Effect of Warming and Elevated O3 Concentration on CO2 Emissions in a Wheat-Soybean Rotation Cropland
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. CO2 Flux Measurement
2.4. Temperature and Soil Moisture Measurement
2.5. Crop Biomass Measurements
2.6. Data Analysis
3. Results
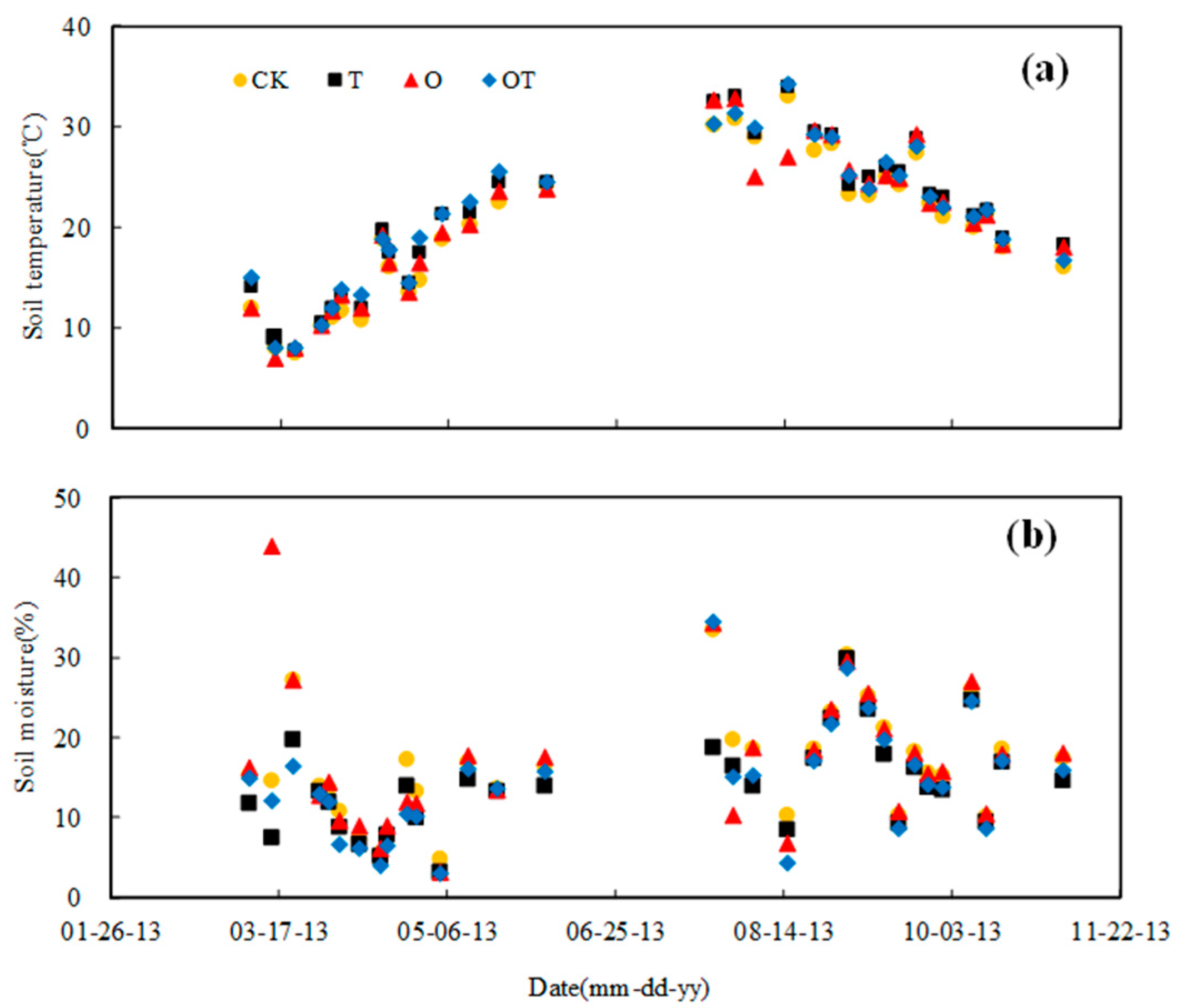
3.1. Changes of Soil Temperature and Moisture
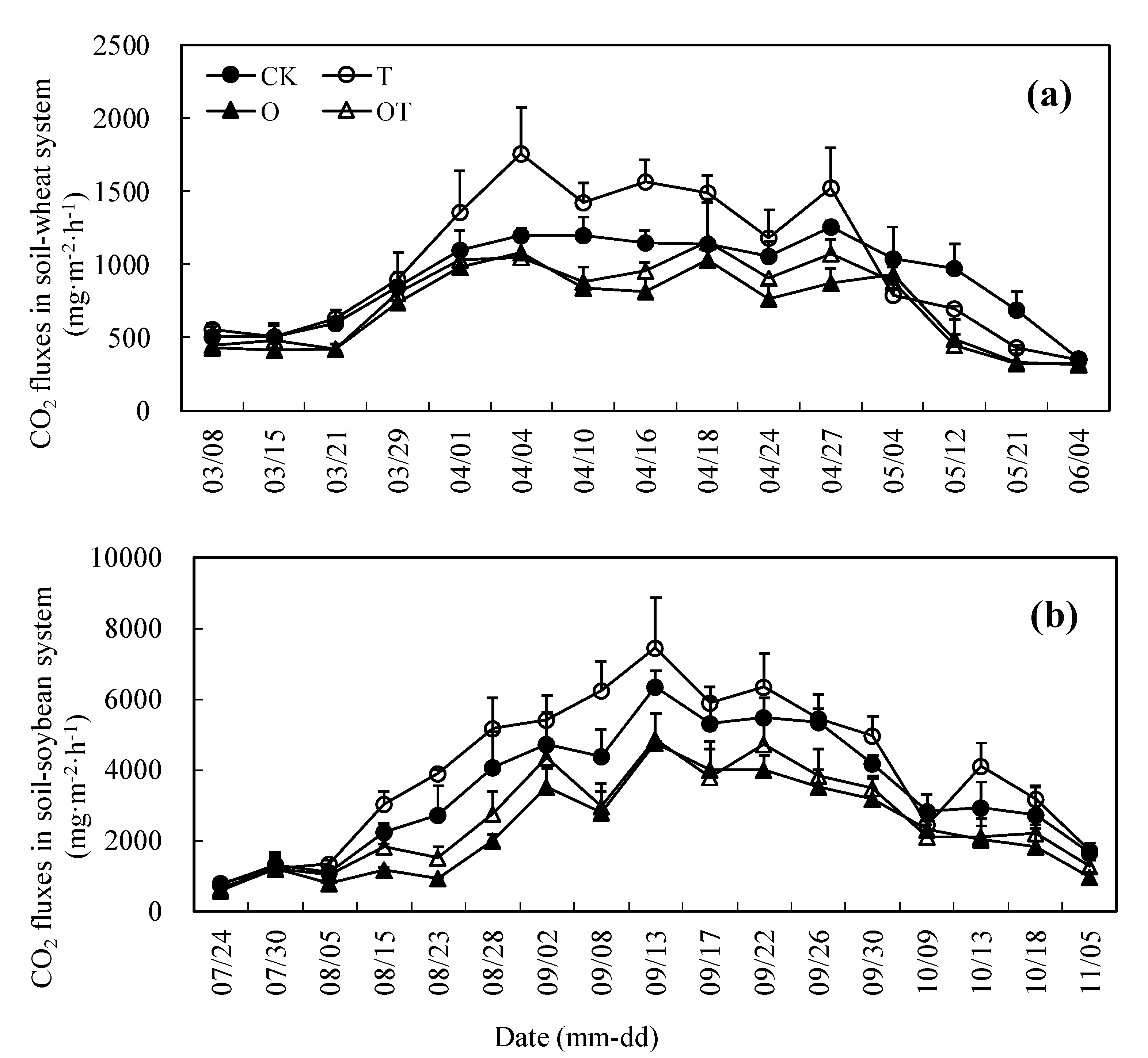
3.2. Seasonal Change of CO2 Emissions in Soil-Crop System
3.3. Effects of Warming and Elevated O3 Concentration on the Mean CO2 Emission from Soil-Crop System
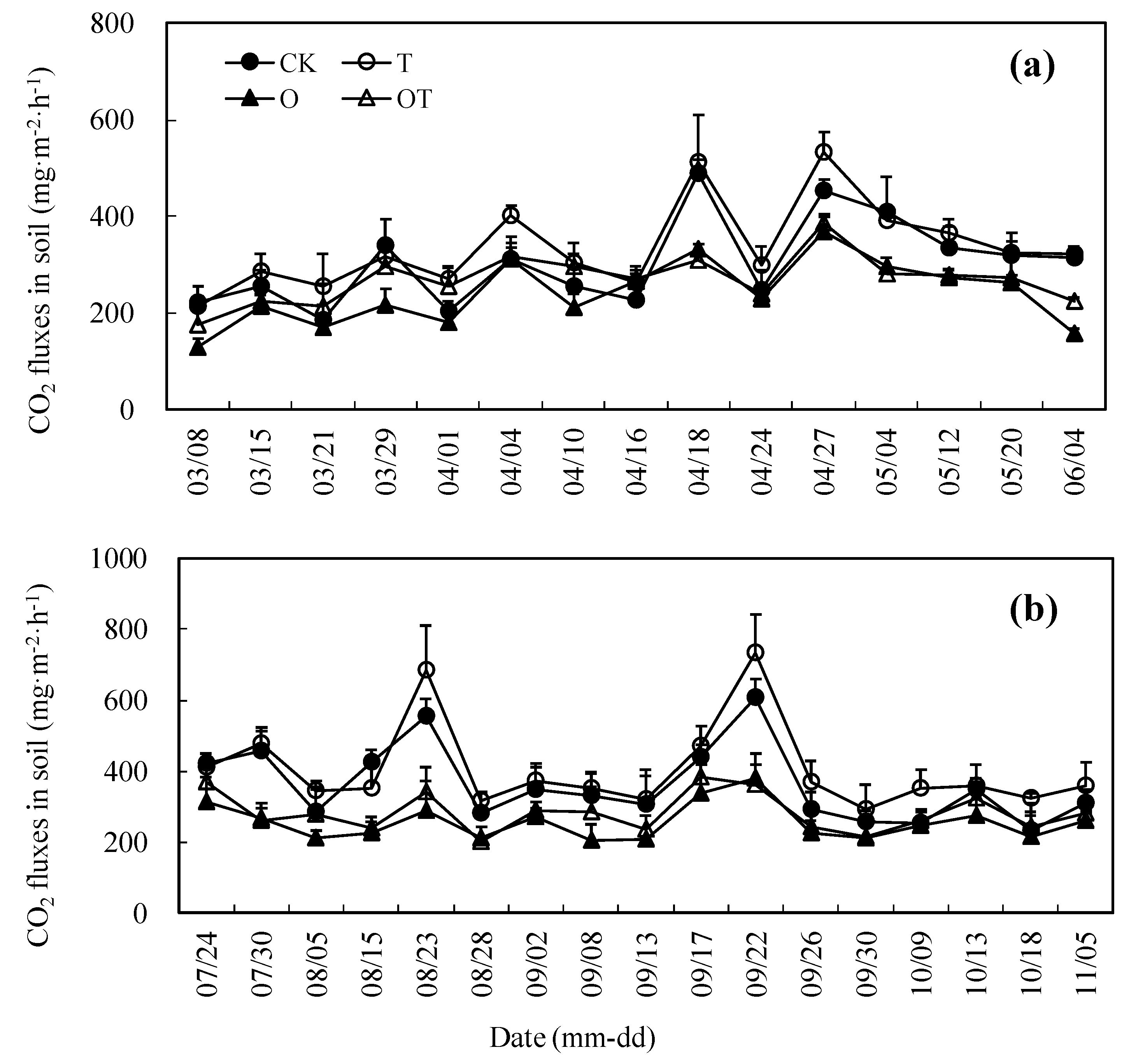
3.4. Seasonal Change of CO2 Emissions from Soil
3.5. Effects of Warming and Elevated O3 Concentration on the Mean CO2 Fluxes from Soil
3.6. Effect of Warming and Elevated O3 Concentration on Cumulative Amount of CO2 Emission from Soil and Soil-Crop System
3.7. Effect of Warming and Elevated O3 Concentration on Biomass of Winter-Wheat and Soybean
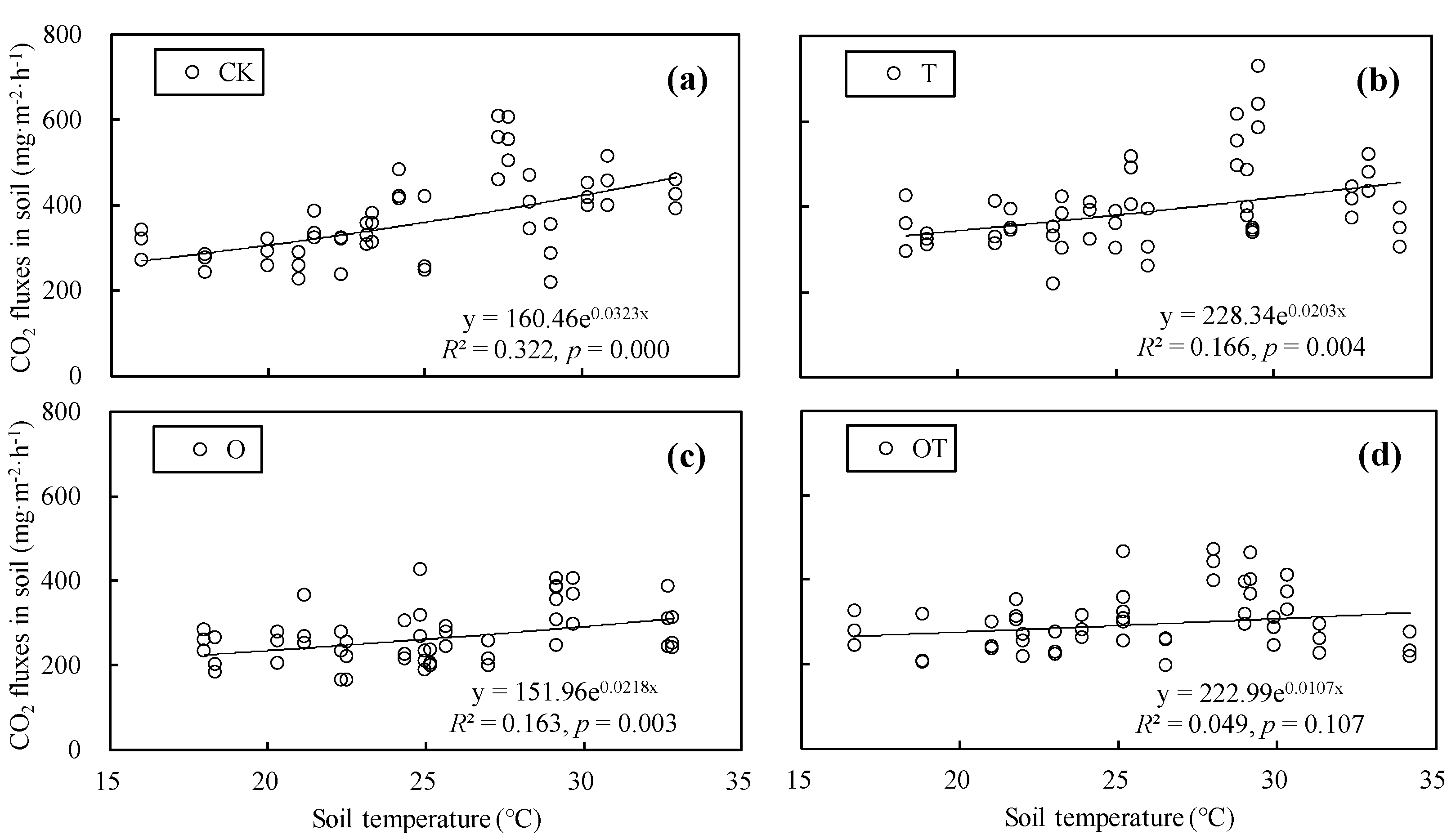
3.8. Effect of Warming and Elevated O3 Concentration on the Temperature Sensitivity of Soil Respiration
4. Discussion
4.1. Warming and Elevated O3 Concentration Affect CO2 Emission Fluxes
4.1.1. Seasonal Change of CO2 Emission Fluxes
4.1.2. Warming Affect CO2 Emission Fluxes
4.1.3. Elevated O3 Concentration Affect CO2 Emission Fluxes
4.1.4. The Combination of Warming and Elevated O3 Concentration Affect CO2 Emission Fluxes
4.2. Warming and Elevated O3 Concentration Affect Crop Biomass
4.3. Warming and Elevated O3 Concentration Affect the Temperature Sensitivity of Soil Respiration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; p. 996. [Google Scholar]
- WMO. Greenhouse Gas Bulletin; No. 14; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Kou, T.J.; Cheng, X.H.; Zhu, J.G.; Xie, Z.B. The influence of ozone pollution on CO2, CH4, and N2O emissions from a Chinese subtropical rice-wheat rotation system under free-air O3 exposure. Agr. Ecosyst. Environ. 2015, 204, 72–81. [Google Scholar] [CrossRef]
- Stevenson, D.S.; Young, P.J.; Naik, V.; Lamarque, J.-F.; Shindell, D.T.; Voulgarakis, A.; Skeie, R.B.; Dalsoren, S.B.; Myhre, G.; Berntsen, T.K.; et al. Tropospheric ozone changes, radiative forcing and attribution to emissions in the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP). Atmos. Chem. Phys. 2013, 13, 3063–3085. [Google Scholar] [CrossRef]
- Vingarzan, R. A review of surface ozone background levels and trends. Atmos. Environ. 2004, 33, 3431–3442. [Google Scholar] [CrossRef]
