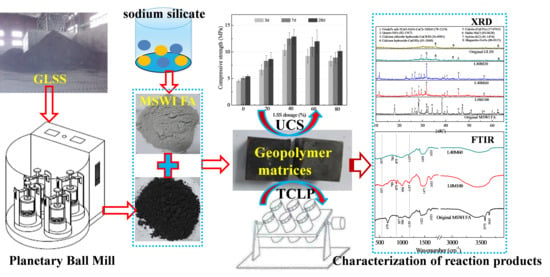
Cotreatment of MSWI Fly Ash and Granulated Lead Smelting Slag Using a Geopolymer System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
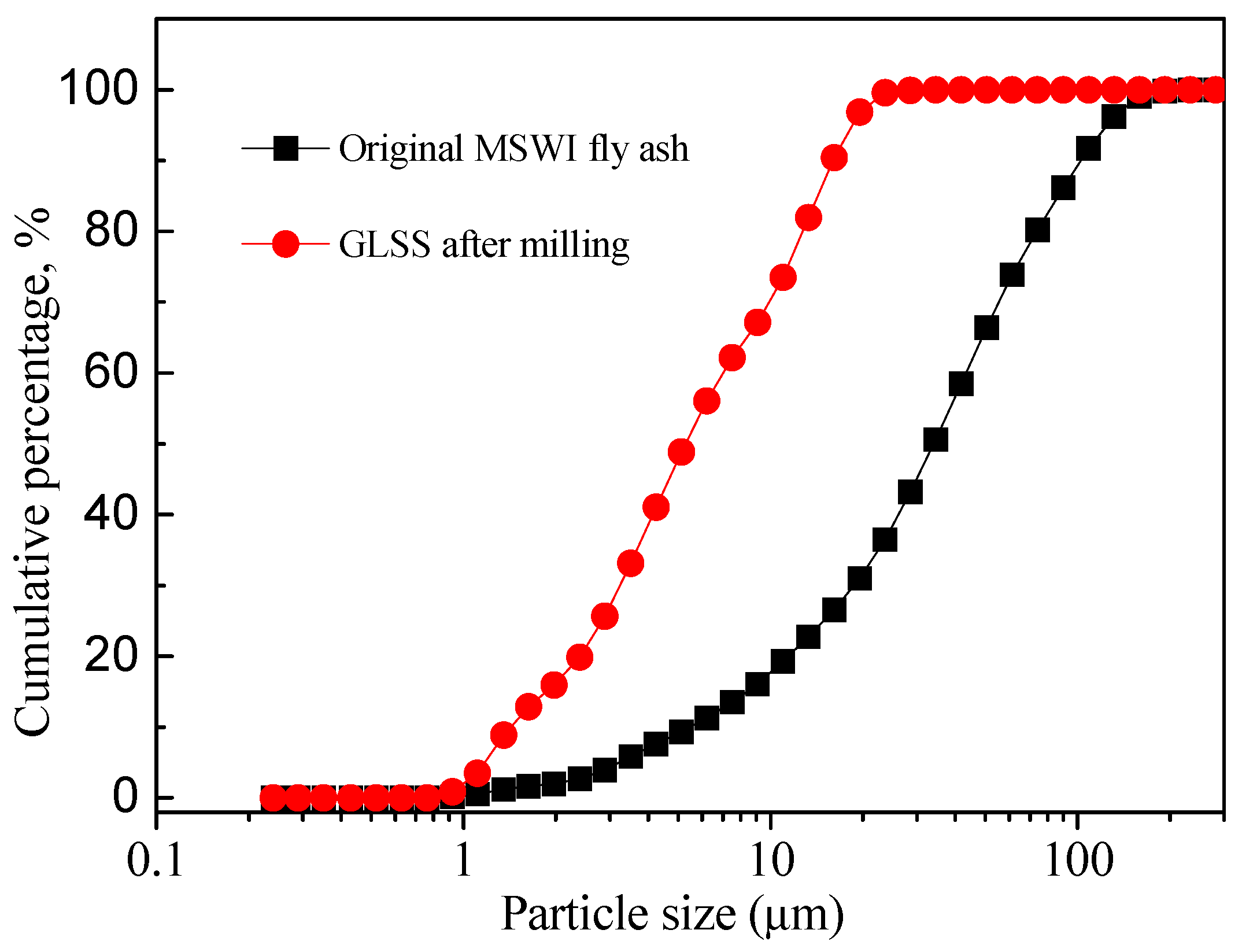
