Changes of Atmospheric and Blood Concentrations of Lead and Cadmium in the General Population of South Korea from 2008 to 2017
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Data Collection
2.2. Determination of Lead and Cadmium in Whole Blood
2.3. Environmental Heavy Metal Measurements
2.4. Statistical Analysis
3. Results
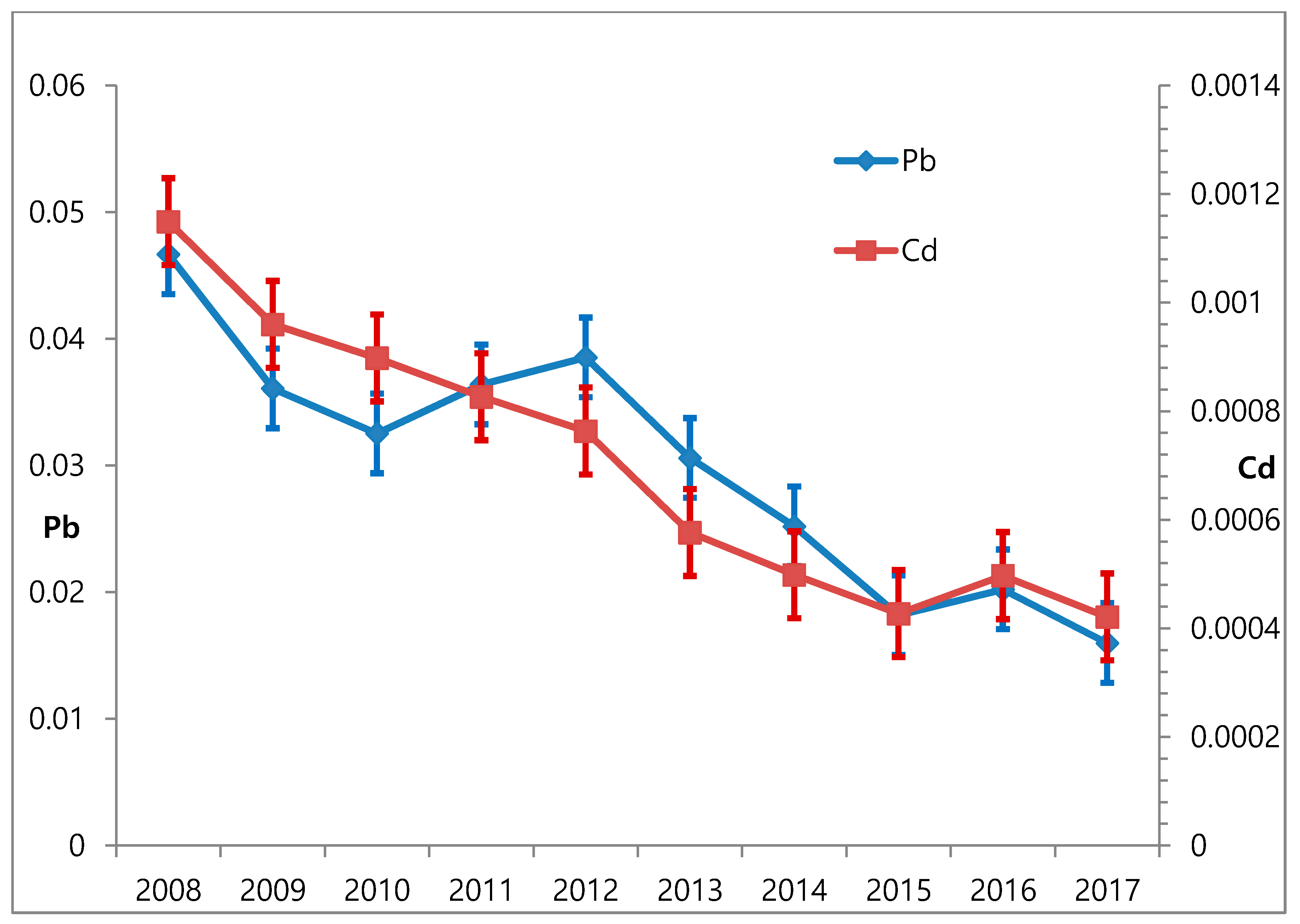
3.1. Changes in Blood Lead and Atmospheric Lead Over Time
3.2. Changes in Blood Cadmium and Atmospheric Cadmium over Time
3.3. Relationship of Atmospheric and Blood Levels of Lead and Cadmium in Men and Women
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abadin, H.; Ashizawa, A.; Llados, F.; Stevens, Y.-W. Toxicological Profile for Lead; ATSDR: Atlanta, GA, USA, 2007.
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; ATSDR: Atlanta, GA, USA, 2012.