- Jaggard, K.W.; Qi, A.; Ober, E.S. Possible changes to arable crop yields by 2050. Philos. Trans. R. SOC. B 2010, 365, 2835–2851. [Google Scholar] [CrossRef]
- Pandey, A.K.; Ghosh, A.; Agrawal, M.; Agrawal, S.B. Effect of elevated ozone and varying levels of soil nitrogen in two wheat (Triticum aestivum L.) cultivars: Growth, gas–exchange, antioxidant status, grain yield and quality. Ecotox. Environ. Safe. 2018, 158, 59–68. [Google Scholar] [CrossRef]
- Hu, Z.H.; Li, C.Z.; Chen, S.T.; Li, H.M.; Yang, Y.P.; Shen, S.H. Effects of Elevated Ozone Concentration on CO2 Emission from Soil-Winter. Environ. Sci. 2011, 32, 46–50. [Google Scholar]
- Fiscus, E.L.; Booker, E.L.; Burkey, K.O. Crop responses to ozone: Uptake modes of action, carbon assimilation and partitioning. Plant Cell Environ. 2005, 28, 997–1011. [Google Scholar] [CrossRef]
- Manning, W.J. Establishing a cause and effect relationship for ambient ozone exposure and tree growth in the forest: Progress and an experimental approach. Environ. Pollut. 2005, 137, 443–453. [Google Scholar] [CrossRef]
- Koponen, H.T.; Flöjt, L.; Martikainen, P.J. Nitrous oxide emission from agricultural soils at low temperatures: A laboratory microcosm study. Soil. Biol. Biochem. 2004, 36, 757–766. [Google Scholar] [CrossRef]
- Zhong, Q.C.; Du, Q.; Gong, J.N.; Zhang, C.; Wang, K.Y. Effects of in situ experimental air warming on the soil respiration in a coastal salt marsh reclaimed for agriculture. Plant Soil 2013, 371, 487–502. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Davidson, E.A.; Trumbore, S.E.; Amundsn, R. Soil warming and organic carbon content. Nature 2000, 408, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.J.; Xiao, J.; Li, Y.F.; Chen, Z.; Cheng, X.Y.; Zhao, C.Z.; Liu, Q. Warming effects on root morphological and physiological traits: The potential consequences on soil C dynamics as altered root exudation. Agric. Fore. Meteorol. 2013, 180, 287–296. [Google Scholar] [CrossRef]
- Wan, S.Q.; Norby, R.J.; Ledford, J.; Weltzin, J.F. Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Glob. Chang. Biol. 2007, 13, 2411–2424. [Google Scholar] [CrossRef]
- Kanerva, T.; Regina, K.; Rämä, K.; Ojanperä, K.; Manninen, S. Fluxes of N2O, CH4 and CO2 in a meadow ecosystem exposed to elevated ozone and carbon dioxide for three years. Environ. Pollut. 2007, 145, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.R.; Mulchi, C.L.; Ali, A.A. Interactions of tropospheric CO2 and O3 enrichments and moisture variations on microbial biomass and respiration in soil. Glob. Chang. Biol. 2000, 6, 255–265. [Google Scholar] [CrossRef]