2.2. Experimental Procedures
2.3. Tests
3. Results and Discussion
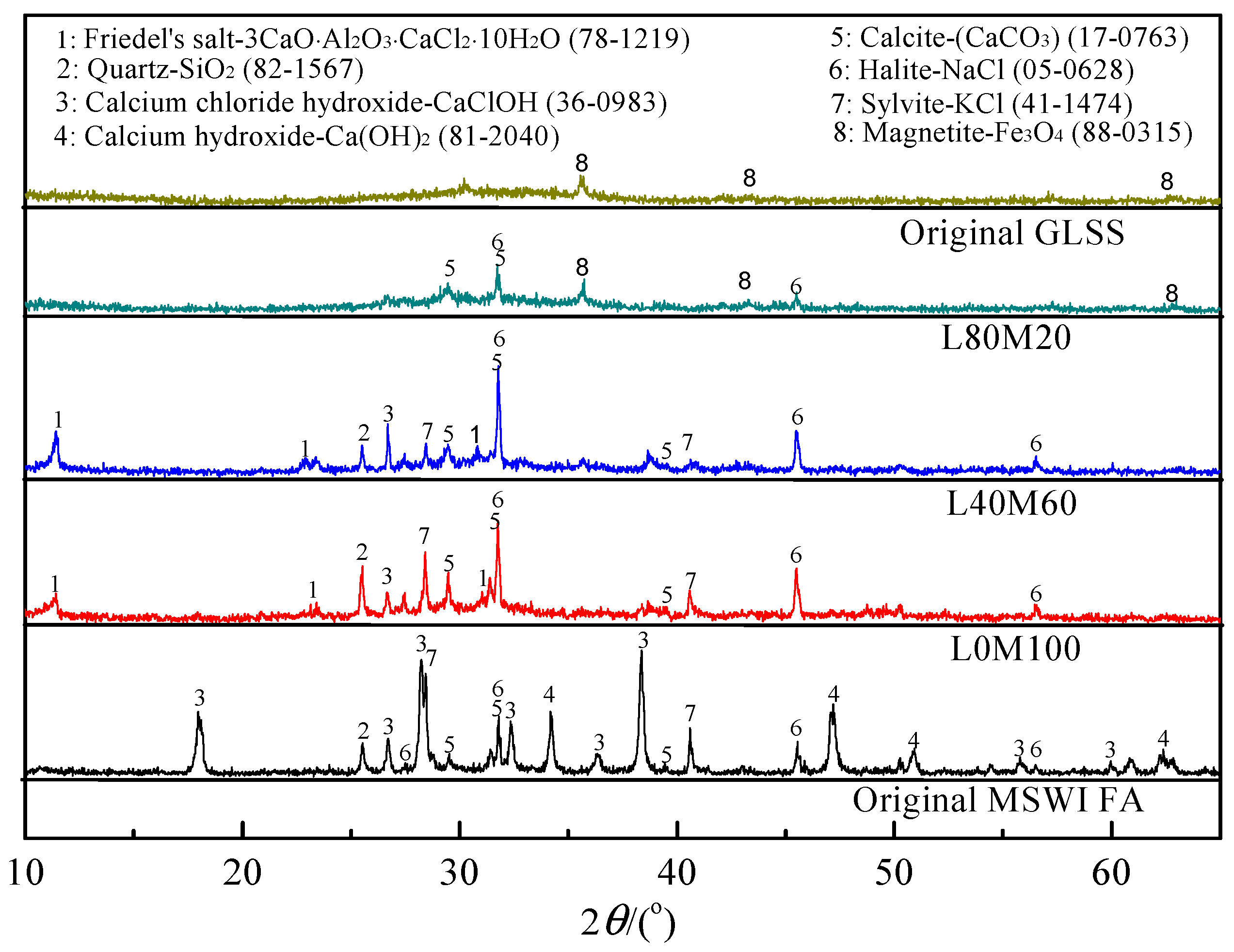
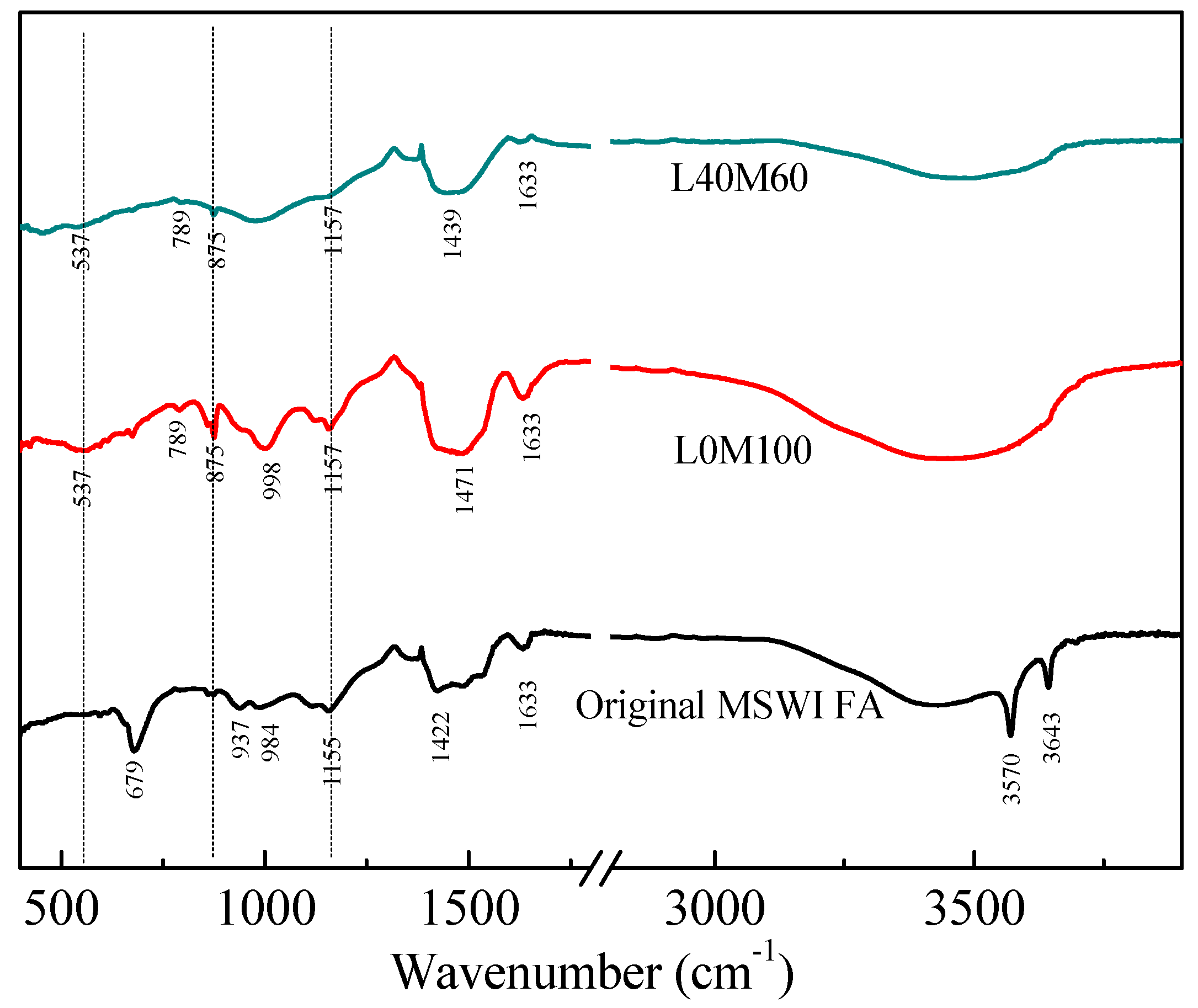
3.1. Characterization of the Reaction Products in the Geopolymer Binder