- Bellinger, D.C. Prenatal exposures to environmental chemicals and children’s neurodevelopment: An update. Saf. Health Work 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP Monograph: Health Effects of Low-Level Lead. In NTP Monograph; National Toxicology Program: Research Triangle Park, NC, USA, 2012. [Google Scholar]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- De Temmerman, L.; Hoenig, M. Vegetable crops for biomonitoring lead and cadmium deposition. J. Atmos. Chem. 2004, 49, 121–135. [Google Scholar] [CrossRef]
- Lockitch, G. Perspectives on lead toxicity. Clin. Biochem. 1993, 26, 371–381. [Google Scholar] [CrossRef]
- Hu, H.; Shih, R.; Rothenberg, S.; Schwartz, B.S. The epidemiology of lead toxicity in adults: Measuring dose and consideration of other methodologic issues. Environ. Health Perspect. 2007, 115, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F., Jr.; Tanus-Santos, J.E.; Gerlach, R.F.; Parsons, P.J. A critical review of biomarkers used for monitoring human exposure to lead: Advantages, limitations, and future needs. Environ. Health Perspect. 2005, 113, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Gao, Y.; Zhang, Y.; Wang, C.; Zhou, Y.; Hu, Y.; Shi, R.; Tian, Y. Effects of prenatal exposure to cadmium on neurodevelopment of infants in Shandong, China. Environ. Pollut. 2016, 211, 67–73. [Google Scholar] [CrossRef]
- Jeong, K.S.; Park, H.; Ha, E.; Hong, Y.-C.; Ha, M.; Park, H.; Kim, B.-N.; Lee, B.-E.; Lee, S.-J.; Lee, K.Y.; et al. Performance IQ in children is associated with blood cadmium concentration in early pregnancy. J. Trace Elem. Med. Biol. 2015, 30, 107–111. [Google Scholar] [CrossRef]
- Sanchez-Martin, M.J.; Sanchez-Camazano, M.; Lorenzo, L.F. Cadmium and lead contents in suburban and urban soils from two medium-sized cities of Spain: Influence of traffic intensity. Bull. Environ. Contam. Toxicol. 2000, 64, 250–257. [Google Scholar] [CrossRef]
- Pan, J.; Plant, J.A.; Voulvoulis, N.; Oates, C.J.; Ihlenfeld, C. Cadmium levels in Europe: Implications for human health. Environ. Geochem. Health 2010, 32, 1–12. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Berglund, M.; Elinder, C.G.; Nordberg, G.; Vahter, M. Health effects of cadmium exposure—A review of the literature and a risk estimate. Scand. J. Work Environ. Health 1998, 24 (Suppl. 1), 1–51. [Google Scholar] [PubMed]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Dillon, H.K.; Ho, M.H. Biological Monitoring of Exposure to Chemicals; John Wiley & Sons.: New York, NY, USA, 1990. [Google Scholar]
- Nordberg, G.; Jin, T.; Bernard, A.; Fierens, S.; Buchet, J.P.; Ye, T.; Kong, Q.; Wang, H. Low bone density and renal dysfunction following environmental cadmium exposure in China. Ambio 2002, 31, 478–481. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. America’s Children and the Environment, 3rd ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 2013.
- Alarcon, W.A. Summary of notifiable noninfectious conditions and disease outbreaks: Elevated blood lead levels among employed adults—United States, 1994–2012. MMWR Morb. Mortal. Wkly. Rep. 2015, 62, 52–75. [Google Scholar] [CrossRef][Green Version]
- Hwang, Y.H.; Ko, Y.; Chiang, C.D.; Hsu, S.P.; Lee, Y.H.; Yu, C.H.; Chiou, C.H.; Wang, J.D.; Chuang, H.Y. Transition of cord blood lead level, 1985–2002, in the Taipei area and its determinants after the cease of leaded gasoline use. Environ. Res. 2004, 96, 274–282. [Google Scholar] [CrossRef]
- Brunekreef, B. The relationship between air lead and blood lead in children: A critical review. Sci. Total Environ. 1984, 38, 79–123. [Google Scholar] [CrossRef]
- Romieu, I.; Carreon, T.; Lopez, L.; Palazuelos, E.; Rios, C.; Manuel, Y.; Hernandez-Avila, M. Environmental urban lead exposure and blood lead levels in children of Mexico City. Environ. Health Perspect. 1995, 103, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, B.K. Associations of blood lead, cadmium, and mercury with estimated glomerular filtration rate in the Korean general population: Analysis of 2008–2010 Korean National Health and Nutrition Examination Survey data. Environ. Res. 2012, 118, 124–129. [Google Scholar] [CrossRef]
- Bono, R.; Pignata, C.; Scursatone, E.; Rovere, R.; Natale, P.; Gilli, G. Updating about reductions of air and blood lead concentrations in Turin, Italy, following reductions in the lead content of gasoline. Environ. Res. 1995, 70, 30–34. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, S.; Zhang, J. Blood lead levels of children and its trend in China. Sci. Total Environ. 2009, 407, 3986–3993. [Google Scholar] [CrossRef] [PubMed]
- Institute of Research on Traffic and Environment. Understanding Regulations for Automobile Environment Management; Korean Ministry of Environment: Sejong, South Korea, 2016.
