Chronic Viral Hepatitis Signifies the Association of Premixed Insulin Analogues with Liver Cancer Risks: A Nationwide Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
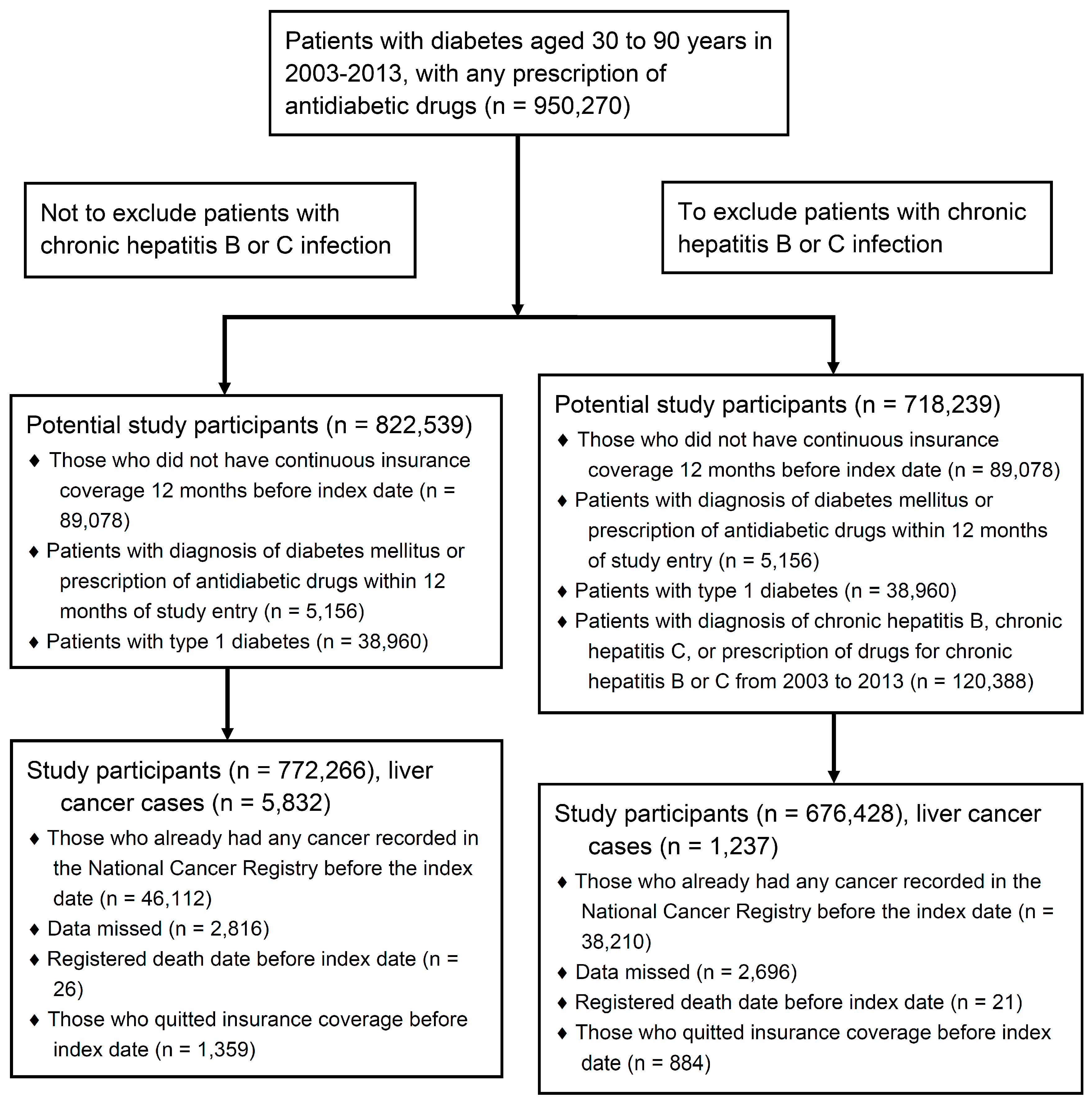
2.2. Study Design and Population
2.3. Cases Ascertainment and Controls Selection
2.4. Exposure Ascertainment and Covariate Adjustment
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Univariate Analyses
3.2. Multivariate Analysis
3.3. Interaction Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGlynn, K.A.; London, W.T. The global epidemiology of hepatocellular carcinoma: Present and future. Clin. Liver Dis. 2011, 15, 223–243. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Liao, S.F.; Yang, H.I.; Lee, M.H.; Chen, C.J.; Lee, W.C. Fifteen-year population attributable fractions and causal pies of risk factors for newly developed hepatocellular carcinomas in 11,801 men in Taiwan. PLoS ONE 2012, 7, e34779. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Lin, J.; Yang, Y.C.; Tsai, M.K.; Tsao, C.K.; Etzel, C.; Huang, M.; Hsu, C.Y.; Ye, Y.; Mishra, L.; et al. Hepatocellular carcinoma risk prediction model for the general population: the predictive power of transaminases. J. Natl. Cancer Inst. 2012, 104, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Aberg, F.; Helenius-Hietala, J.; Puukka, P.; Farkkila, M.; Jula, A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology 2018, 67, 2141–2149. [Google Scholar] [CrossRef]
- Brower, V. Illuminating the diabetes-cancer link. J. Natl. Cancer Inst. 2012, 104, 1048–1050. [Google Scholar] [CrossRef]
- Chen, C.L.; Yang, H.I.; Yang, W.S.; Liu, C.J.; Chen, P.J.; You, S.L.; Wang, L.Y.; Sun, C.A.; Lu, S.N.; Chen, D.S.; et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: A follow-up study in Taiwan. Gastroenterology 2008, 135, 111–121. [Google Scholar] [CrossRef]
- Davila, J.A.; Morgan, R.O.; Shaib, Y.; McGlynn, K.A.; El-Serag, H.B. Diabetes increases the risk of hepatocellular carcinoma in the United States: A population based case control study. Gut 2005, 54, 533–539. [Google Scholar] [CrossRef]
- Inoue, M.; Kurahashi, N.; Iwasaki, M.; Tanaka, Y.; Mizokami, M.; Noda, M.; Tsugane, S. Metabolic factors and subsequent risk of hepatocellular carcinoma by hepatitis virus infection status: A large-scale population-based cohort study of Japanese men and women (JPHC Study Cohort II). Cancer Causes Control 2009, 20, 741–750. [Google Scholar] [CrossRef]