3.2. Compressive Strength
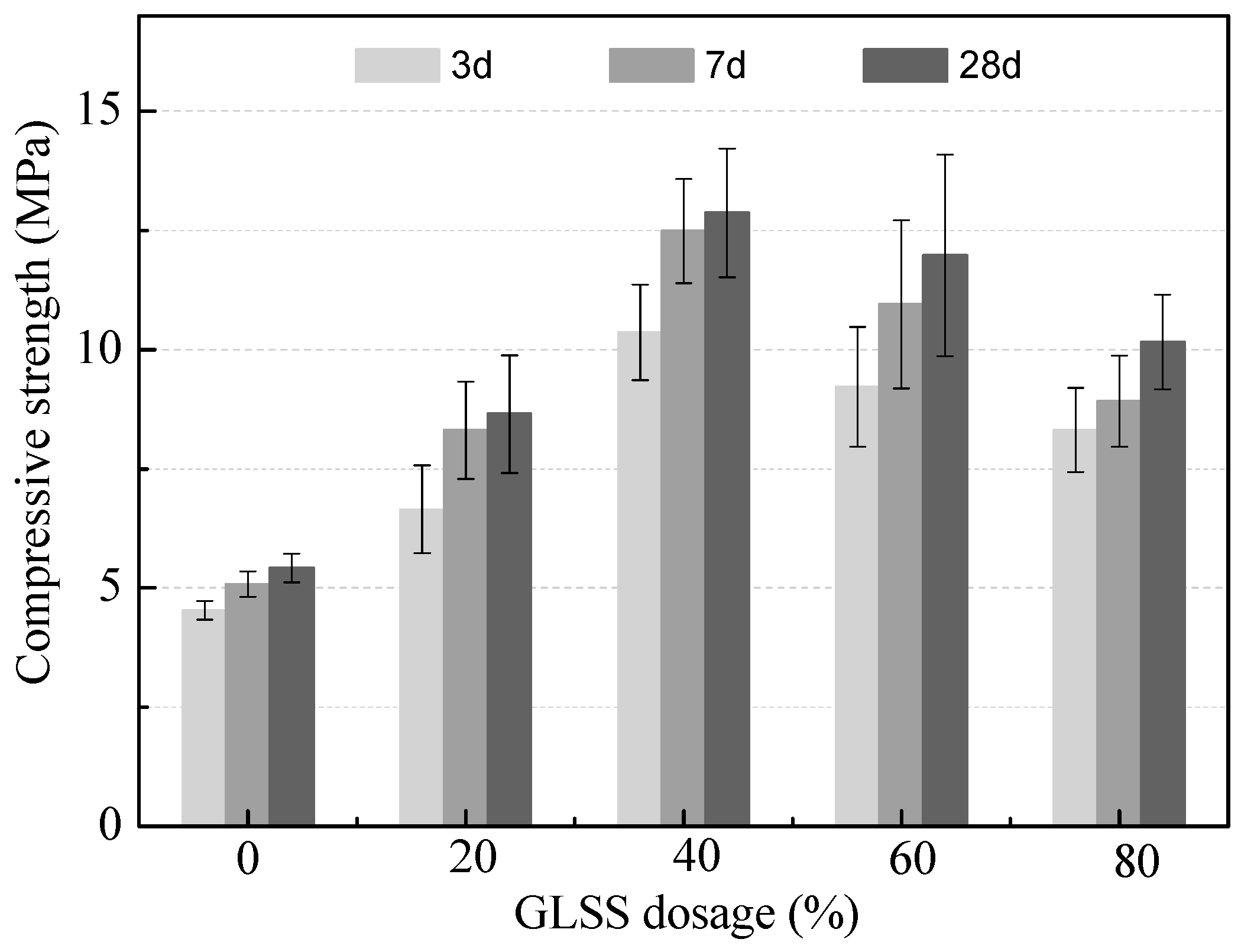
3.2.1. Effect of GLSS Addition
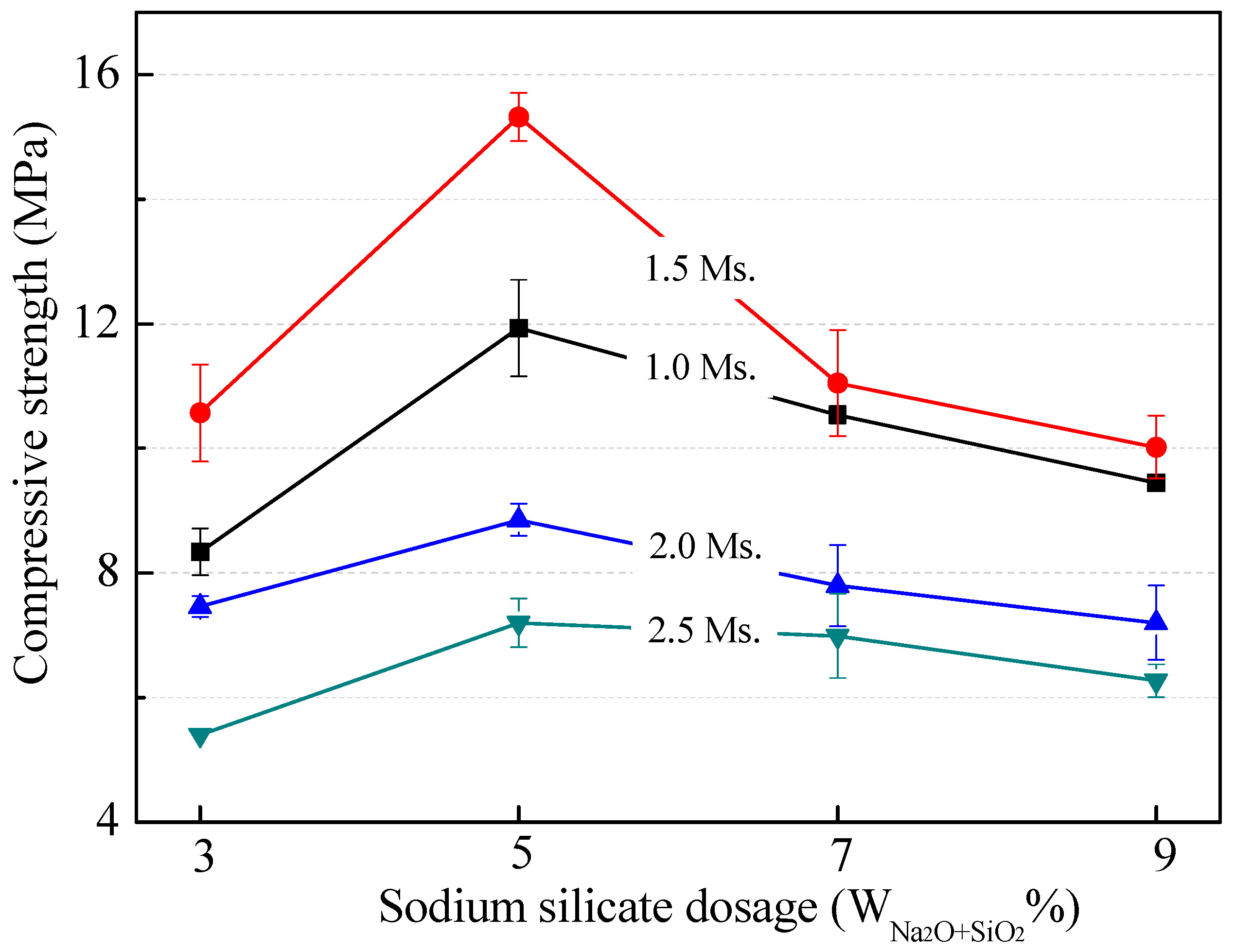
3.2.2. Effect of Modulus and Dosage of Sodium Silicate
3.3. Other Physical Properties
3.4. Heavy Metals Leachability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Bureau of Statistics of China. China Statistical Yearbook 2016; China Statistical Press: Beijing, China, 2016.