- Park, K.S. A Study of Lead Concentrations in Ambient Air of Major Cities. Ph.D. Thesis, Hanyang University, Seoul, South Korea, 1993. [Google Scholar]
- Oh, S.E.; Kim, G.B.; Hwang, S.H.; Ha, M.; Lee, K.M. Longitudinal trends of blood lead levels before and after leaded gasoline regulation in Korea. Environ. Health Toxicol. 2017, 32, e2017019. [Google Scholar] [CrossRef] [PubMed]
- Lee, D. Annual variation of atmospheric lead concentration in Seoul (1984–1993). J. Korean Air Pollut. Res. Assoc. 1994, 10, 170–174. [Google Scholar]
- Moon, C.S.; Zhang, Z.W.; Shimbo, S.; Watanabe, T.; Moon, D.H.; Lee, C.U.; Lee, B.K.; Ahn, K.D.; Lee, S.H.; Ikeda, M. Dietary intake of cadmium and lead among the general population in Korea. Environ. Res. 1995, 71, 46–54. [Google Scholar] [CrossRef]
- Moon, C.-S.; Yang, H.-R.; Nakatsuka, H.; Ikeda, M. Time trend of cadmium intake in Korea. Environ. Health Prev. Med. 2016, 21, 118–128. [Google Scholar] [CrossRef]
- Ikeda, M.; Ezaki, T.; Tsukahara, T.; Moriguchi, J. Dietary cadmium intake in polluted and non-polluted areas in Japan in the past and in the present. Int. Arch. Occup. Environ. Health 2004, 77, 227–234. [Google Scholar] [CrossRef]
- Ikeda, M.; Zhang, Z.W.; Shimbo, S.; Watanabe, T.; Nakatsuka, H.; Moon, C.S.; Matsuda-Inoguchi, N.; Higashikawa, K. Urban population exposure to lead and cadmium in east and south-east Asia. Sci. Total Environ. 2000, 249, 373–384. [Google Scholar] [CrossRef]
- Guan, S.; Palermo, T.; Meliker, J. Seafood intake and blood cadmium in a cohort of adult avid seafood consumers. Int. J. Hyg. Environ. Health 2015, 218, 147–152. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Fourth National Report on Human Exposure to Environmental Chemicals Updated Table. Available online: https://www.cdc.gov/exposurereport/index.html (accessed on 3 May 2019).
- Environmental Protection Agency. Lead Trends; Environmental Protection Agency: Washington, DC, USA, 2019.
- European Commission. Ambient Air Pollution by Arsenic, Cadmium and Nickel Compounds; European Commission: Luxemburg, 2001. [Google Scholar]
- Park, S.K.; Lee, S.; Basu, N.; Franzblau, A. Associations of blood and urinary mercury with hypertension in U.S. adults: The NHANES 2003–2006. Environ. Res. 2013, 123, 25–32. [Google Scholar] [CrossRef]
- Lee, B.K.; Kim, Y. Sex-specific profiles of blood metal levels associated with metal-Iron interactions. Saf. Health Work 2014, 5, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Kim, S.H.; Kim, N.S.; Ham, J.O.; Kim, Y. Iron deficiency increases blood cadmium levels in adolescents surveyed in KNHANES 2010–2011. Biol. Trace Elem. Res. 2014, 159, 52–58. [Google Scholar] [CrossRef]
- Zoller, H.; Koch, R.O.; Theurl, I.; Obrist, P.; Pietrangelo, A.; Montosi, G.; Haile, D.J.; Vogel, W.; Weiss, G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology 2001, 120, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.M.; Brantsaeter, A.L.; Borch-Iohnsen, B.; Ellingsen, D.G.; Alexander, J.; Thomassen, Y.; Stigum, H.; Ydersbond, T.A. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ. Res. 2010, 110, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Baecklund, M.; Pedersen, N.L.; Bjorkman, L.; Vahter, M. Variation in blood concentrations of cadmium and lead in the elderly. Environ. Res. 1999, 80, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Kim, Y. Association between bone mineral density and blood lead level in menopausal women: Analysis of 2008–2009 Korean National Health and Nutrition Examination Survey data. Environ. Res. 2012, 115, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Kim, Y. Association of blood cadmium level with metabolic syndrome after adjustment for confounding by serum ferritin and other factors: 2008–2012 Korean National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2016, 171, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.L.; Nguyen, L.M. Geographic region of residence and blood lead levels in US children: Results of the National Health and Nutrition Examination Survey. Int. Arch. Occup. Environ. Health 2011, 84, 513–522. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines for Europe; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices, 7th ed.; ACGIH: Cincinnati, OH, USA, 2018. [Google Scholar]
| Classification | Total | Men | Women | |
|---|---|---|---|---|
| (n = 16,873) | (n = 8117) | (n = 8756) | ||
| Age, NS | <30 | 3011 (25.2) | 1451 (26.4) | 1560 (23.6) |
| 30–39 | 3258 (20.1) | 1570 (20.6) | 1688 (19.5) | |
| 40–49 | 3341 (21.4) | 1621 (21.4) | 1720 (21.3) | |
| 50–59 | 3376 (18.8) | 1612 (18.7) | 1764 (18.9) | |
| 60≤– | 3887 (14.6) | 1863 (12.9) | 2024 (16.8) | |
| Employment* | Manual | 4102 (26.4) | 2319 (29.7) | 1783 (22.4) |
| Non-manual | 6397 (38.2) | 3722 (45.2) | 2675 (29.7) | |
| Others | 6374 (35.4) | 2076 (25.1) | 4298 (47.9) | |
| Smoking* | Non-smoker | 9461 (52) | 1758 (23.8) | 7703 (86.4) |
| Past smoker | 3344 (19.7) | 2853 (30.7) | 491 (6.2) | |
| Current smoker | 4068 (28.3) | 3506 (45.4) | 562 (7.4) | |
| Alcohol* | None | 4274 (21.8) | 1302 (14.3) | 2972 (30.9) |
| Mild | 8414 (50.5) | 3493 (44.7) | 4921 (57.6) | |
| Moderate | 2004 (12.6) | 1442 (17.1) | 562 (7.2) | |
| Heavy | 2181 (15.1) | 1880 (24) | 301 (4.3) | |
| Residence, NS | Urban | 13671 (83.4) | 6544 (83) | 7127 (83.9) |
| Rural | 3202 (16.6) | 1573 (17) | 1629 (16.1) | |
| Outdoor activity | Exercise, NS | 2923 (21.5) | 1395 (21.3) | 1528 (21.9) |
| No exercise | 13950 (78.5) | 6722 (78.7) | 7228 (78.1) | |
| Regular walk* | 5005 (27.6) | 2608 (30.2) | 2397 (24.5) | |
| No regular walk | 11868 (72.4) | 5509 (69.8) | 6359 (75.5) | |
| Classification | All | p-Value | Year | Changes in Blood Level Relative to Initial Level.(%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2016 | 2017 | |||||
| All | 1.95 (1.93–1.97) | - | 2.37 (2.32–2.42) | 2.32 (2.26–2.37) | 2.21 (2.17–2.25) | 2.12 (2.07–2.17) | 1.98 (1.94–2.03) | 1.92 (1.88–1.97) | 1.74 (1.70–1.78) | 1.46 (1.43–1.49) | 61.6 | |
| Gender | Men | 2.13 (2.10–2.15) | ** | 2.71 (2.65–2.78) | 2.64 (2.58–2.71) | 2.55 (2.49–2.61) | 2.40 (2.34–2.47) | 2.23 (2.17–2.28) | 2.18 (2.12–2.25) | 1.95 (1.89–2.00) | 1.65 (1.61–1.69) | 60.9 |
| Women | 1.74 (1.72–1.77) | 2.00 (1.94–2.06) | 1.97 (1.91–2.04) | 1.86 (1.81–1.90) | 1.82 (1.76–1.88) | 1.73 (1.68–1.79) | 1.65 (1.60–1.71) | 1.51 (1.47–1.55) | 1.25 (1.22–1.28) | 62.5 | ||
| Age | <30 | 1.55 (1.53–1.58) | ** | 1.94 (1.86–2.02) | 1.85 (1.77–1.92) | 1.75 (1.68–1.81) | 1.66 (1.60–1.72) | 1.51 (1.46–1.57) | 1.55 (1.47–1.62) | 1.34 (1.27–1.42) | 1.14 (1.09–1.19) | 58.8 |