- Li, J.L.; Mahalov, A.; Hydec, P. Simulating the effects of chronic ozone exposure on hydrometeorology and crop productivity using a fully coupled crop, meteorology and air quality modeling system. Agri. Forest Meteorol. 2018, 260, 287–299. [Google Scholar] [CrossRef]
- Changey, F.; Bagard, M.; Souleymane, M.; Lerch, T.Z. Cascading effects of elevated ozone on wheat rhizosphere microbial communities depend on temperature and cultivar sensitivity. Environ. Pollut. 2018, 242, 113–125. [Google Scholar] [CrossRef]
- King, J.S.; Preziger, K.S.; Zak, D.R.; Sober, J.; Isebrands, J.G.; Dickson, R.E.; Hendrey, G.R.; Karnosky, D.F. Fine-root biomass and fluxes of soil carbon in young stands of paper birch and trembling aspen as affected by elevated atmospheric CO2 and tropospheric O3. Oecologia 2001, 128, 237–250. [Google Scholar] [CrossRef]
- Andersen, C.P. Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Sami, K.M.; Jaana, K.H.; Riikka, R.; Paivi, T.; Sanna, S.; Jouko, S.; Toini, H.; Perttij, M. Long-term ozone effects on vegetation, microbial community and methane dynamics of boreal peatland microcosms in open-field conditions. Glob. Chang. Biol. 2008, 14, 1891–1903. [Google Scholar]
- Chen, Z.; Wang, X.K.; Yao, F.F.; Zheng, F.X.; Feng, Z.Z. Elevated ozone changed soil microbial community in a rice paddy. Soil Biol. Biochem. 2010, 74, 829–837. [Google Scholar] [CrossRef]
- Lu, Y.; Conrad, R. In situ stable isotope probing of methanogenic archaea in the rice rhizosphere. Science 2005, 309, 1088–1090. [Google Scholar] [CrossRef]
- Xiong, J.; Peng, F.; Sun, H.; Zhang, H.; Xue, X.; Chu, H. Divergent responses of soil fungi functional groups to short-term warming. Microb. Ecol. 2014, 68, 708–715. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, S.Q.; Luo, Y.Q. Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Glob. Chang. Biol. 2007, 13, 761–775. [Google Scholar]
- Liu, W.X.; Zhang, Z.; Wan, S.Q. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Glob. Chang. Biol. 2009, 15, 184–195. [Google Scholar] [CrossRef]
- Verburg, P.S.J.; Larsen, J.; Johnson, D.W.; Schorran, D.E.; Arnone, J.A. Impacts of an anomalously warm year on soil CO2 efflux in experimentally manipulated tallgrass prairie ecosystems. Glob. Chang. Biol. 2005, 11, 1720–1732. [Google Scholar] [CrossRef]
- Reth, S.; Graf, W.; Reichstein, M.; Munch, J.C. Sustained stimulation of soil respiration after 10 years of experimental warming. Environ. Res. Lett. 2009, 4, 1–5. [Google Scholar] [CrossRef]
- Edwards, N.T. Root and soil respiration responses to ozone in Pinus taeda L. seedlings. New Phytol. 1991, 118, 315–321. [Google Scholar] [CrossRef]