- Lai, M.S.; Hsieh, M.S.; Chiu, Y.H.; Chen, T.H. Type 2 diabetes and hepatocellular carcinoma: A cohort study in high prevalence area of hepatitis virus infection. Hepatology 2006, 43, 1295–1302. [Google Scholar] [CrossRef]
- Chiang, C.H.; Lee, L.T.; Hung, S.H.; Lin, W.Y.; Hung, H.F.; Yang, W.S.; Sung, P.K.; Huang, K.C. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology 2014, 59, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Elkrief, L.; Chouinard, P.; Bendersky, N.; Hajage, D.; Larroque, B.; Babany, G.; Kutala, B.; Francoz, C.; Boyer, N.; Moreau, R.; et al. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology 2014, 60, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.C.; Huang, Y.W.; Wang, T.C.; Hu, J.T.; Chen, D.S.; Yang, S.S. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with new onset diabetes: a nationwide cohort study. Aliment. Pharmacol. Ther. 2015, 41, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, F.; Saito, E.; Lin, Y.; Song, M.; Luu, H.N.; Gupta, P.C.; Sawada, N.; Tamakoshi, A.; Shu, X.O.; et al. Association between type 2 diabetes and risk of cancer mortality: A pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia 2017, 60, 1022–1032. [Google Scholar] [CrossRef]
- Wang, P.; Kang, D.; Cao, W.; Wang, Y.; Liu, Z. Diabetes mellitus and risk of hepatocellular carcinoma: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2012, 28, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Wang, P.; Wang, B.; Fu, Z.J.; Zhao, W.J.; Yan, S.L. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: A systematic review and meta-analysis. PloS ONE 2014, 9, e95485. [Google Scholar] [CrossRef]
- Chen, J.; Han, Y.; Xu, C.; Xiao, T.; Wang, B. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases: A meta-analysis of cohort studies. Eur. J. Cancer Prev. 2015, 24, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Kim, Y.J.; Kim, M.S.; Suh, K.S.; Kim, S.B.; Han, C.J.; Kim, Y.J.; Jang, W.I.; Kang, S.H.; Tchoe, H.J.; et al. Association of Metformin Use With Cancer-Specific Mortality in Hepatocellular Carcinoma After Curative Resection: A Nationwide Population-Based Study. Medicine 2016, 95, e3527. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.P.; Roberts, L.R.; Sanchez, W. Chemopreventive strategies in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 45–54. [Google Scholar] [CrossRef]
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006, 29, 254–258. [Google Scholar] [CrossRef]