| 30–39 | 1.83 (1.80–1.85) | 2.24 (2.15–2.32) | 2.20 (2.12–2.28) | 2.08 (2.01–2.15) | 2.01 (1.93–2.10) | 1.89 (1.82–1.97) | 1.86 (1.80–1.93) | 1.62 (1.56–1.68) | 1.31 (1.26–1.37) | 58.5 | ||
| 40–49 | 2.04 (2.01–2.07) | 2.49 (2.39–2.59) | 2.47 (2.36–2.59) | 2.38 (2.28–2.49) | 2.23 (2.15–2.32) | 2.09 (2.00–2.19) | 2.05 (1.97–2.13) | 1.82 (1.76–1.89) | 1.56 (1.51–1.62) | 62.7 | ||
| 50–59 | 2.31 (2.28–2.35) | 2.78 (2.67–2.89) | 2.76 (2.66–2.87) | 2.66 (2.57–2.77) | 2.57 (2.46–2.69) | 2.46 (2.33–2.59) | 2.29 (2.20–2.38) | 2.13 (2.06–2.21) | 1.78 (1.72–1.84) | 64.0 | ||
| 60≤ | 2.35 (2.31–2.39) | 2.65 (2.53–2.77) | 2.57 (2.45–2.69) | 2.46 (2.34–2.58) | 2.44 (2.36–2.53) | 2.43 (2.31–2.56) | 2.14 (2.04–2.26) | 2.13 (2.06–2.20) | 1.80 (1.74–1.87) | 67.9 | ||
| Employment | Manual | 1.99 (1.97–2.02) | ** | 2.71 (2.62–2.79) | 2.60 (2.52–2.69) | 2.46 (2.39–2.53) | 2.39 (2.31–2.48) | 2.19 (2.11–2.28) | 2.14 (2.07–2.21) | 1.92 (1.86–1.98) | 1.64 (1.59–1.69) | 60.5 |
| Non-manual | 1.95 (1.93–1.98) | 2.22 (2.12–2.32) | 2.26 (2.18–2.34) | 2.11 (2.05–2.18) | 2.00 (1.93–2.08) | 1.95 (1.88–2.02) | 1.85 (1.78–1.92) | 1.69 (1.64–1.75) | 1.33 (1.29–1.37) | 59.9 | ||
| Others | 1.89 (1.87–1.92) | 2.18 (2.11–2.24) | 2.10 (2.03–2.17) | 2.03 (1.97–2.09) | 1.92 (1.85–1.98) | 1.79 (1.72–1.87) | 1.74 (1.67–1.80) | 1.60 (1.55–1.65) | 1.38 (1.34–1.42) | 63.3 | ||
| Smoking | Non-smoker | 1.88 (1.86–1.91) | ** | 2.06 (2.00–2.12) | 2.03 (1.97–2.10) | 1.94 (1.89–1.99) | 1.89 (1.82–1.95) | 1.75 (1.69–1.80) | 1.72 (1.67–1.77) | 1.54 (1.50–1.58) | 1.29 (1.26–1.33) | 62.6 |
| Past smoker | 1.94 (1.91–1.97) | 2.66 (2.56–2.76) | 2.55 (2.44–2.65) | 2.47 (2.37–2.58) | 2.40 (2.30–2.50) | 2.21 (2.11–2.31) | 2.12 (2.03–2.22) | 1.96 (1.90–2.03) | 1.62 (1.56–1.68) | 60.9 | ||
| Current smoker | 2.07 (2.04–2.10) | 2.82 (2.72–2.91) | 2.75 (2.66–2.84) | 2.60 (2.52–2.68) | 2.41 (2.33-2.51) | 2.33 (2.25–2.41) | 2.21 (2.12–2.30) | 1.98 (1.90–2.05) | 1.69 (1.63–1.75) | 59.9 | ||
| Alcohol | None | 1.82 (1.80–1.85) | ** | 2.20 (2.11–2.29) | 2.15 (2.07–2.24) | 2.10 (2.01–2.20) | 2.03 (1.94–2.12) | 1.88 (1.80–1.97) | 1.86 (1.78–1.95) | 1.67 (1.61–1.72) | 1.42 (1.36–1.48) | 64.5 |
| Mild | 1.89 (1.87–1.91) | 2.23 (2.17–2.29) | 2.16 (2.09–2.23) | 2.05 (2.00–2.10) | 1.98 (1.92–2.04) | 1.80 (1.75–1.85) | 1.77 (1.72–1.82) | 1.61 (1.56–1.66) | 1.36 (1.33–1.39) | 61.0 | ||
| Moderate | 2.09 (2.05–2.13) | 2.66 (2.53–2.79) | 2.66 (2.53–2.79) | 2.54 (2.43–2.66) | 2.36 (2.21–2.51) | 2.28 (2.17–2.40) | 2.10 (1.98–2.22) | 1.96 (1.86–2.06) | 1.59 (1.51–1.68) | 59.8 | ||
| Heavy | 2.21 (2.17–2.25) | 2.96 (2.83–3.09) | 2.91 (2.79–3.04) | 2.66 (2.55–2.78) | 2.60 (2.48–2.72) | 2.60 (2.47–2.74) | 2.48 (2.35–2.63) | 2.17 (2.08–2.26) | 1.79 (1.71–1.87) | 60.5 | ||
| Residence | Urban | 1.94 (1.92–1.95) | * | 2.35 (2.29–2.40) | 2.29 (2.23–2.34) | 2.18 (2.14–2.22) | 2.07 (2.03–2.12) | 1.96 (1.92–2.01) | 1.89 (1.85–1.94) | 1.71 (1.67–1.75) | 1.43 (1.40–1.46) | 60.9 |
| Rural | 1.99 (1.94–2.03) | 2.48 (2.39–2.59) | 2.46 (2.32–2.61) | 2.35 (2.24–2.47) | 2.37 (2.19–2.56) | 2.08 (1.96–2.20) | 2.10 (1.93–2.27) | 1.89 (1.80–1.99) | 1.59 (1.50–1.69) | 64.1 | ||
| Outdoor activity | Exercise | 1.94 (1.92–1.97) | NS | 2.41 (2.32–2.50) | 2.36 (2.29–2.44) | 2.20 (2.13–2.27) | 2.11 (2.02–2.21) | 1.99 (1.90–2.09) | 1.97 (1.88–2.05) | 1.71 (1.65–1.77) | 1.46 (1.41–1.51) | 60.6 |