- Coleman, M.D.; Dickson, R.E.; Isebrands, J.G.; Karnosky, D.F. Root growth and physiology of potted and field-grown trembling aspen exposed to ozone. Tree Physiol. 1996, 16, 145–152. [Google Scholar] [CrossRef]
- Pregitzer, K.; Loya, W.; Kubiske, M.; Zak, D. Soil respiration in northern forests exposed to elevated atmospheric carbon dioxide and ozone. Oecologia 2006, 148, 503–516. [Google Scholar] [CrossRef]
- Chen, S.T.; Zhang, Y.; Chen, H.S.; Hu, Z.H. Effects of elevated O3 on soil respiration in a winter wheat-soybean rotation cropland. Soil Res. 2012, 50, 500–506. [Google Scholar] [CrossRef]
- Andersen, C.P.; Scagel, C.F. Nutrient availability alters belowground respiration of ozone-exposed ponderosa pine. Tree Physiol. 1997, 17, 377–387. [Google Scholar] [CrossRef]
- Scagel, C.F.; Andersen, C.P. Seasonal changes in root and soil respiration of ozone-exposed ponderosa pine (Pinus ponderosa) grown in different substrates. New Phytol. 1997, 136, 627–643. [Google Scholar] [CrossRef]
- Kasurinen, A.; Gonzales, P.K.; Riikonen, J.; Vapaavuori, E.; Holopainen, T. Soil CO2 efflux of two silver birch clones exposed to elevated CO2 and O3 levels during three growing seasons. Glob. Chang. Biol. 2004, 10, 1654–1665. [Google Scholar] [CrossRef]
- Tingey, D.T.; Johnson, M.G.; Lee, E.H.; Wise, C.; Waschmann, R.; Olszyk, D.M.; Watrud, L.S.; Donegan, K.K. Effects of elevated CO2 and O3 on soil respiration under ponderosa pine. Soil Biol. Biochem. 2006, 38, 1764–1778. [Google Scholar] [CrossRef]
- Liu, X.J.; Liu, H.; Zhao, P.; Sun, G.C.; Lin, Y.B.; Rao, X.Q.; Wang, Y.S. Characteristics of CO2, CH4 and N2O emissions from winter-fallowed paddy fields in hilly areas of South China. Front. Agric. China. 2007, 1, 418–423. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wang, Y.H. Quick measurement of CH4, CO2 and N2O emission from a short-plant ecosystem. Adv. Atmos. Sci. 2003, 20, 842–844. [Google Scholar]
- Daniel, L.K.; Burger, J.A.; Edwards, G.S. Estimating root respiration, microbial respiration in the rhizosphere, and root-free soil respiration in forest soils. Soil Biol. Biochem. 1998, 30, 961–968. [Google Scholar]
- Lu, X.Y.; Fan, J.H.; Yan, Y.; Wang, X.D. Responses of Soil CO2 Fluxes to Short-Term Experimental Warming in Alpine Steppe Ecosystem, Northern Tibet. PLoS ONE 2013, 8, e59054. [Google Scholar] [CrossRef]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.; Mitchell, M.; Hartley, A.; Cornelissen, J.; Gurevitch, J. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef]
