Prenatal Exposure to Perfluoroalkyl Substances and Birth Outcomes; An Updated Analysis from the Danish National Birth Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Birh Outcomes
2.3. Exposure Assessment
2.4. Maternal and Newborn Covariates
2.5. Statistical Analysis
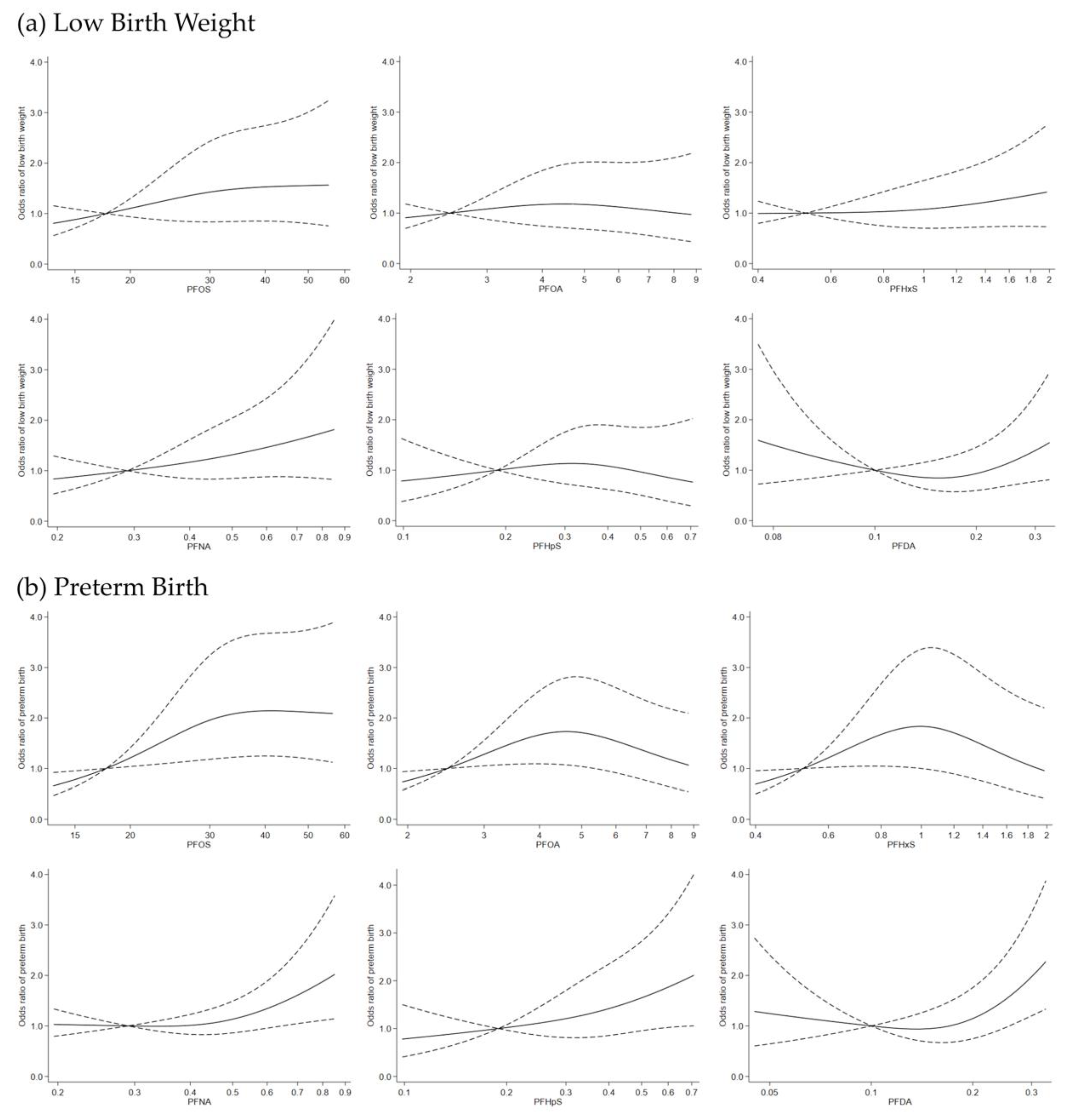
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.C. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Wong, L.Y.; Jia, L.T.; Kuklenyik, Z.; Calafat, A.M. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ. Sci. Technol. 2011, 45, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Nost, T.H.; Vestergren, R.; Berg, V.; Nieboer, E.; Odland, J.O.; Sandanger, T.M. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: Assessing time trends, compound correlations and relations to age/birth cohort. Environ. Int. 2014, 67, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjerregaard-Olesen, C.; Bach, C.C.; Long, M.; Ghisari, M.; Bossi, R.; Bech, B.H.; Nohr, E.A.; Henriksen, T.B.; Olsen, J.; Bonefeld-Jorgensen, E.C. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013. Environ. Int. 2016, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; van Asseldonk, L.; van Leeuwen, S.P.J. Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol. 2017, 51, 11057–11065. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Letcher, R.J.; McGoldrick, D.J.; Backus, S.M. A New Fluorinated Surfactant Contaminant in Biota: Perfluorobutane Sulfonamide in Several Fish Species. Environ. Sci. Technol. 2016, 50, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Arevalo, E.; Strynar, M.; Lindstrom, A.; Richardson, M.; Kearns, B.; Pickett, A.; Smith, C.; Knappe, D.R.U. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett. 2016, 3, 415–419. [Google Scholar] [CrossRef]
- Lau, C.; Butenhoff, J.L.; Rogers, J.M. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol. Appl. Pharmacol. 2004, 198, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, C.; Thibodeaux, J.R.; Hanson, R.G.; Narotsky, M.G.; Rogers, J.M.; Lindstrom, A.B.; Strynar, M.J. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. 2006, 90, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Luebker, D.J.; York, R.G.; Hansen, K.J.; Moore, J.A.; Butenhoff, J.L. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: Dose-response, and biochemical and pharamacokinetic parameters. Toxicology 2005, 215, 149–169. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, J.C.; Peden-Adams, M.M.; Keller, J.M.; Germolec, D.R. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol. Pathol. 2012, 40, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Abedi-Valugerdi, M.; Xie, Y.; Zhao, X.Y.; Moller, G.; Nelson, B.D.; DePierre, J.W. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int. Immunopharmacol. 2002, 2, 389–397. [Google Scholar] [CrossRef]