- Diabetes Association Of The Republic Of China, T. Executive summary of the DAROC clinical practice guidelines for diabetes care- 2018. J. Formos. Med. Assoc. 2019. [Google Scholar] [CrossRef] [PubMed]
- Taiwan National Health Insurance Drug List. Available online: https://reurl.cc/No409 (accessed on 7 June 2019).
- Petrick, J.L.; Freedman, N.D.; Demuth, J.; Yang, B.; Van Den Eeden, S.K.; Engel, L.S.; McGlynn, K.A. Obesity, diabetes, serum glucose, and risk of primary liver cancer by birth cohort, race/ethnicity, and sex: Multiphasic health checkup study. Cancer epidemiol. 2016, 42, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Si, W.K.; Chung, J.W.; Cho, J.; Baeg, J.Y.; Jang, E.S.; Yoon, H.; Kim, J.; Shin, C.M.; Park, Y.S.; Hwang, J.H.; et al. Predictors of Increased Risk of Hepatocellular Carcinoma in Patients with Type 2 Diabetes. PloS ONE 2016, 11, e0158066. [Google Scholar] [CrossRef] [PubMed]
- Universal Health Coverage in Taiwan. Available online: https://reurl.cc/OoGvD (accessed on 7 June 2019).
- Tseng, C.H. Type 2 diabetes, smoking, insulin use, and mortality from hepatocellular carcinoma: A 12-year follow-up of a national cohort in Taiwan. Hepatol. Int. 2013, 7, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Miuma, S.; Ichikawa, T.; Taura, N.; Shibata, H.; Takeshita, S.; Akiyama, M.; Motoyoshi, Y.; Ozawa, E.; Fujimoto, M.; Kawashimo, H.; et al. The level of fasting serum insulin, but not adiponectin, is associated with the prognosis of early stage hepatocellular carcinoma. Oncol. Rep. 2009, 22, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Franchi, M.; Nicotra, F.; Asciutto, R.; Merlino, L.; La Vecchia, C.; Corrao, G. Insulin and other antidiabetic drugs and hepatocellular carcinoma risk: A nested case-control study based on Italian healthcare utilization databases. Pharmacoepidemiol. Drug Saf. 2015, 24, 771–778. [Google Scholar] [CrossRef]
- Donadon, V.; Balbi, M.; Ghersetti, M.; Grazioli, S.; Perciaccante, A.; Della Valentina, G.; Gardenal, R.; Dal Mas, M.; Casarin, P.; Zanette, G.; et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J. Gastroenterol. 2009, 15, 2506–2511. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Curley, S.A.; Li, D.H.; Kaseb, A.; Davila, M.; Abdalla, E.K.; Javle, M.; Moghazy, D.M.; Lozano, R.D.; Abbruzzese, J.L.; et al. Association of Diabetes Duration and Diabetes Treatment With the Risk of Hepatocellular Carcinoma. Cancer 2010, 116, 1938–1946. [Google Scholar] [CrossRef]