- Bergner, B.; Johntone, J.; Treseder, K.K. Experimental warming and burn severity alter soil CO2 flux and soil functional groups in a recently burned boreal forest. Glob. Chang. Biol. 2004, 10, 1996–2004. [Google Scholar] [CrossRef]
- Melillo, J.M.; Steudler, P.A.; Aber, J.D.; Newkirk, K.; Lux, H.; Bowles, F.P.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Wan, S.Q.; Hui, D.F.; Wallace, L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 2001, 413, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Ren, W.W.; Ding, L.L.; Liu, Z.F.; Fang, C.M. Responses of a rice-wheat rotation agroecosystem to experimental warming. Ecol. Res. 2013, 28, 959–967. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.T.; Wang, L.X.; Shen, X.S.; Hu, Z.H.; Shi, Y.S. Effects of elevated ozone concentration on soil respiration, nitrification and denitrification in a winter wheat farmland. Environ. Sci. 2010, 31, 2988–2994. [Google Scholar]
- Lütz, C.; Anegg, S.; Gerant, D.; Alaoui-Sossé, B.; Gérard, J.; Dizengremel, P. Beech trees exposed to high CO2 and to simulated summer ozone levels: Effects on photosynthesis, chloroplast components and leaf enzyme activity. Physiol. Plantarum 2000, 109, 252–259. [Google Scholar] [CrossRef]
- Shah, N.H.; Paulsen, G.M. Interaction of drought and high temperature on photosynthesis and grainfilling of wheat. Plant Soil 2003, 257, 219–226. [Google Scholar] [CrossRef]
- Tacarindua, C.R.P.; Shiraiwa, T.; Homma, K.; Kumagai, E.; Sameshima, R. The response of soybean seed growth characteristics to increased temperature under nearfield conditions in a temperature gradient chamber. Field Crop. Res. 2012, 131, 26–31. [Google Scholar] [CrossRef]
- Wu, Z.T.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Chen, S.T.; Liu, Y.; Zhai, X.Y.; Hu, Z.H. Experimental Warming Effects on Soil Respiration, Nitrification, and Denitrification in a Winter Wheat-Soybean Rotation Cropland. Commun. Soil Sci. Plan. 2017, 48, 148–161. [Google Scholar] [CrossRef]
- Wada, N.; Shimono, M.; Miyamoto, M.; Kojima, S. Warming effects on shoot developmental growth and biomass production in sympatric evergreen alpine dwarf shrubs Empetrum nigrum and Loiseleuria Procumbens. Ecol. Res. 2002, 17, 125–132. [Google Scholar] [CrossRef]
- Walther, G.R.; Biessner, S.; Burga, C.A. Trends in the upward shift of alpine plant. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- Zhang, W.W.; Feng, Z.Z.; Wang, X.K.; Niu, J.F. Elevated ozone negatively affects photosynthesis of current-year leaves but not previous-year leaves in evergreen Cyclobalanopsis glauca seedlings. Environ. Pollut. 2014, 184, 676–681. [Google Scholar] [CrossRef]