| No exercise | 1.95 (1.93–1.96) | 2.36 (2.30–2.41) | 2.31 (2.24–2.37) | 2.21 (2.17–2.26) | 2.12 (2.07–2.17) | 1.98 (1.93–2.03) | 1.91 (1.86–1.97) | 1.75 (1.71–1.79) | 1.46 (1.42–1.49) | 61.9 | ||
| Regular walk | 1.93 (1.91–1.96) | NS | 2.30 (2.24–2.37) | 2.28 (2.22–2.35) | 2.20 (2.13–2.26) | 2.08 (2.02–2.14) | 1.93 (1.86–1.99) | 1.88 (1.82–1.94) | 1.70 (1.65–1.76) | 1.44 (1.40–1.49) | 62.6 | |
| No regular walk | 1.95 (1.93–1.97) | 2.42 (2.36–2.48) | 2.33 (2.27–2.40) | 2.21 (2.16–2.26) | 2.14 (2.08–2.20) | 2.02 (1.96–2.07) | 1.95 (1.89–2.01) | 1.77 (1.73–1.81) | 1.47 (1.44–1.51) | 60.7 | ||
| Menopause (women in their 50s) | Yes (n = 1394) | 2.08 (2.03–2.13) | ** | 2.45 (2.29–2.62) | 2.42 (2.25–2.60) | 2.30 (2.17–2.44) | 2.27 (2.15–2.40) | 2.26 (2.09–2.43) | 2.16 (2.04–2.28) | 1.96 (1.86–2.05) | 1.57 (1.49–1.65) | 75.5 |
| No (n = 370) | 1.92 (1.83–2.01) | 2.59 (2.37–2.83) | 2.56 (2.34–2.79) | 2.06 (1.81–2.34) | 2.09 (1.80–2.42) | 1.87 (1.66–2.10) | 1.79 (1.57–2.04) | 1.71 (1.56–1.86) | 1.36 (1.24–1.50) | 70.8 | ||
| Classification | All | p-Value | Year | Changes in Blood Level Relative to Initial Level.(%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2016 | 2017 | |||||
| All | 0.87 (0.86–0.88) | 0.88 (0.85–0.92) | 0.91 (0.87–0.95) | 0.97 (0.94–1.00) | 0.95 (0.92–0.98) | 0.91 (0.88–0.94) | 0.80 (0.77–0.82) | 0.87 (0.84–0.89) | 0.72 (0.70–0.74) | 81.8 | ||
| Gender | Men | 0.73 (0.72–0.75) | ** | 0.80 (0.76–0.84) | 0.83 (0.79–0.87) | 0.88 (0.85–0.91) | 0.87 (0.83–0.90) | 0.83 (0.80–0.87) | 0.74 (0.71–0.77) | 0.80 (0.77–0.83) | 0.64 (0.62–0.67) | 80.0 |
| Women | 1.07 (1.05–1.09) | 1.00 (0.96–1.05) | 1.01 (0.96–1.06) | 1.08 (1.04–1.13) | 1.06 (1.02–1.10) | 1.02 (0.98–1.06) | 0.88 (0.85–0.91) | 0.96 (0.92–0.99) | 0.83 (0.80–0.86) | 83.0 | ||
| Age | <30 | 0.57 (0.55–0.58) | ** | 0.55 (0.51–0.59) | 0.57 (0.53–0.62) | 0.64 (0.60–0.67) | 0.61 (0.57–0.65) | 0.57 (0.54–0.61) | 0.50 (0.47–0.53) | 0.54 (0.50–0.58) | 0.42 (0.39–0.45) | 76.4 |
| 30–39 | 0.77 (0.76–0.79) | 0.84 (0.78–0.91) | 0.83 (0.77–0.89) | 0.89 (0.84–0.94) | 0.86 (0.81–0.91) | 0.82 (0.78–0.87) | 0.72 (0.68–0.76) | 0.74 (0.70–0.78) | 0.60 (0.57–0.64) | 71.4 | ||
| 40–49 | 1.02 (1.00–1.04) | 1.01 (0.96–1.07) | 1.06 (1.00–1.12) | 1.16 (1.10–1.22) | 1.11 (1.06–1.16) | 1.09 (1.03–1.16) | 0.94 (0.89–0.99) | 1.01 (0.96–1.05) | 0.89 (0.84–0.94) | 88.1 | ||
| 50–59 | 1.14 (1.12–1.16) | 1.16 (1.09–1.23) | 1.23 (1.16–1.30) | 1.23 (1.16–1.29) | 1.24 (1.17–1.31) | 1.20 (1.15–1.25) | 1.05 (0.99–1.10) | 1.19 (1.14–1.25) | 1.00 (0.96–1.05) | 86.2 | ||
| 60≤ | 1.20 (1.18–1.23) | 1.22 (1.15–1.29) | 1.22 (1.16–1.29) | 1.22 (1.17–1.28) | 1.25 (1.19–1.32) | 1.24 (1.18–1.30) | 1.15 (1.10–1.21) | 1.25 (1.21–1.30) | 1.08 (1.04–1.13) | 88.5 | ||
| Employment | Manual | 0.88 (0.86–0.89) | ** | 1.02 (0.96–1.09) | 1.04 (0.99–1.10) | 1.02 (0.98–1.07) | 1.05 (1.00–1.11) | 1.01 (0.96–1.06) | 0.87 (0.83–0.92) | 0.94 (0.90–0.98) | 0.80 (0.76–0.84) | 78.4 |
| Non-manual | 0.85 (0.83–0.87) | 0.74 (0.70–0.79) | 0.78 (0.72–0.85) | 0.91 (0.86–0.96) | 0.81 (0.77–0.85) | 0.76 (0.72–0.81) | 0.71 (0.68–0.75) | 0.74 (0.71–0.78) | 0.60 (0.57–0.63) | 81.1 | ||