- Spector, S.A.; Olson, E.T.; Gumbs, A.A.; Friess, H.; Buchler, M.W.; Seymour, N.E. Human insulin receptor and insulin signaling proteins in hepatic disease. J. Surg. Res. 1999, 83, 32–35. [Google Scholar] [CrossRef]
- Yu, J.; Shen, J.; Sun, T.T.; Zhang, X.; Wong, N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin. Cancer Biol. 2013, 23, 483–491. [Google Scholar] [CrossRef]
- Chettouh, H.; Lequoy, M.; Fartoux, L.; Vigouroux, C.; Desbois-Mouthon, C. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver Int. 2015, 35, 2203–2217. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.X.; Buddha, H.; Castelino-Prabhu, S.; Zhang, Z.; Britton, R.S.; Bacon, B.R.; Neuschwander-Tetri, B.A. Activation of Insulin-PI3K/Akt-p70S6K Pathway in Hepatic Stellate Cells Contributes to Fibrosis in Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2017, 62, 968–978. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Barthel, S.R.; Medvedev, R.; Heinrich, T.; Buchner, S.M.; Kettern, N.; Hildt, E. Hepatitis B virus inhibits insulin receptor signaling and impairs liver regeneration via intracellular retention of the insulin receptor. Cell. Mol. Life Sci. 2016, 73, 4121–4140. [Google Scholar] [CrossRef]
- Parvaiz, F.; Manzoor, S.; Iqbal, J.; Sarkar-Dutta, M.; Imran, M.; Waris, G. Hepatitis C virus NS5A promotes insulin resistance through IRS-1 serine phosphorylation and increased gluconeogenesis. World J. Gastroenterol. 2015, 21, 12361–12369. [Google Scholar] [CrossRef] [PubMed]
| Factors | Cases (N = 1237) | Controls (N = 4948) | Crude OR (95% CI) |
|---|---|---|---|
| Age | 64.8 (11.5) | 64.5 (11.5) | − |
| Male | 932 (75.3) | 3728 (75.3) | − |
| Year of initiating antidiabetic agents | |||
| 2003 | 201 (16.3) | 804 (16.3) | − |
| 2004 | 179 (14.5) | 716 (14.5) | − |
| 2005 | 182 (14.7) | 728 (14.7) | − |
| 2006 | 130 (10.5) | 520 (10.5) | − |
| 2007 | 155 (12.5) | 620 (12.5) | − |
| 2008 | 115 (9.3) | 460 (9.3) | − |
| 2009 | 103 (8.3) | 412 (8.3) | − |
| 2010 | 84 (6.8) | 336 (6.8) | − |
| 2011 | 51 (4.1) | 204 (4.1) | − |
| 2012 | 32 (2.6) | 128 (2.6) | − |
| 2013 | 5 (0.4) | 20 (0.4) | − |
| Socioeconomic status (monthly income in NTD) | |||
| ≤17,280 | 530 (42.9) | 2253 (45.5) | Reference |
| 17,281~22,800 | 483 (39.1) | 1774 (35.9) | 1.16 (1.01–1.33) |
| 22,801~28,800 | 54 (4.4) | 202 (4.1) | 1.14 (0.83–1.57) |
| 28,801~36,300 | 56 (4.5) | 219 (4.4) | 1.09 (0.79–1.49) |
| 36,301~45,800 | 65 (5.3) | 240 (4.9) | 1.15 (0.85–1.55) |
| >45,800 | 49 (4.0) | 260 (5.3) | 0.80 (0.58–1.11) |
| Medication use before cancer diagnosis | |||