- Feng, Z.Z.; Wang, S.G.; Szantoi, Z.; Chen, S.; Wang, X.K. Protection of plants from ambient ozone by applications of ethylenediurea (EDU): A meta-analytic review. Environ. Pollut. 2010, 158, 3236–3242. [Google Scholar] [CrossRef]
- Li, L.; William, J.M.; Tong, L.; Wang, X.K. Chronic drought stress reduced but not protected Shantung maple (Acer truncatum Bunge) from adverse effects of ozone (O3) on growth and physiology in the suburb of Beijing, China. Environ. Pollut. 2015, 201, 34–41. [Google Scholar] [CrossRef]
- Han, M.G.; Shi, B.K.; Jin, G.Z. Temporal variations of soil respiration at multiple timescales in a spruce-fir valley forest, northeastern China. J. Soils Sediments 2016, 16, 2385–2394. [Google Scholar] [CrossRef]
- Giardina, C.P.; Litton, C.M.; Crow, S.E.; Asner, G.P. Warming-related increases in soil CO2 efflux are explained by increased below-ground carbon flux. Nat. Clim. Chang. 2014, 4, 822–827. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J.B. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 2001, 33, 55–165. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]


| Date | Winter-Wheat | Date | Soybean |
|---|---|---|---|
| 2012-11-18 | Sow, fertilization 40 g/m2 (N 26%, P2O5 11.5%) | 2013-06-29 | Sow, fertilization 32 g/m2 (N 18%, P2O5 46%) |
| 2012-11-20 | Seedling | 2013-07-04 | Seedling |
| 2013-01-05 | Fertilization (urea, 19 g/m2) | 2013-07-14 | Trefoil |
| 2013-02-06 | Turning green | 2013-07-24 | Branching |
| 2013-02-26 | Fertilization (urea, 19 g/m2) | 2013-08-15 | Flowering |
| 2013-03-21 | Elongation | 2013-09-08 | Pod |
| 2013-03-26 | Booting | 2013-09-22 | Grain filling |
| 2013-04-16 | Heading | 2013-11-05 | Harvest |
| 2013-04-18 | Flowering | ||
| 2013-04-27 | Grain filling | ||
| 2013-05-20 | Maturity | ||
| 2013-06-04 | Harvest |
| Crop | Growth Stages | Treatments | The Mean CO2 Emission Fluxes |
|---|---|---|---|
| Wheat | Turning green | CK | 535.00 ± 3.43 |
| T | 565.40 ± 25.51 (+5.7%) | ||
| O | 423.06 ± 53.70 (−20.9%) ** | ||
| OT | 453.46 ± 10.13 (−15.2%) ** | ||
| Elongation-Booting | CK | 1050.57 ± 64.46 | |
| T | 1339.72 ± 309.56 (+27.5%) * | ||
| O | 936.21 ± 62.18 (−10.9%) | ||
| OT | 964.46 ± 35.38 (−8.2%) | ||
| Heading-flowering | CK | 1112.87 ± 25.02 | |
| T | 1412.70 ± 5.08 (+26.9%) ** | ||
| O | 870.94 ± 17.08 (−21.7%) * | ||
| OT | 1008.19 ± 56.73 (−9.4%) | ||
| Grain filling-maturity | CK | 864.35 ± 6.77 | |
| T | 759.61 ± 64.72 (−12.1%) * | ||
| O | 588.22 ± 78.84 (−31.9%) ** | ||
| OT | 609.01 ± 31.68 (−29.5%) ** | ||
| Whole growth stage | CK | 890.70 ± 23.21 | |