- Goudarzi, H.; Araki, A.; Itoh, S.; Sasaki, S.; Miyashita, C.; Mitsui, T.; Nakazawa, H.; Nonomura, K.; Kishi, R. The Association of Prenatal Exposure to Perfluorinated Chemicals with Glucocorticoid and Androgenic Hormones in Cord Blood Samples: The Hokkaido Study. Environ. Health Perspect. 2017, 125, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, C.; McLaughlin, J.K.; Tarone, R.E.; Olsen, J. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environ. Health Perspect. 2007, 115, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Starling, A.P.; Adgate, J.L.; Hamman, R.F.; Kechris, K.; Calafat, A.M.; Ye, X.; Dabelea, D. Perfluoroalkyl Substances during Pregnancy and Offspring Weight and Adiposity at Birth: Examining Mediation by Maternal Fasting Glucose in the Healthy Start Study. Environ. Health Perspect. 2017, 125, 067016. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Rifas-Shiman, S.L.; Fleisch, A.F.; Webster, T.F.; Calafat, A.M.; Ye, X.; Gillman, M.W.; Oken, E. Early-Pregnancy Plasma Concentrations of Perfluoroalkyl Substances and Birth Outcomes in Project Viva: Confounded by Pregnancy Hemodynamics? Am. J. Epidemiol. 2018, 187, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.R.; Savitz, D.A.; Dougan, M. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am. J. Epidemiol. 2009, 170, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Melbye, M.; Olsen, S.F.; Sorensen, T.I.; Aaby, P.; Andersen, A.M.; Taxbol, D.; Hansen, K.D.; Juhl, M.; Schow, T.B.; et al. The Danish National Birth Cohort—Its background, structure and aim. Scand. J. Public Health 2001, 29, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; von Ehrenstein, O.S.; Bech, B.H.; Nohr, E.A.; Fei, C.; Bossi, R.; Henriksen, T.B.; Bonefeld-Jorgensen, E.C.; Olsen, J. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: A nested case-control study in the Danish National Birth Cohort. Environ. Health Perspect. 2015, 123, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Kesmodel, U.S.; Underbjerg, M.; Kilburn, T.R.; Bakketeig, L.; Mortensen, E.L.; Landro, N.I.; Schendel, D.; Bertrand, J.; Grove, J.; Ebrahim, S.; et al. Lifestyle during pregnancy: Neurodevelopmental effects at 5 years of age. The design and implementation of a prospective follow-up study. Scand. J. Public Health 2010, 38, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Bonefeld-Jorgensen, E.C.; Henriksen, T.B.; Nohr, E.A.; Bech, B.H.; Fei, C.; Bossi, R.; von Ehrenstein, O.S.; Streja, E.; et al. Prenatal exposure to perfluoroalkyl substances and the risk of congenital cerebral palsy in children. Am. J. Epidemiol. 2014, 180, 574–581. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and UNICEF. Low Birthweight: Country, Regional and Global Estimates. Available online: http://apps.who.int/iris/bitstream/handle/10665/43184/9280638327.pdf;jsessionid=165C2FC9CB3D2AC0688561A57A5C9A67?sequence=1 (accessed on 22 July 2018).