- National Bureau of Statistics of China. China Statistical Yearbook 2004; China Statistical Press: Beijing, China, 2004.
- Hjelmar, O. Disposal strategies for municipal solid waste incineration residues. J. Hazard. Mater. 1996, 47, 345–368. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, L.; Li, G. Chemical stabilization of MSW incinerator fly ashes. J. Hazard. Mater. 2002, 95, 47–63. [Google Scholar]
- Lam, C.H.K.; Ip, A.W.M.; Barford, J.P.; McKay, G. Use of incineration MSW ash: A review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Sirini, P.; Testa, F. Properties of Portland cement-stabilised MSWI fly ashes. J. Hazard. Mater. 2001, 88, 123–138. [Google Scholar] [CrossRef]
- Liu, D.G.; Min, X.B.; Chai, L.Y.; Ke, Y.; Liang, Y.J.; Li, Y.C.; Yao, L.W.; Wang, Z.B. Co-treatment of flotation waste, neutralization sludge and arsenic-containing gypsum sludge from copper smelting: Solidification/stabilization of arsenic and heavy metals with minimal cement clinker. Environ. Sci. Pollut. Res. 2018, 25, 7600–7607. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Min, X.B.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liyang, W.J. Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals. J. Environ. Manag. 2016, 181, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Anamul, M.H.; Rahman, J.; Tanvir, M. Zn and Ni of bottom ash as a potential diffuse pollutant and their application as “fine aggregate”. J. Civ. Eng. Res. 2012, 2, 64–72. [Google Scholar] [CrossRef]
- Hájková, P. Kaolinite claystone-based geopolymer materials: Effect of chemical composition and curing conditions. Minerals 2018, 8, 444. [Google Scholar] [CrossRef]
- Singh, N.B. Fly ash-based geopolymer binder: A future construction material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef]
- Li, Y.; Min, X.; Ke, Y.; Liu, D.; Tang, C. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, H.; Liu, J. Rational utilization of fine unclassified tailings and activated blast furnace slag with high calcium. Minerls 2018, 7, 48. [Google Scholar] [CrossRef]
- Favier, A.; Habert, G.; Roussel, N.; Lacaillerie, J.B.D.E.D. A multinuclear static NMR study of geopolymerisation. Cem. Concr. Res. 2015, 75, 104–109. [Google Scholar] [CrossRef]
- Lancellotti, I.; Kamseu, E.; Michelazzi, M.; Barbieri, L.; Corradi, A.; Leonelli, C. Chemical stability of geopolymers containing municipal solid waste incinerator fly ash. Waste Manag. 2010, 30, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, W.; Shi, Y. The effects of alkaline dosage and Si/Al ratio on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere 2010, 79, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Galiano, Y.L.; Pereira, C.F.; Vale, J. Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. J. Hazard. Mater. 2011, 185, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Chen, Y.; Yang, J.; Liang, S.; Hu, Y.; Xiao, B.; Huang, Q.; Shi, Y.; Hu, J.; Wu, X. Co-disposal of MSWI fly ash and bayer red mud using an one-part geopolymeric system. J. Hazard. Mater. 2016, 318, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Silva, D.P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.Q.; Tan, J.; Yin, Z.L.; Chen, Q.Y.; Liao, Z.; Zhang, P.M.; Liu, Y. Recovery of iron from lead-zinc metallurgical slags by bath smelting. J. Cent. South Univ. 2015, 22, 1256–1263. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Zhang, L.; Liu, F.; Peng, B.; Chai, L.; Liu, D.; Liu, D.; Wang, T.; Liu, H.; et al. Formation mechanism of zinc-doped fayalite (Fe2−xZnxSiO4) slag during copper smelting. J. Hazard. Mater. 2019, 364, 488–498. [Google Scholar] [CrossRef]
- Ettler, V.; Komárková, M.; Jehlička, J.; Coufal, P.; Hradil, D.; Machovič, V.; Delorme, F. Leaching of lead metallurgical slag in citric solutions—Implications for disposal and weathering in soil environments. Chemosphere 2004, 57, 567–577. [Google Scholar] [CrossRef]
- Chai, L.Y.; Shi, M.Q.; Liang, Y.J.; Tang, J.W.; Li, Q.Z. Behavior, distribution and environmental influence of arsenic in a typical lead smelter. J Cent. South Univ. 2015, 22, 1276–1286. [Google Scholar] [CrossRef]
- Ke, Y.; Peng, N.; Xue, K.; Min, X.; Chai, L.; Pan, Q.; Liang, Y.; Xiao, R.; Wang, Y.; Tang, C.; et al. Sulfidation behavior and mechanism of zinc silicate roasted with pyrite. Appl. Surf. Sci. 2018, 435, 1011–1019. [Google Scholar] [CrossRef]