| Premixed insulin analogues | 73 (5.9) | 127 (2.6) | 2.38 (1.77–3.20) |
| Oral antidiabetic drugs | 1058 (85.5) | 4464 (90.2) | 0.63 (0.52–0.76) |
| ACEi/ARBs | 99 (8.0) | 389 (7.9) | 1.02 (0.81–1.29) |
| Beta-blockers | 155 (12.5) | 435 (8.8) | 1.50 (1.23–1.83) |
| Calcium channel blockers | 73 (5.9) | 273 (5.5) | 1.08 (0.82–1.42) |
| Diuretics | 85 (6.9) | 151 (3.1) | 2.40 (1.82–3.17) |
| Statins | 147 (11.9) | 915 (18.5) | 0.58 (0.48–0.70) |
| Fibrates | 137 (11.1) | 658 (13.3) | 0.81 (0.66–0.98) |
| Comorbidities | |||
| Liver cirrhosis | 283 (22.9) | 36 (0.7) | 39.7 (27.0–58.6) |
| Hypertension | 551 (44.5) | 1934 (39.1) | 1.26 (1.11–1.44) |
| Hyperlipidemia | 140 (11.3) | 837 (16.9) | 0.63 (0.52–0.76) |
| Ischemic heart disease | 134 (10.8) | 446 (9.0) | 1.23 (1.00–1.51) |
| Myocardial infarction | 15 (1.2) | 52 (1.1) | 1.16 (0.65–2.06) |
| Heart failure | 73 (5.9) | 153 (3.1) | 2.01 (1.50–2.69) |
| Atrial fibrillation | 20 (1.6) | 66 (1.3) | 1.22 (0.73–2.01) |
| Cerebrovascular disease | 117 (9.5) | 488 (9.9) | 0.95 (0.77–1.18) |
| Stroke | 68 (5.5) | 319 (6.5) | 0.84 (0.64–1.11) |
| Peripheral vascular disease | 9 (0.7) | 32 (0.7) | 1.13 (0.54–2.36) |
| Chronic kidney disease | 60 (4.9) | 103 (2.1) | 2.41 (1.74–3.34) |
| Depression | 15 (1.2) | 63 (1.3) | 0.95 (0.54–1.68) |
| Charlson index | 2.6 (2.6) | 1.0 (1.3) | 1.56 (1.50–1.63) |
| Resource utilization | |||
| Number of A1C measurements | 4.8 (5.9) | 4.8 (6.4) | 1.00 (0.99–1.01) |
| Number of lipid measurements | 4.3 (5.3) | 4.5 (5.8) | 0.99 (0.98–1.00) |
| Number of outpatient visits | 88.2 (95.9) | 78.6 (86.2) | 1.00 (1.00–1.00) |
| Number of hospitalizations | 2.0 (3.24) | 1.1 (2.2) | 1.15 (1.12–1.18) |
| Length of hospital stay >7 days | 1.0 (2.0) | 0.6 (1.4) | 1.19 (1.14–1.24) |
| After exclusion of Chronic Hepatitis B or C | Before Exclusion of Chronic Hepatitis B or C | |||||||
|---|---|---|---|---|---|---|---|---|
| Factors | HCCs | Controls | Crude OR | Adjusted OR † | HCCs | Controls | Crude OR | Adjusted OR ‡ |
| Non-use | 1164 | 4821 | 1.0 | 1.0 | 5469 | 22,718 | 1.0 | 1.0 |
| Any use | 73 | 127 | 2.38 (1.77–3.20) | 1.35 (0.92–1.98) | 363 | 610 | 2.48 (2.17–2.84) | 1.27 (1.04–1.55) |
| Current use | 42 | 60 | 2.89 (1.94–4.31) | 1.15 (0.67–1.98) | 220 | 310 | 2.97 (2.49–3.54) | 1.45 (1.12–1.89) |
| Recent use | 9 | 14 | 2.65 (1.15–6.13) | 1.41 (0.49–4.03) | 36 | 67 | 2.21 (1.48–3.32) | 0.70 (0.39–1.25) |
| Past use | 22 | 53 | 1.73 (1.04–2.86) | 1.63 (0.90–2.96) | 107 | 233 | 1.92 (1.52–2.42) | 1.25 (0.90–1.72) |