| T | 1019.36 ± 85.93 (+14.4%) * | ||
| O | 704.61 ± 13.32 (−20.9%) ** | ||
| OT | 758.78 ± 33.48 (−14.8%) * | ||
| Soybean | Branching | CK | 1046.71 ± 105.45 |
| T | 1123.03 ± 101.88 (+7.3%) | ||
| O | 881.31 ± 71.99 (−15.8%) | ||
| OT | 952.61 ± 78.37 (−9.0%) | ||
| Flowering-pod | CK | 4267.64 ± 307.79 | |
| T | 5305.85 ± 222.64 (+24.3%) ** | ||
| O | 2740.76 ± 462.05 (−35.8%) ** | ||
| OT | 342.75 ± 16.85 (−92.0%) ** | ||
| Grain filling-maturity | CK | 3916.66 ± 2.00 | |
| T | 4522.57 ± 56.23 (+15.5%) ** | ||
| O | 2745.45 ± 474.87 (−29.9%) ** | ||
| OT | 3095.37 ± 111.01 (−21.0%) ** | ||
| Whole growth stage | CK | 3077.00 ± 68.11 | |
| T | 3650.48 ± 59.00 (+18.6%) ** | ||
| O | 2122.51 ± 222.35 (−31.0%) ** | ||
| OT | 2399.88 ± 90.89 (−22.0%) ** |
| Crop | Growth Stages | Treatments | The Mean CO2 Emission Fluxes |
|---|---|---|---|
| Wheat | Turning green | CK | 221.44 ± 1.23 |
| T | 252.77 ± 20.20 (+14.1%) | ||
| O | 171.79 ± 23.51 (−22.4%) * | ||
| OT | 206.07 ± 25.12 (−6.9%) | ||
| Elongation-Booting | CK | 286.15 ± 19.28 | |
| T | 329.79 ± 3.38 (+15.3%) ** | ||
| O | 237.31±0.16 (−17.1%) ** | ||
| OT | 289.53 ± 0.59 (+1.2%) | ||
| Heading-flowering | CK | 321.56 ± 28.61 | |
| T | 380.27 ± 57.63 (+18.3%) * | ||
| O | 276.23 ± 30.20 (−14.1%) | ||
| OT | 273.97 ± 5.37 (−14.8%) | ||
| Grain filling-maturity | CK | 366.49 ± 10.11 | |
| T | 387.41 ± 4.31 (+5.7%) * | ||
| O | 271.90 ± 3.48 (−25.8%) ** | ||
| OT | 289.02 ± 6.95 (−21.1%) ** | ||
| Whole growth stage | CK | 298.91 ± 9.14 | |
| T | 337.56 ± 19.23 (+12.9%) ** | ||
| O | 239.30 ± 0.84 (−19.9%) ** | ||
| OT | 264.65 ± 6.53 (−11.5%) ** | ||
| Soybean | Branching | CK | 365.61 ± 29.50 |
| T | 400.23 ± 52.07 (+9.5%) | ||
| O | 289.84 ± 39.23 (−20.7%) * | ||
| OT | 347.85 ± 9.84 (−4.9%) | ||
| Flowering-pod | CK | 395.29 ± 7.85 | |
| T | 430.66 ± 39.70 (+8.9%) | ||
| O | 314.82 ± 15.13 (−20.4%) * | ||
| OT | 342.75 ± 16.85 (−13.3%) * | ||
| Grain filling-maturity | CK | 349.55 ± 36.37 | |
| T | 371.56 ± 22.94 (+6.3%) | ||
| O | 275.33 ± 38.17 (−21.2%) ** | ||
| OT | 309.10 ± 0.51 (−11.6%) | ||
| Whole growth stage | CK | 370.15 ± 4.90 | |
| T | 400.82 ± 38.24 (+8.3%) | ||
| O | 293.33 ± 30.85 (−20.8%) * | ||
| OT | 333.23 ± 2.17 (−10.0%) |
| Crop | Growth Stages | Treatments | Shoot Biomass | Root Biomass | Total Biomass |
|---|---|---|---|---|---|
| Wheat | Elongation-booting | CK | 12.10 ± 2.14 | 1.25 ± 0.13 | 13.35 ± 2.27 |
| T | 18.05 ± 1.49 (+49.2%) ** | 1.28 ± 0.18 (+2.4%) | 19.33 ± 1.32 (+44.8) ** | ||
| O | 10.23 ± 2.19 (−15.5%) | 1.04 ± 0.08 (−16.8%) | 11.27 ± 2.25 (−15.6%) | ||