- Yao, L.; Min, X.; Xu, H.; Ke, Y.; Liang, Y.; Yang, K. Hydrothermal treatment of arsenic sulfide residues from arsenic-bearing acid wastewater. Int. J. Environ. Res. Public Health 2018, 15, 1863. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, F.; Shen, T.; Yang, L.; Chen, D.; Luo, J.; Luo, X.; Min, X.; Wang, F. Exceptional adsorption of arsenic by zirconium metal-organic frameworks: Engineering exploration and mechanism insight. J. Colloid Interface Sci. 2019, 539, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Chai, L.Y.; Wang, Y.Y.; Yang, Z.H.; Wang, H.Y.; Wu, X. Potential health risk of arsenic and cadmium in groundwater near Xiangjiang River, China: A case study for risk assessment and management of toxic substances. Environ. Monit. Assess. 2011, 175, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chai, L.; Yang, Z.; Wang, Y.; Wang, H. Identifying sources and assessing potential risk of heavy metals in soils from direct exposure to children in a mine-impacted city, Changsha, China. J. Environ. Qual. 2010, 39, 1616. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.C.; Min, X.B.; Wang, Z.X.; Pang, Z.H.; Liang, Y.J.; Ke, Y. Health and ecological risk assessment of heavy metals pollution in an antimony mining region: A case study from South China. Environ. Sci. Pollut. Res. 2017, 24, 27573–27586. [Google Scholar] [CrossRef]
- Xie, X.D.; Min, X.B.; Chai, L.Y.; Tang, C.J.; Liang, Y.J.; Li, M.; Ke, Y.; Chen, J.; Wang, Y. Quantitative evaluation of environmental risks of flotation tailings from hydrothermal sulfidation-flotation process. Environ. Sci. Pollut. Res. 2013, 20, 6050–6058. [Google Scholar] [CrossRef]
- Gao, W.; Wang, C.Y.; Yin, F.; Chen, Y.Q.; Yang, W.J. Situation and technology progress of lead smelting in China. Adv. Mater. Res. 2012, 581–582, 904–911. [Google Scholar] [CrossRef]
- Renew, J.E.; Huang, C.H.; Burns, S.E.; Carrasquillo, M.; Sun, W.L.; Ellison, K.M. Immobilization of heavy metals by solidification/stabilization of co-disposed flue gas desulfurization brine and coal fly ash. Energy Fuels 2016, 30, 5042–5051. [Google Scholar] [CrossRef]
- Prasanna, S.V.; Kamath, P.V. Synthesis and characterization of arsenate-intercalated layered double hydroxides (LDHs): Prospects for arsenic mineralization. J. Colloid Interface Sci. 2009, 331, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Cao, Y.; Chui, P.; Tay, J. Utilization of MSWI fly ash for stabilization/solidification of industrial waste sludge. J. Hazard. Mater. 2006, 129, 274–281. [Google Scholar] [CrossRef]
- Qian, G.R.; Shi, J.; Cao, Y.L.; Xu, Y.F.; Chui, P.C. Properties of MSW fly ash-calcium sulfoaluminate cement matrix and stabilization/solidification on heavy metals. J. Hazard. Mater. 2008, 152, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, X.; Tian, Q.; Xu, Z.; Xu, R.; Zhang, L. Synthesis and application of friedel’s salt in arsenic removal from caustic solution. Chem. Eng. J. 2017, 323, 304–311. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Zhang, J.; Chi, Y.; Ruan, X.; Liu, J.; Qian, G. Effective removal of zinc from aqueous solution by hydrocalumite. Chem. Eng. J. 2011, 175, 33–38. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Chemical activation of lime-slag blends. Int. Concr. Abstr. Portal 1995, 153, 1165–1178. [Google Scholar]
- Cheng, Y.; Li, Z.G.; Huang, X.; Bai, X.H. Effect of friedel’s salt on strength enhancement of stabilized chloride saline soil. J. Cent. South Univ. 2017, 24, 937–946. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. The effect of fiber properties on high performance alkali-activated slag/silica fume mortars. Compos. Part B-Eng. 2013, 45, 63–69. [Google Scholar] [CrossRef]
- Xu, H.; Li, Q.; Shen, L.; Wang, W.; Zhai, J. Synthesis of thermostable geopolymer from circulating fluidized bed combustion (CFBC) bottom ashes. J. Hazard. Mater. 2010, 175, 198–204. [Google Scholar] [CrossRef]
- Batchelor, B. Overview of waste stabilization with cement. Waste Manag. 2006, 26, 689–698. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Toxicity Characteristic Leaching Procedure; EPA: Washington, DC, USA, 1992.