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Ehresman, D.J.; Froehlich, J.W.; Olsen, G.W.; Chang, S.C.; Butenhoff, J.L. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007, 103, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Bech, B.H.; Nohr, E.A.; Vaeth, M.; Henriksen, T.B.; Olsen, J. Coffee and fetal death: A cohort study with prospective data. Am. J. Epidemiol. 2005, 162, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Howards, P.P.; Winquist, A.; Steenland, K. PFOA and PFOS serum levels and miscarriage risk. Epidemiology 2014, 25, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Olsen, J.; Cui, X.; Ritz, B.; Arah, O.A. Bias from conditioning on live birth in pregnancy cohorts: An illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int. J. Epidemiol. 2015, 44, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Salgado, C.B.; Casas, M.; Lopez-Espinosa, M.J.; Ballester, F.; Iniguez, C.; Martinez, D.; Costa, O.; Santa-Marina, L.; Pereda-Pereda, E.; Schettgen, T.; et al. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ. Int. 2017, 108, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.C.; Liew, Z.; Bech, B.H.; Nohr, E.A.; Fei, C.; Bonefeld-Jorgensen, E.C.; Henriksen, T.B.; Olsen, J. Perfluoroalkyl acids and time to pregnancy revisited: An update from the Danish National Birth Cohort. Environ. Health 2015, 14, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matilla-Santander, N.; Valvi, D.; Lopez-Espinosa, M.J.; Manzano-Salgado, C.B.; Ballester, F.; Ibarluzea, J.; Santa-Marina, L.; Schettgen, T.; Guxens, M.; Sunyer, J.; et al. Exposure to Perfluoroalkyl Substances and Metabolic Outcomes in Pregnant Women: Evidence from the Spanish INMA Birth Cohorts. Environ. Health Perspect. 2017, 125, 117004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, Z.; Ritz, B.; Virk, J.; Arah, O.A.; Olsen, J. Prenatal Use of Acetaminophen and Child IQ: A Danish Cohort Study. Epidemiology 2016, 27, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Bach, C.C.; Asarnow, R.F.; Bech, B.H.; Nohr, E.A.; Bossi, R.; Henriksen, T.B.; Bonefeld-Jorgensen, E.C.; Olsen, J. Prenatal Exposure to Perfluoroalkyl Substances and IQ Scores at Age 5; A Study in the Danish National Birth Cohort. Environ. Health Perspect. 2018, 126, 067004. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, T.I.; Fei, C.; Olsen, J.; Lipworth, L.; McLaughlin, J.K.; Olsen, S.F. Dietary predictors of perfluorinated chemicals: A study from the Danish National Birth Cohort. Environ. Sci. Technol. 2008, 42, 8971–8977. [Google Scholar] [CrossRef] [PubMed]