| Others | 0.88 (0.86–0.89) | 0.91 (0.86–0.95) | 0.93 (0.87–0.98) | 0.96 (0.91–1.01) | 0.96 (0.92–1.01) | 0.93 (0.88–0.98) | 0.77 (0.73–0.81) | 0.87 (0.83–0.90) | 0.73 (0.70–0.76) | 80.2 | ||
| Smoking | Non-smoker | 0.75 (0.74–0.76) | ** | 0.84 (0.81-0.88) | 0.86 (0.81–0.90) | 0.92 (0.88–0.96) | 0.91 (0.88–0.94) | 0.89 (0.85–0.93) | 0.74 (0.72–0.77) | 0.83 (0.80–0.85) | 0.67 (0.65–0.69) | 79.8 |
| Past smoker | 0.80 (0.78–0.82) | 0.76 (0.71–0.81) | 0.77 (0.71–0.83) | 0.86 (0.81–0.91) | 0.78 (0.74–0.82) | 0.80 (0.76–0.84) | 0.72 (0.67–0.76) | 0.78 (0.75–0.82) | 0.64 (0.61–0.68) | 84.2 | ||
| Current smoker | 1.21 (1.18–1.24) | 1.06 (1.00–1.12) | 1.15 (1.09–1.21) | 1.15 (1.10–1.21) | 1.16 (1.11–1.22) | 1.07 (1.02–1.13) | 0.98 (0.93–1.03) | 1.00 (0.94–1.06) | 0.90 (0.85–0.94) | 84.9 | ||
| Alcohol | None | 0.86 (0.85–0.88) | ** | 0.98 (0.93–1.04) | 1.03 (0.97–1.10) | 1.06 (1.00–1.13) | 1.08 (1.02–1.14) | 1.00 (0.95–1.06) | 0.93 (0.87–0.99) | 1.01 (0.97–1.05) | 0.85 (0.82–0.90) | 86.7 |
| Mild | 0.84 (0.83–0.86) | 0.81 (0.78–0.85) | 0.84 (0.79–0.89) | 0.92 (0.88–0.95) | 0.85 (0.82–0.89) | 0.86 (0.82–0.89) | 0.72 (0.69–0.75) | 0.79 (0.76–0.82) | 0.65 (0.63–0.67) | 80.2 | ||
| Moderate | 0.89 (0.86–0.91) | 0.87 (0.79–0.97) | 0.88 (0.81–0.95) | 0.95 (0.89–1.01) | 0.98 (0.90–1.07) | 0.90 (0.83–0.97) | 0.84 (0.78–0.92) | 0.83 (0.79–0.88) | 0.73 (0.68–0.78) | 83.9 | ||
| Heavy | 0.95 (0.93–0.98) | 1.01 (0.94–1.09) | 1.04 (0.97–1.12) | 1.01 (0.94–1.08) | 1.08 (1.00–1.16) | 1.00 (0.93–1.07) | 0.90 (0.84–0.96) | 0.97 (0.91–1.03) | 0.80 (0.75–0.85) | 79.2 | ||
| Residence | Urban | 0.87 (0.86–0.88) | NS | 0.86 (0.83–0.90) | 0.90 (0.86–0.95) | 0.96 (0.93–0.99) | 0.92 (0.89–0.95) | 0.88 (0.85–0.91) | 0.79 (0.76–0.81) | 0.85 (0.82–0.88) | 0.71 (0.69–0.73) | 82.6 |
| Rural | 0.88 (0.85–0.91) | 1.00 (0.91–1.09) | 0.93 (0.86–1.01) | 1.02 (0.94–1.10) | 1.09 (1.00–1.20) | 1.03 (0.96–1.11) | 0.85 (0.80–0.91) | 0.96 (0.90–1.02) | 0.82 (0.77–0.86) | 82.0 | ||
| Outdoor activity | Exercise | 0.85 (0.83–0.86) | * | 0.84 (0.79–0.89) | 0.86 (0.80–0.92) | 0.87 (0.82–0.93) | 0.91 (0.85–0.96) | 0.87 (0.81–0.94) | 0.74 (0.69–0.79) | 0.76 (0.72–0.79) | 0.63 (0.60–0.66) | 75.0 |
| No exercise | 0.88 (0.87–0.89) | 0.90 (0.87–0.94) | 0.93 (0.89–0.97) | 1.01 (0.97–1.04) | 0.97 (0.94–1.00) | 0.93 (0.90–0.96) | 0.82 (0.80–0.85) | 0.91 (0.88–0.94) | 0.76 (0.74–0.78) | 84.4 | ||
| Regular walk | 0.86 (0.85–0.87) | * | 0.81 (0.78–0.85) | 0.83 (0.79–0.88) | 0.88 (0.85–0.92) | 0.89 (0.85–0.93) | 0.85 (0.82–0.89) | 0.76 (0.73–0.80) | 0.83 (0.80–0.86) | 0.69 (0.66–0.72) | 85.2 | |
| No regular walk | 0.88 (0.87–0.89) | 0.93 (0.90–0.98) | 0.96 (0.92–1.01) | 1.02 (0.99–1.06) | 0.98 (0.95–1.02) | 0.94 (0.91–0.98) | 0.82 (0.79–0.85) | 0.90 (0.87–0.93) | 0.75 (0.73–0.78) | 80.6 | ||
| Menopause (women in their 50s) | Yes (n = 1394) | 1.29 (1.25–1.32) | ** | 1.31 (1.19–1.45) | 1.36 (1.23–1.50) | 1.40 (1.29–1.51) | 1.38 (1.28–1.49) | 1.45 (1.37–1.54) | 1.16 (1.09–1.24) | 1.29 (1.22–1.36) | 1.09 (1.02–1.17) | 83.2 |
| No (n = 370) | 1.44 (1.36–1.52) | 1.42 (1.28–1.57) | 1.53 (1.35–1.72) | 1.38 (1.16–1.64) | 1.67 (1.32–2.11) | 1.51 (1.24–1.84) | 1.20 (1.06–1.36) | 1.44 (1.26–1.64) | 1.38 (1.21–1.56) | 97.2 | ||
| Classification | Men (n = 3764) | Women (n = 4135) | All | ||||
|---|---|---|---|---|---|---|---|
