Ince, N.H. Catalytic ozonation of ibuprofen with ultrasound and Fe-based catalysts. Catal. Today 2015, 240, 2–8. [Google Scholar] [CrossRef]
- Muruganandham, M.; Wu, J.J. Granular α-FeOOH—A stable and efficient catalyst for the decomposition of dissolved ozone in water. Catal. Commun. 2007, 8, 668–672. [Google Scholar] [CrossRef]
- Ince, N.H. Ultrasound-assisted advanced oxidation processes for water decontamination. Ultrason Sonochem. 2018, 40, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Neppolian, B.; Park, J.S.; Choi, H. Effect of Fenton-like oxidation on enhanced oxidative degradation of para-chlorobenzoic acid by ultrasonic irradiation. Ultrason Sonochem. 2004, 11, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Leighton, T.G. The Acoustic Bubble; Academic Press: Cambridge, MA, USA, 1997; ISBN 0124419216. [Google Scholar]
- Wang, Y.; Zhang, H.; Chen, L. Ultrasound enhanced catalytic ozonation of tetracycline in a rectangular air-lift reactor. Catal. Today 2011, 175, 283–292. [Google Scholar] [CrossRef]
- Ministry of Environment Protection. Determination of Ammonia Nitrogen by Nessler’s Reagent Spectrophotometry. Available online: http://kjs.mee.gov.cn/hjbhbz/bzwb/jcffbz/201001/t20100112_184155.shtml (accessed on 21 May 2019).
- Ministry of Environment Protection. Determination of Nitrite by Spectrophotometry. Available online: http://kjs.mee.gov.cn/hjbhbz/bzwb/jcffbz/198708/t19870801_66628.shtml (accessed on 21 May 2019).
- Weavers, L.K.; Hoffmann, M.R. Sonolytic decomposition of ozone in aqueous solution: Mass transfer effects. Environ. Sci. Technol. 1998, 32, 3941–3947. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K. Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ. Chem. Lett. 2016, 14, 259–290. [Google Scholar] [CrossRef]
- Holgne, J.; Bader, H. Ozonation of water: Kinetics of oxidation of ammonia by ozone and hydroxyl radicals. Environ. Sci. Technol. 1978, 12, 79–84. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahdavianpour, M. The selective direct oxidation of ammonium in the contaminated water to nitrogen gas using the chemical-less VUV photochemical continuous-flow reactor. Chem. Eng. J. 2016, 295, 57–63. [Google Scholar] [CrossRef]
- Kuo, C.H.; Yuan, F.; Hill, D.O. Kinetics of oxidation of ammonia in solutions containing ozone with or without hydrogen peroxide. Ind. Eng. Chem. Res. 1997, 36, 4106–4113. [Google Scholar] [CrossRef]
- Huang, Y.X.; Cui, C.C.; Zhang, D.F.; Li, L.; Pan, D. Heterogeneous catalytic ozonation of dibutyl phthalate in aqueous solution in the presence of iron-loaded activated carbon. Chemosphere 2015, 119, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation for the treatment of synthetic and industrial effluents—Application of mesoporous materials: A review. J. Environ. Manag. 2018, 211, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Hua, I.; Hoffmann, M.R. Optimization of ultrasonic irradiation as an advanced oxidation technology. Environ. Sci. Technol. 1997, 31, 2237–2243. [Google Scholar] [CrossRef]
- Gurol, M.D.; Akata, A. Kinetics of ozone photolysis in aqueous solution. Am. Inst. Chem. Eng. 1996, 42, 3258–3292. [Google Scholar] [CrossRef]
- Chen, Z.L.; Qi, F.; Xu, B.B.; Shen, J.M.; Yue, B.; Ye, M.M. Mechanism discussion of γ-alumina catalyzed ozonation for 2-methylisoborneol removal. Environ. Sci. 2007, 28, 563–568. [Google Scholar]
- Buxtion, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 2, 513–886. [Google Scholar] [CrossRef]
- Acero, J.L.; Gunten, U.V. Influence of carbonate on the ozone/hydrogen peroxide based advanced oxidation process for drinking water treatment. Ozone Sci. Technol. 2000, 22, 305–328. [Google Scholar] [CrossRef]
- Xiao, Y.J.; Zhang, L.F.; Yue, J.Q.; Webster, R.D.; Lim, T. Kinetic modeling and energy efficiency of UV/H2O2 treatment of iodinated trihalomethanes. Water Res. 2015, 75, 259–269. [Google Scholar] [CrossRef]
- Ludvikova, J.; Jablonska, M.; Jiratova, K.; Chmielarz, L.; Balabanova, J.; Kovanda, F.; Obalova, L. Co-Mn-Al mixed oxides as catalysts for ammonia oxidation to N2O. Res. Chem. Intermed. 2016, 42, 2669–2690. [Google Scholar] [CrossRef]
- Rang, R.Q.; Yang, R.T. Selective catalytic oxidation of ammonia to nitrogen over Fe2O3-TiO2 prepared with a sol-gel method. J. Catal. 2002, 207, 158–165. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).