- Verner, M.A.; Loccisano, A.E.; Morken, N.H.; Yoon, M.; Wu, H.; McDougall, R.; Maisonet, M.; Marcus, M.; Kishi, R.; Miyashita, C.; et al. Associations of Perfluoroalkyl Substances (PFAS) with Lower Birth Weight: An Evaluation of Potential Confounding by Glomerular Filtration Rate Using a Physiologically Based Pharmacokinetic Model (PBPK). Environ. Health Perspect. 2015, 123, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Morgen, C.S.; Andersen, P.K.; Mortensen, L.H.; Howe, L.D.; Rasmussen, M.; Due, P.; Sorensen, T.I.; Andersen, A.N. Socioeconomic disparities in birth weight and body mass index during infancy through age 7 years: A study within the Danish National Birth Cohort. BMJ Open 2017, 7, e011781. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.C.; Bech, B.H.; Brix, N.; Nohr, E.A.; Bonde, J.P.; Henriksen, T.B. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review. Crit. Rev. Toxicol. 2015, 45, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Goudarzi, H.; Oulhote, Y. Developmental Exposures to Perfluoroalkyl Substances (PFASs): An Update of Associated Health Outcomes. Curr. Environ. Health Rep. 2018, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.C.; Bech, B.H.; Nohr, E.A.; Olsen, J.; Matthiesen, N.B.; Bonefeld-Jorgensen, E.C.; Bossi, R.; Henriksen, T.B. Perfluoroalkyl Acids in Maternal Serum and Indices of Fetal Growth: The Aarhus Birth Cohort. Environ. Health Perspect. 2016, 124, 848–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitworth, K.W.; Haug, L.S.; Baird, D.D.; Becher, G.; Hoppin, J.A.; Skjaerven, R.; Thomsen, C.; Eggesbo, M.; Travlos, G.; Wilson, R.; et al. Perfluorinated compounds in relation to birth weight in the Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2012, 175, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, J.R.; Hanson, R.G.; Rogers, J.M.; Grey, B.E.; Barbee, B.D.; Richards, J.H.; Butenhoff, J.L.; Stevenson, L.A.; Lau, C. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: Maternal and prenatal evaluations. Toxicol. Sci. 2003, 74, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Koustas, E.; Lam, J.; Sutton, P.; Johnson, P.I.; Atchley, D.S.; Sen, S.; Robinson, K.A.; Axelrad, D.A.; Woodruff, T.J. The Navigation Guide—Evidence-based medicine meets environmental health: Systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ. Health Perspect. 2014, 122, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K.; Ji, K.; Seo, J.; Kho, Y.; Park, J.; Kim, S.; Park, S.; Hwang, I.; Jeon, J.; et al. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ. Sci. Technol. 