- Kougemitrou, I.; Godelitsas, A.; Tsabaris, C.; Stathopoulos, V.; Papandreou, A.; Gamaletsos, P.; Economou, G.; Papadopoulos, D. Characterisation and management of ash produced in the hospital waste incinerator of Athens, Greece. J. Hazard. Mater. 2011, 187, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Sata, V.; Sathonsaowaphak, A.; Chindaprasirt, P. Resistance of lignite bottom ash geopolymer mortar to sulfate and sulfuric acid attack. Cem. Concr. Comp. 2012, 34, 700–708. [Google Scholar] [CrossRef]
- Phair, J.W.; Deventer, J.S.J.V. Effect of the silicate activator pH on the microstructural characteristics of waste-based geopolymers. Int. J. Miner. Process. 2002, 66, 121–143. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Deventer, J.S.J.V. Structural reorganisation of class F fly ash in alkaline silicate solutions. Colloid Surf. A 2002, 211, 49–66. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Colangelo, F.; Grieco, T.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, C.; Wang, W.; Shi, Y.; Gao, X. Immobilization of MSWI fly ash through geopolymerization: Effects of water-wash. Waste Manag. 2011, 31, 311–317. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Birnin-Yauri1, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Songpiriyakij, S.; Kubprasit, T.; Jaturapitakkul, C.; Chindaprasirt, P. Compressive strength and degree of reaction of biomass- and fly ash-based geopolymer. Constr. Build. Mater. 2010, 24, 236–240. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Deventer, J.S.J.v. Effects of anions on the formation of aluminosilicate gel in geopolymers. Ind. Eng. Chem. Res. 2002, 41, 4550–4558. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Deventer, J.S.J.V. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Messina, F.; Santoro, L.; Cioffi, R. Recycling of pre-washed municipal solid waste incinerator fly ash in the manufacturing of low temperature setting geopolymer materials. Materials 2013, 6, 3420–3437. [Google Scholar] [CrossRef] [PubMed]
- Rowles, M.; O’Connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Li, Y.C.; Min, X.B.; Ke, Y.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liu, D.G. Utilization of red mud and Pb/Zn smelter waste for the synthesis of a red mud-based cementitious material. J. Hazard. Mater. 2018, 344, 343–349. [Google Scholar] [CrossRef]
- Vempati, R.K.; Mollah, M.Y.A.; Chinthala, A.K.; Cocke, D.L.; Beeghly, J.H. Solidification/stabilization of toxic metal wastes using coke and coal combustion by-products. Waste Manag. 1995, 15, 433–440. [Google Scholar] [CrossRef]
- Kara, İ.; Yilmazer, D.; TunaliAkar, S. Metakaolin based geopolymer as an effective adsorbent for adsorption of zinc(II) and nickel(II) ions from aqueous solutions. Appl. Clay Sci. 2017, 139, 54–63. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, W.; Qiao, W.; Shi, Y.; Liu, X. Immobilization of Cu2+, Zn2+, Pb2+, and Cd2+ during geopolymerization. Front. Environ. Sci. Eng. 2015, 9, 642–648. [Google Scholar] [CrossRef]
- Min, X.B.; Liu, D.G.; Chai, L.Y.; Ke, Y.; Liang, Y.J.; Shi, M.Q.; Li, Y.C.; Tang, C.J.; Wang, Y.Y.; Wang, Z.B. Comparison of arsenic immobilization properties among calcium silicate hydrate, ettringite, and Friedel’s salt in a slag-based binder. Environ. Prog. Sustain. Energy 2018. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, Z.; Pu, Q.; Cao, Y.; Zhou, J.; Sun, Y.; Qian, G. Stabilization mechanism of Friedel’s salt on Pb2+. Acta Sci. Circumst. 2009, 29, 594–599. [Google Scholar]