| Blood Lead | Blood Cadmium | Blood Lead | Blood Cadmium | Blood Lead | Blood Cadmium | ||
| Per doubling of air concentration | 0.08 (−0.01 to 0.16) | −0.02 (−0.08 to 0.04) | 0.10 (0.03 to 0.17) | 0.00 (−0.05 to 0.055) | 0.09 (0.03 to 0.15) | −0.01 (−0.05 to 0.03) | |
| Gender | Men | - | - | - | - | Reference | Reference |
| Women | −0.40 (−0.47 to −0.34) | 0.31 (0.28 to 0.35) | |||||
| Age | <30 | Reference | Reference | Reference | Reference | Reference | Reference |
| 30–39 | 0.34 (0.24 to 0.45) | 0.12 (0.07 to 0.20) | 0.15 (0.08 to 0.23) | 0.29 (0.24 to 0.34) | 0.27 (0.20 to 0.34) | 0.20 (0.17 to 0.24) | |
| 40–49 | 0.66 (0.52 to 0.80) | 0.32 (0.26 to 0.38) | 0.36 (0.27 to 0.45) | 0.66 (0.59 to 0.72) | 0.53 (0.45 to 0.61) | 0.48 (0.44 to 0.52) | |
| 50–59 | 0.86 (0.73 to 1.00) | 0.47 (0.40 to 0.54) | 0.75 (0.65 to 0.84) | 0.76 (0.68 to 0.84) | 0.82 (0.74 to 0.91) | 0.60 (0.552 to 0.65) | |
| 60- | 1.04 (0.87 to 1.21) | 0.52 (0.45 to 0.58) | 0.70 (0.60 to 0.81) | 0.89 (0.80 to 0.97) | 0.90 (0.79 to 1.01) | 0.69 (0.64 to 0.74) | |
| Smoking status | Non-smoker | Reference | Reference | Reference | Reference | Reference | Reference |
| Past smoker | 0.03 (−0.07 to 0.14) | 0.07 (0.02 to 0.11) | 0.02 (−0.12 to 0.16) | −0.03 (−0.10 to 0.04) | 0.06 (−0.02 to 0.1) | −0.03 (−0.07 to 0.01) | |
| Current smoker | 0.17 (0.06 to 0.27) | 0.48 (0.42 to 0.53) | 0.11 (−0.03 to 0.26) | 0.25 (0.14 to 0.35) | 0.18 (0.09 to 0.26) | 0.39 (0.35 to 0.44) | |
| Alcohol | None | Reference | Reference | Reference | Reference | Reference | Reference |
| Mild | 0.06 (−0.05 to 0.17) | −0.02 (−0.07 to 0.04) | 0.10 (0.05 to 0.16) | 0.01 (−0.04 to 0.06) | 0.09 (0.03 to 0.14) | −0.01 (−0.05 to 0.03) | |
| Moderate | 0.39 (0.24 to 0.55) | 0.01 (-0.05 to 0.08) | 0.34 (0.22 to 0.46) | 0.08 (−0.02 to 0.17) | 0.39 (0.29 to 0.50) | 0.03 (−0.03 to 0.08) | |
| Heavy | 0.50 (0.373 to 0.63) | 0.11 (0.05 to 0.18) | 0.51 (0.30 to 0.72) | 0.14 (0.01 to 0.27) | 0.52 (0.42 to 0.62) | 0.11 (0.06 to 0.17) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.; Kim, N.-S.; Lee, B.-K.; Oh, I.; Kim, Y. Changes of Atmospheric and Blood Concentrations of Lead and Cadmium in the General Population of South Korea from 2008 to 2017. Int. J. Environ. Res. Public Health 2019, 16, 2096. https://doi.org/10.3390/ijerph16122096
Ahn J, Kim N-S, Lee B-K, Oh I, Kim Y. Changes of Atmospheric and Blood Concentrations of Lead and Cadmium in the General Population of South Korea from 2008 to 2017. International Journal of Environmental Research and Public Health. 2019; 16(12):2096. https://doi.org/10.3390/ijerph16122096
Chicago/Turabian StyleAhn, Jaeouk, Nam-Soo Kim, Byung-Kook Lee, Inbo Oh, and Yangho Kim. 2019. "Changes of Atmospheric and Blood Concentrations of Lead and Cadmium in the General Population of South Korea from 2008 to 2017" International Journal of Environmental Research and Public Health 16, no. 12: 2096. https://doi.org/10.3390/ijerph16122096
APA StyleAhn, J., Kim, N.-S., Lee, B.-K., Oh, I., & Kim, Y. (2019). Changes of Atmospheric and Blood Concentrations of Lead and Cadmium in the General Population of South Korea from 2008 to 2017. International Journal of Environmental Research and Public Health, 16(12), 2096. https://doi.org/10.3390/ijerph16122096