| Cumulative dosage | ||||||||
| High | 26 | 39 | 2.74 (1.66–4.52) | 1.62 (0.86–3.05) | 139 | 182 | 3.18 (2.54–3.97) | 1.62 (1.16–2.26) |
| Intermediate | 23 | 48 | 1.99 (1.20–3.29) | 1.04 (0.53–2.05) | 98 | 229 | 1.77 (1.40–2.25) | 1.05 (0.75–1.47) |
| Low | 24 | 40 | 2.51 (1.50–4.19) | 1.42 (0.75–2.70) | 126 | 199 | 2.68 (2.13–3.36) | 1.21 (0.87–1.68) |
| Cumulative duration | ||||||||
| Long | 26 | 40 | 2.67 (1.62–4.39) | 1.48 (0.78–2.82) | 135 | 188 | 2.99 (2.39–3.75) | 1.80 (1.30–-2.51) |
| Intermediate | 25 | 43 | 2.42 (1.47–3.98) | 1.28 (0.68–2.41) | 92 | 220 | 1.74 (1.36–2.22) | 0.72 (0.50–1.05) |
| Short | 22 | 44 | 2.08 (1.24–3.50) | 1.28 (0.65–2.52) | 136 | 202 | 2.83 (2.27–3.53) | 1.41 (1.03–1.93) |
| Premixed Insulin Analogs | Chronic Viral Hepatitis | HCCs | Controls | Crude OR | Adjusted OR † |
|---|---|---|---|---|---|
| Non-use | Yes | 2998 | 1154 | 22.7 (20.9–24.5) | 6.99 (5.14–9.51) |
| Any use | No | 132 | 534 | 2.16 (1.78–2.62) | 1.51 (1.21–1.89) |
| Any use | Yes | 231 | 76 | 26.5 (20.4–34.5) | 8.16 (7.42–8.97) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-H.; Kuo, C.-S.; Lin, W.-W.; Su, J.-H.; Chen, J.-D.; Huang, K.-C. Chronic Viral Hepatitis Signifies the Association of Premixed Insulin Analogues with Liver Cancer Risks: A Nationwide Population-Based Study. Int. J. Environ. Res. Public Health 2019, 16, 2097. https://doi.org/10.3390/ijerph16122097
Chiang C-H, Kuo C-S, Lin W-W, Su J-H, Chen J-D, Huang K-C. Chronic Viral Hepatitis Signifies the Association of Premixed Insulin Analogues with Liver Cancer Risks: A Nationwide Population-Based Study. International Journal of Environmental Research and Public Health. 2019; 16(12):2097. https://doi.org/10.3390/ijerph16122097
Chicago/Turabian StyleChiang, Chien-Hsieh, Chia-Sheng Kuo, Wan-Wan Lin, Jun-Han Su, Jin-De Chen, and Kuo-Chin Huang. 2019. "Chronic Viral Hepatitis Signifies the Association of Premixed Insulin Analogues with Liver Cancer Risks: A Nationwide Population-Based Study" International Journal of Environmental Research and Public Health 16, no. 12: 2097. https://doi.org/10.3390/ijerph16122097
APA StyleChiang, C.-H., Kuo, C.-S., Lin, W.-W., Su, J.-H., Chen, J.-D., & Huang, K.-C. (2019). Chronic Viral Hepatitis Signifies the Association of Premixed Insulin Analogues with Liver Cancer Risks: A Nationwide Population-Based Study. International Journal of Environmental Research and Public Health, 16(12), 2097. https://doi.org/10.3390/ijerph16122097