2011, 45, 7465–7472. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.D. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod. Toxicol. 2009, 27, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Starling, A.P.; Haug, L.S.; Eggesbo, M.; Becher, G.; Thomsen, C.; Travlos, G.; King, D.; Hoppin, J.A.; Rogan, W.J.; et al. Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: A cross-sectional study. Environ. Health 2013, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.S.; Bonefeld-Jorgensen, E.C. Perfluorinated compounds affect the function of sex hormone receptors. Environ. Sci. Pollut. Res. Int. 2013, 20, 8031–8044. [Google Scholar] [CrossRef] [PubMed]
- Govarts, E.; Remy, S.; Bruckers, L.; Den Hond, E.; Sioen, I.; Nelen, V.; Baeyens, W.; Nawrot, T.S.; Loots, I.; Van Larebeke, N.; et al. Combined Effects of Prenatal Exposures to Environmental Chemicals on Birth Weight. Int. J. Environ. Res. Public Health 2016, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Valvi, D.; Oulhote, Y.; Weihe, P.; Dalgard, C.; Bjerve, K.S.; Steuerwald, U.; Grandjean, P. Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ. Int. 2017, 107, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, M.G.; Seals, R.M.; Webster, T.F. Bias Amplification in Epidemiologic Analysis of Exposure to Mixtures. Environ. Health Perspect. 2018, 126, 047003. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.M.; Lanphear, B.P.; Braun, J.M.; McCandless, L.C. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: A Bayesian analysis of the HOME Study. Environ. Health 2017, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Rappazzo, K.M.; Coffman, E.; Hines, E.P. Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int. J. Environ. Res. Public Health 2017, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Andersen, L.B.; Kyhl, H.B.; Nielsen, F.; Christesen, H.T.; Grandjean, P. Association between perfluorinated compound exposure and miscarriage in Danish pregnant women. PLoS ONE 2015, 10, e0123496. [Google Scholar] [CrossRef] [PubMed]
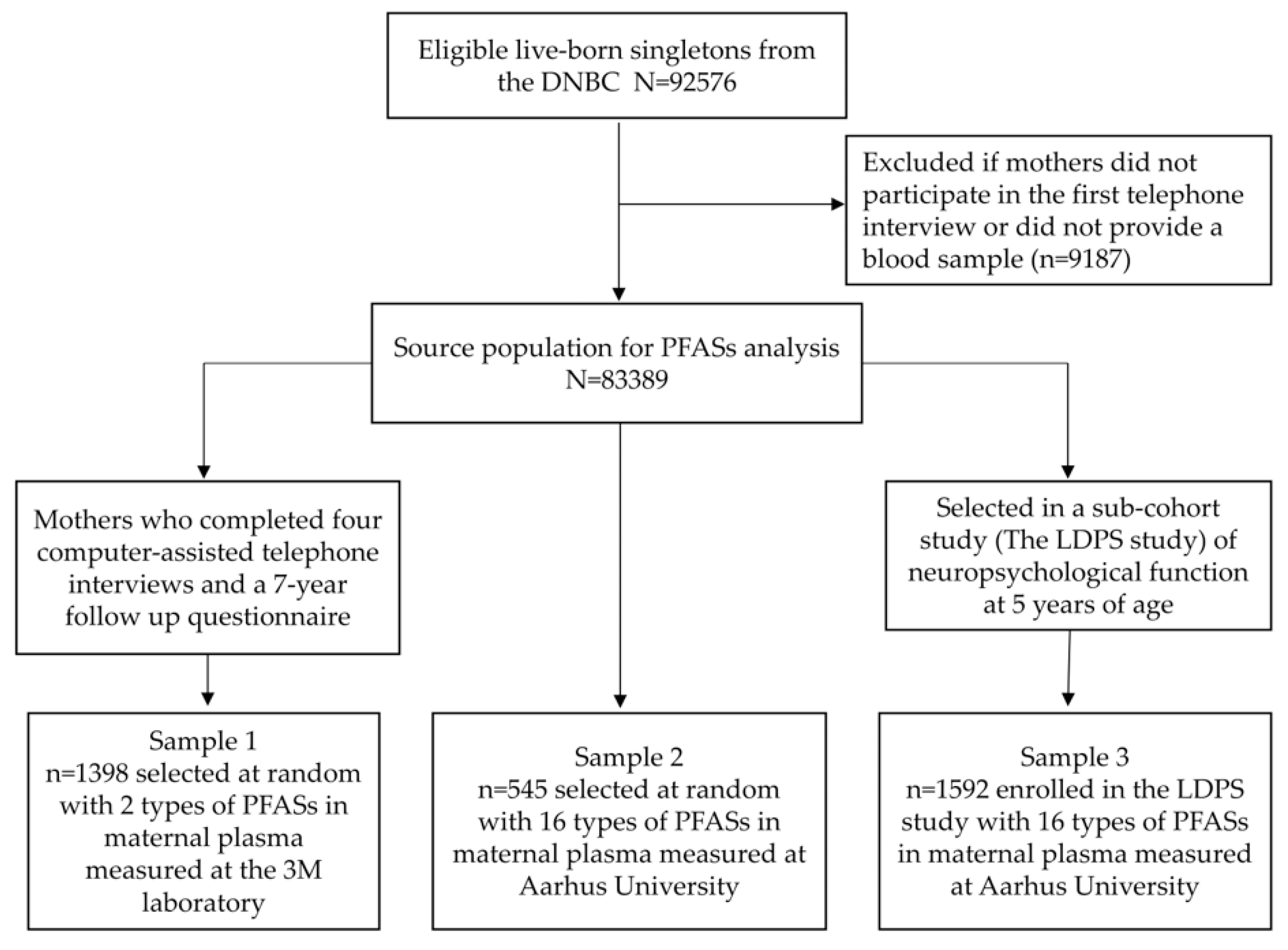
| Median (Interquartile Range) or N (%) | ||||
|---|---|---|---|---|
| Total | Sample 1 | Sample 2 | Sample 3 | |
| Maternal Characteristics | ||||
| PFAS (ng/mL) | ||||
| PFOS | 30.1 (22.9–39.0) | 33.4 (26.1–43.3) | 27.4 (20.4–35.6) | 28.1 (21.6–35.8) |
| PFOA | 4.6 (3.3–6.0) | 5.2 (3.9–7.0) | 4.0 (3.0–5.4) | 4.3 (3.2–5.5) |
| PFHxS | 1.0 (0.7–1.3) | N/A | 0.9 (0.7–1.2) | 1.1 (0.8–1.4) |
| PFNA | 0.5 (0.4–0.6) | N/A | 0.4 (0.4–0.6) | 0.5 (0.4–0.6) |
| PFHpS | 0.4 (0.3–0.5) | N/A | 0.3 (0.2–0.4) | 0.4 (0.3–0.5) |