| OT | 16.56 ± 2.70 (+36.9%) * | 1.02 ± 0.05 (−18.4%) * | 17.58 ± 2.66 (31.7%) * | ||
| Heading-flowering | CK | 15.27 ± 1.59 | 1.74 ± 0.28 | 17.01 ± 1.74 | |
| T | 20.38 ± 1.57 (+33.5) * | 1.95 ± 0.40 (+12.1%) | 22.33 ± 1.97 (+31.3%) * | ||
| O | 14.80 ± 2.74 (−3.1%) | 1.47 ± 0.29 (−15.5%) | 16.27 ± 2.92 (−4.4%) | ||
| OT | 20.34 ± 1.58 (+33.2%) * | 1.24 ± 0.58 (−28.7%) | 21.58 ± 2.02 (+26.9%) * | ||
| Grain filling | CK | 24.08 ± 1.55 | 2.50 ± 0.43 | 26.58 ± 1.87 | |
| T | 27.97 ± 1.76 (+16.2%) * | 3.07 ± 0.43 (+22.8%) | 31.04 ± 2.06 (+16.8%) ** | ||
| O | 18.62 ± 1.79 (−22.7%) ** | 2.05 ± 0.30 (−18.0%) | 20.67 ± 1.49 (−22.2%) ** | ||
| OT | 24.86 ± 2.39 (+3.2%) | 2.33 ± 0.71 (−6.8%) | 27.19 ± 2.15 (+2.3%) | ||
| Maturity | CK | 35.54 ± 1.89 | 2.47 ± 0.41 | 38.01 ± 1.68 | |
| T | 29.40 ± 2.36 (−17.3%) * | 0.74 ± 0.18 (−70%) ** | 30.14 ± 2.41 (−20.7%) ** | ||
| O | 27.37 ± 1.86 (−23.0%) ** | 1.53 ± 0.14 (−38.1%) ** | 28.90 ± 1.75 (−24.0%) ** | ||
| OT | 26.04 ± 3.09 (−26.7%) ** | 0.97 ± 0.16 (−60.7) ** | 27.01 ± 3.21 (−28.9) ** | ||
| Soybean | Maturity | CK | 83.90 ± 8.28 | 4.13 ± 0.45 | 88.03 ± 8.56 |
| T | 74.40 ± 10.20 (−11.3%) | 4.07 ± 0.42 (−1.5%) | 78.47 ± 10.03 (−10.9%) | ||
| O | 52.71 ± 14.10 (−37.2%) ** | 2.86 ± 0.64 (−30.8%) | 55.57±14.69 (−36.9%)* | ||
| OT | 50.37 ± 13.29 (−40.0%) ** | 3.30 ± 0.60 (−20.1%) | 53.67±13.89 (−39.0)** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, Z.; Islam, A.R.M.T.; Chen, S.; Shang, D.; Xue, Y. Effect of Warming and Elevated O3 Concentration on CO2 Emissions in a Wheat-Soybean Rotation Cropland. Int. J. Environ. Res. Public Health 2019, 16, 1755. https://doi.org/10.3390/ijerph16101755
Wang Y, Hu Z, Islam ARMT, Chen S, Shang D, Xue Y. Effect of Warming and Elevated O3 Concentration on CO2 Emissions in a Wheat-Soybean Rotation Cropland. International Journal of Environmental Research and Public Health. 2019; 16(10):1755. https://doi.org/10.3390/ijerph16101755
Chicago/Turabian StyleWang, Yuanyuan, Zhenghua Hu, A. R. M. Towfiqul Islam, Shutao Chen, Dongyao Shang, and Ying Xue. 2019. "Effect of Warming and Elevated O3 Concentration on CO2 Emissions in a Wheat-Soybean Rotation Cropland" International Journal of Environmental Research and Public Health 16, no. 10: 1755. https://doi.org/10.3390/ijerph16101755
APA StyleWang, Y., Hu, Z., Islam, A. R. M. T., Chen, S., Shang, D., & Xue, Y. (2019). Effect of Warming and Elevated O3 Concentration on CO2 Emissions in a Wheat-Soybean Rotation Cropland. International Journal of Environmental Research and Public Health, 16(10), 1755. https://doi.org/10.3390/ijerph16101755