- Zhang, J.J.; Zhao, H.; Cao, H.B.; Li, H.P.; Li, Z.B. Removal of Cd2+ from water by Friedel’s salt (FS: 3CaO∙Al2O3∙CaCl2∙10H2O): Sorption characteristics and mechanisms. J. Environ. Sci. China 2013, 25, 1719–1725. [Google Scholar] [CrossRef]
- Zhang, D.; Jia, Y.; Ma, J.; Li, Z. Removal of arsenic from water by friedel’s salt (FS: 3CaO·Al2O3·CaCl2·10H2O). J. Hazard. Mater. 2011, 195, 398–404. [Google Scholar] [CrossRef] [PubMed]
| Element | Ag | Al | As | Ba | Be | Ca | Cd | Cr | Cu | Fe | Hg |
| MSWI FA | 0.0005 | 2.49 | 0.030 | 0.087 | <0.0001 | 28.7 | 0.017 | 0.016 | 0.049 | 1.32 | <0.0005 |
| GLSS | 0.0007 | 4.32 | 0.011 | 0.49 | 0.0002 | 9.55 | 0.0025 | 0.14 | 0.19 | 26.9 | <0.0005 |
| Element | K | Mg | Mn | Na | Ni | Pb | S | Zn | Se | Si | Cl |
| MSWI FA | 2.57 | 1.39 | 0.053 | 1.73 | 0.0056 | 0.16 | 2.60 | 0.57 | 0.0016 | 2.62 | 17.98 |
| GLSS | 0.95 | 1.18 | 1.44 | 0.51 | 0.035 | 0.20 | 0.22 | 2.81 | 0.0018 | 15.93 | - |
| Element | Zn | Pb | As | Cd | Ni | Cr | Ba | Cu | Ag | Hg | Be | Se |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USEPA Limits | - | 5 | 5 | 1 | - | 5 | 100 | 100 | - | 0.2 | - | - |
| GLSS | 167.16 | 0.15 | 0.05 | 0.22 | 0.45 | 0.01 | 12.53 | 0.07 | <0.01 | <0.01 | <0.01 | <0.01 |
| MSWI FA | 0.42 | 8.47 | 0.63 | <0.01 | <0.01 | 0.09 | 1.94 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Element | Zn | Pb | As | Cd | Ni | Cr | Ba | Cu | Ag | Hg | Be | Se |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US EPA Limits | - | 5 | 5 | 1 | - | 5 | 100 | 100 | - | 0.2 | - | - |
| L0M100 | <0.01 | 2.16 | 0.12 | <0.01 | <0.01 | 0.02 | 0.85 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| L20M80 | <0.01 | 0.25 | 0.12 | <0.01 | <0.01 | 0.02 | 0.68 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| L40M60 | <0.01 | <0.01 | 0.10 | <0.01 | <0.01 | 0.03 | 0.59 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| L60M40 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.42 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| L80M20 | 3.30 | <0.01 | <0.01 | 0.1 | 0.1 | <0.01 | 0.45 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Optimal matrix | <0.01 | <0.01 | 0.12 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.-G.; Ke, Y.; Min, X.-B.; Liang, Y.-J.; Wang, Z.-B.; Li, Y.-C.; Fei, J.-C.; Yao, L.-W.; Xu, H.; Jiang, G.-H. Cotreatment of MSWI Fly Ash and Granulated Lead Smelting Slag Using a Geopolymer System. Int. J. Environ. Res. Public Health 2019, 16, 156. https://doi.org/10.3390/ijerph16010156
Liu D-G, Ke Y, Min X-B, Liang Y-J, Wang Z-B, Li Y-C, Fei J-C, Yao L-W, Xu H, Jiang G-H. Cotreatment of MSWI Fly Ash and Granulated Lead Smelting Slag Using a Geopolymer System. International Journal of Environmental Research and Public Health. 2019; 16(1):156. https://doi.org/10.3390/ijerph16010156
Chicago/Turabian StyleLiu, De-Gang, Yong Ke, Xiao-Bo Min, Yan-Jie Liang, Zhong-Bing Wang, Yuan-Cheng Li, Jiang-Chi Fei, Li-Wei Yao, Hui Xu, and Guang-Hua Jiang. 2019. "Cotreatment of MSWI Fly Ash and Granulated Lead Smelting Slag Using a Geopolymer System" International Journal of Environmental Research and Public Health 16, no. 1: 156. https://doi.org/10.3390/ijerph16010156
APA StyleLiu, D.-G., Ke, Y., Min, X.-B., Liang, Y.-J., Wang, Z.-B., Li, Y.-C., Fei, J.-C., Yao, L.-W., Xu, H., & Jiang, G.-H. (2019). Cotreatment of MSWI Fly Ash and Granulated Lead Smelting Slag Using a Geopolymer System. International Journal of Environmental Research and Public Health, 16(1), 156. https://doi.org/10.3390/ijerph16010156