| PFDA | 0.2 (0.1–0.2) | N/A | 0.2 (0.1–0.2) | 0.2 (0.1–0.2) |
| Age (years) | ||||
| 19–29 | 1638 (46.3) | 664 (47.5) | 273 (50.1) | 701 (44.0) |
| 30–34 | 1321 (37.4) | 504 (36.1) | 200 (36.7) | 617 (38.8) |
| 35–39 | 576 (16.3) | 230 (16.4) | 72 (13.2) | 274 (17.2) |
| Socio-occupational status | ||||
| High | 2366 (67.2) | 899 (64.5) | 336 (61.9) | 1131 (71.3) |
| Medium | 1057 (30.0) | 453 (32.5) | 192 (35.4) | 412 (26.0) |
| Low | 100 (2.8) | 42 (3.0) | 15 (2.7) | 43 (2.7) |
| Missing | 12 | 4 | 2 | 6 |
| Parity | ||||
| 0 | 1622 (47.1) | 607 (44.4) | 245 (46.2) | 770 (49.8) |
| 1 | 1212 (35.2) | 498 (36.4) | 209 (39.4) | 505 (32.7) |
| >1 | 610 (17.7) | 263 (19.2) | 76 (14.4) | 271 (17.5) |
| Missing | 91 | 30 | 15 | 46 |
| Alcohol intake during pregnancy | ||||
| Never | 766 (21.7) | 400 (28.6) | 159 (29.2) | 207 (13.0) |
| ≤1 per week | 629 (17.8) | 352 (25.2) | 139 (25.5) | 138 (8.7) |
| >1 per week | 2140 (60.5) | 646 (46.2) | 247 (45.3) | 1247 (78.3) |
| Smoking during pregnancy | ||||
| No | 2534 (71.7) | 1050 (75.1) | 407 (74.7) | 1077 (67.7) |
| Yes | 1001 (28.3) | 348 (24.9) | 138 (25.3) | 515 (32.3) |
| Pre-pregnancy BMI (kg/m2) | ||||
| <18.5 | 143 (4.1) | 58 (4.3) | 24 (4.5) | 61 (3.9) |
| 18.5–24.9 | 2355 (68.0) | 904 (66.4) | 358 (66.4) | 1093 (70.0) |
| 25.0–29.9 | 705 (20.4) | 298 (21.9) | 115 (21.3) | 292 (18.7) |
| ≥30.0 | 258 (7.5) | 101 (7.4) | 42 (7.8) | 115 (7.4) |
| Missing | 74 | 37 | 6 | 31 |
| Newborn Characteristics | ||||
| Sex | ||||
| Female | 1559 (44.1) | 688 (49.2) | 110 (20.2) | 761 (47.8) |
| Male | 1976 (55.9) | 710 (50.8) | 435 (79.8) | 831 (52.2) |
| Weight (g) | 3600 (3270–3960) | 3630 (3260–4000) | 3628 (3250–3970) | 3600 (3280–3925) |
| Gestational age (days) | 281 (275–288) | 281 (274–288) | 281 (274–287) | 282 (275–288) |
| Low birth weight | ||||
| Yes | 61 (1.7) | 24 (1.7) | 14 (2.6) | 23 (1.5) |
| No | 3446 (98.3) | 1363 (98.3) | 526 (97.4) | 1557 (98.5) |
| Missing | 28 | 11 | 5 | 12 |
| Preterm birth | ||||
| Yes | 112 (3.2) | 53 (3.8) | 17 (3.1) | 42 (2.6) |
| No | 3410 (96.8) | 1337 (96.2) | 528 (96.9) | 1545 (97.4) |
| Missing | 13 | 8 | 0 | 5 |
| Exposure Level b | Birth Weight | Gestational Age | ||
|---|---|---|---|---|
| n | Adjusted Difference a in Birth Weight (β and 95%CI) | n | Adjusted Difference a in Gestational Age (β and 95%CI) | |
| Pooled sample 1, 2 and 3 | ||||
| PFOS | ||||
| Per doubling of exposure | 3507 | −45.2 (−76.8, −13.6) | 3522 | −1.1 (−1.7, −0.4) |
| Q1 | 885 | ref | 889 | ref |
| Q2 | 875 | 24.7 (−24.8, 74.1) | 879 | −1.1 (−2.1, −0.1) |
| Q3 | 872 | −50.1 (−101.1, 0.9) | 877 | −2.0 (−3.1, −1.0) |
| Q4 | 875 | −48.2 (−99.0, 2.5) | 877 | −1.5 (−2.6, −0.5) |
| PFOA | ||||
| Per doubling of exposure | 3507 | −35.6 (−66.3, −5.0) | 3522 | −0.4 (−1.0, 0.3) |
| Q1 | 885 | ref | 888 | ref |
| Q2 | 873 | −20.4 (−70.0, 29.2) | 874 | −1.4 (−2.4, −0.3) |
| Q3 | 873 | −25.9 (−77.7, 25.9) | 878 | −1.2 (−2.2, −0.1) |
| Q4 | 876 | −117.0 (−172.3, −61.6) | 882 | −1.7 (−2.9, −0.6) |
| Pooled sample 2 and 3 | ||||
| PFHxS | ||||
| Per doubling of exposure | 2120 | 1.2 (−28.3, 30.7) | 2132 | −0.2 (−0.8, 0.4) |
| Q1 | 535 | ref | 537 | ref |
| Q2 | 544 | 37.3 (−25.7, 100.2) | 545 | −1.2 (−2.5, 0.1) |
| Q3 | 510 | 7.6 (−58.1, 73.2) | 519 | −0.4 (−1.7, 1.0) |
| Q4 | 531 | 8.6 (−59.7, 76.9) | 531 | −0.9 (−2.3, 0.5) |
| PFNA | ||||
| Per doubling of exposure | 2120 | −36.3 (−70.6, −2.0) | 2132 | −1.0 (−1.7, −0.3) |
| Q1 | 556 | ref | 562 | ref |
| Q2 | 537 | −9.1 (−71.6, 53.3) | 536 | −1.4 (−2.7, −0.2) |
| Q3 | 513 | −21.7 (−86.3, 42.8) | 519 | −1.1 (−2.4, 0.2) |
| Q4 | 514 | −81.2 (−147.1, −15.4) | 515 | −1.5 (−2.8, −0.2) |
| PFHpS | ||||
| Per doubling of exposure | 2120 | −38.9 (−72.6, −5.1) | 2132 | −1.2 (−1.9, −0.5) |
| Q1 | 547 | ref | 552 | ref |
| Q2 | 520 | −62.1 (−124.6, 0.4) | 521 | −1.7 (−3.0, −0.4) |
| Q3 | 538 | −110.8 (−177.7, −43.8) | 542 | −2.6 (−4.0, −1.3) |
| Q4 | 515 | −102.6 (−169.0, −36.2) | 517 | −2.0 (−3.3, −0.7) |
| PFDA | ||||
| Per doubling of exposure | 2120 | −9.0 (−43.2, 25.2) | 2132 | −0.6 (−1.3, 0.1) |
| Q1 | 655 | ref | 660 | ref |
| Q2 | 456 | −22.6 (−87.2, 42.1) | 462 | 0.6 (−0.7, 1.9) |
| Q3 | 518 | 16.3 (−45.3, 77.9) | 517 | −0.2 (−1.4, 1.1) |
| Q4 | 491 | −16.0 (−80.0, 47.9) | 493 | −0.5 (−1.8, 0.8) |
| Exposure Level b | Low Birth Weight | Preterm Birth | ||
|---|---|---|---|---|
| n | Adjusted OR a and 95% CI | n | Adjusted OR a and 95% CI | |
| Pooled sample 1,2 and 3 | ||||
| PFOS | ||||
| Per doubling of exposure | 61 | 1.3 (0.9, 2.0) | 112 | 1.5 (1.1, 2.2) |
| Q1 | 10 (1.1%) | ref | 19 (2.1%) | ref |
| Q2 | 16 (1.8%) | 1.4 (0.7, 2.8) | 28 (3.2%) | 2.0 (1.1, 3.6) |
| Q3 | 16 (1.8%) | 1.8 (0.9, 3.6) | 37 (4.2%) | 3.3 (1.8, 5.8) |
| Q4 | 19 (2.2%) | 1.2 (0.6, 2.4) | 28 (3.2%) | 1.9 (1.0, 3.5) |
| PFOA | ||||
| Per doubling of exposure | 61 | 1.0 (0.7, 1.5) | 112 | 1.1 (0.8, 1.5) |
| Q1 | 12 (1.4%) | ref | 18 (2.0%) | ref |
| Q2 | 14 (1.6%) | 1.5 (0.8, 3.1) | 32 (3.7%) | 3.2 (1.8, 5.6) |
| Q3 | 13 (1.5%) | 1.2 (0.5, 2.5) | 31 (3.5%) | 1.7 (0.9, 3.2) |
| Q4 | 22 (2.5%) | 1.5 (0.7, 3.3) | 31 (3.5%) | 1.9 (1.0, 3.6) |
| Pooled sample 2 and 3 | ||||
| PFHxS | ||||
| Per doubling of exposure | 37 | 1.2 (0.8, 1.7) | 59 | 1.1 (0.8, 1.5) |
| Q1 | 9 (1.7%) | ref | 13 (2.4%) | ref |
| Q2 | 11 (2.0%) | 1.1 (0.5, 2.4) | 18 (3.3%) | 2.3 (1.1, 4.6) |
| Q3 | 5 (1.0%) | 0.5 (0.2, 1.3) | 14 (2.7%) | 1.5 (0.7, 3.2) |
| Q4 | 12 (2.3%) | 0.8 (0.4, 1.9) | 14 (2.6%) | 1.0 (0.5, 2.3) |
| PFNA | ||||
| Per doubling of exposure | 37 | 1.5 (0.9, 2.4) | 59 | 1.4 (0.9, 2.1) |
| Q1 | 9 (1.6%) | ref | 13 (2.3%) | ref |
| Q2 | 4 (0.7%) | 1.0 (0.4, 2.5) | 14 (2.6%) | 1.2 (0.6, 2.5) |
| Q3 | 11 (2.1%) | 1.4 (0.6, 3.2) | 14 (2.7%) | 0.9 (0.4, 1.9) |
| Q4 | 13 (2.5%) | 1.5 (0.6, 3.6) | 18 (3.5%) | 1.7 (0.8, 3.3) |
| PFHpS | ||||
| Per doubling of exposure | 37 | 1.0 (0.6, 1.5) | 59 | 1.5 (1.0, 2.1) |
| Q1 | 12 (2.2%) | ref | 16 (2.9%) | ref |
| Q2 | 7 (1.3%) | 1.1 (0.5, 2.4) | 8 (1.5%) | 1.5 (0.7, 3.0) |
| Q3 | 8 (1.5%) | 1.2 (0.5, 2.6) | 19 (3.5%) | 1.6 (0.7, 3.3) |
| Q4 | 10 (1.9%) | 0.5 (0.2, 1.4) | 16 (3.1%) | 1.8 (0.8, 3.7) |
| PFDA | ||||
| Per doubling of exposure | 37 | 1.2 (0.8, 1.9) | 59 | 1.7 (1.2, 2.5) |
| Q1 | 13 (2.0%) | ref | 18 (2.7%) | ref |
| Q2 | 7 (1.5%) | 0.8 (0.4, 1.8) | 9 (1.9%) | 1.0 (0.5, 2.0) |
| Q3 | 4 (0.8%) | 0.4 (0.2, 1.0) | 15 (2.9%) | 1.1 (0.5, 2.1) |
| Q4 | 13 (2.6%) | 0.9 (0.4, 2.0) | 17 (3.4%) | 1.6 (0.8, 3.0) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Inoue, K.; Ritz, B.; Olsen, J.; Liew, Z. Prenatal Exposure to Perfluoroalkyl Substances and Birth Outcomes; An Updated Analysis from the Danish National Birth Cohort. Int. J. Environ. Res. Public Health 2018, 15, 1832. https://doi.org/10.3390/ijerph15091832
Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. Prenatal Exposure to Perfluoroalkyl Substances and Birth Outcomes; An Updated Analysis from the Danish National Birth Cohort. International Journal of Environmental Research and Public Health. 2018; 15(9):1832. https://doi.org/10.3390/ijerph15091832
Chicago/Turabian StyleMeng, Qi, Kosuke Inoue, Beate Ritz, Jørn Olsen, and Zeyan Liew. 2018. "Prenatal Exposure to Perfluoroalkyl Substances and Birth Outcomes; An Updated Analysis from the Danish National Birth Cohort" International Journal of Environmental Research and Public Health 15, no. 9: 1832. https://doi.org/10.3390/ijerph15091832
APA StyleMeng, Q., Inoue, K., Ritz, B., Olsen, J., & Liew, Z. (2018). Prenatal Exposure to Perfluoroalkyl Substances and Birth Outcomes; An Updated Analysis from the Danish National Birth Cohort. International Journal of Environmental Research and Public Health, 15(9), 1832. https://doi.org/10.3390/ijerph15091832






