Allostatic Load and Effort-Reward Imbalance: Associations over the Working-Career
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Variables
2.2.1. Effort-Reward Imbalance
2.2.2. Allostatic Load
2.2.3. Covariates
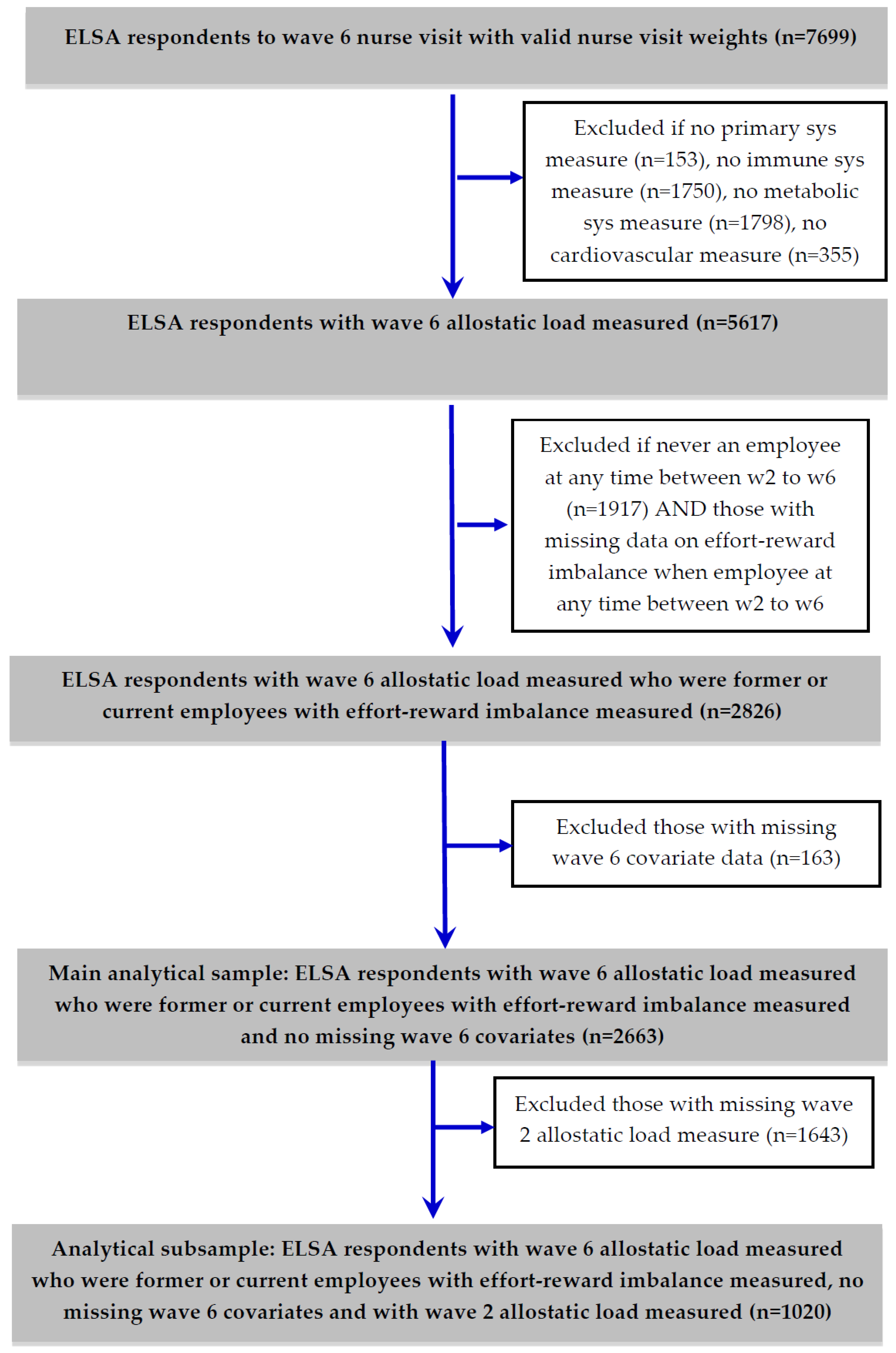
2.3. Analytical Sample and Statistical Models
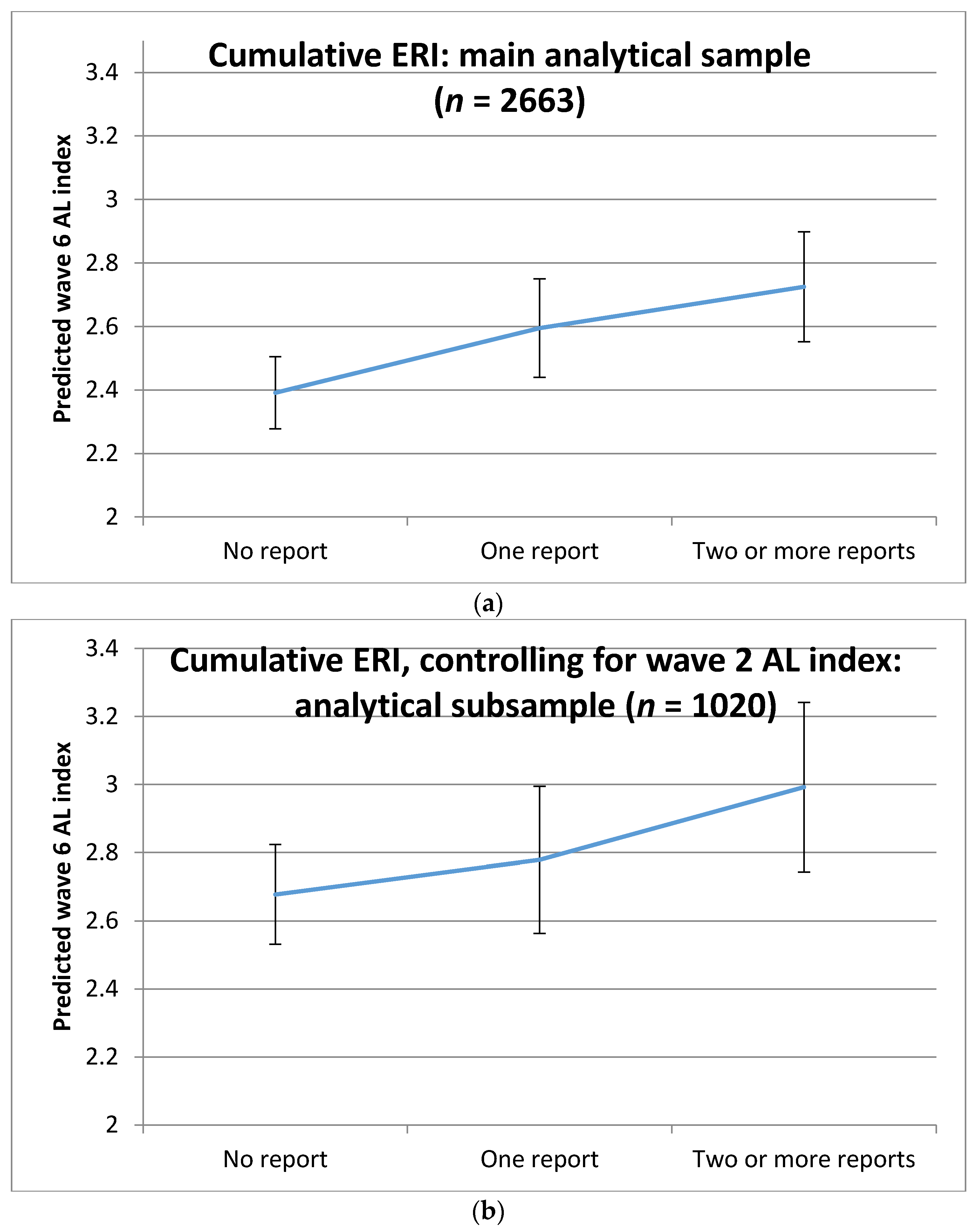
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harvey, S.B.; Modini, M.; Joyce, S.; Milligan-Saville, J.S.; Tan, L.; Mykletun, A.; Bryant, R.A.; Christensen, H.; Mitchell, P.B. Can work make you mentally ill? A systematic meta-review of work-related risk factors for common mental health problems. Occup. Environ. Med. 2017, 74, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Rugulies, R.; Aust, B.; Madsen, I.E. Effort–reward imbalance at work and risk of depressive disorders. A systematic review and meta-analysis of prospective cohort studies. Scand. J. Work. Environ. Health 2017, 43, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Knardahl, S.; Johannessen, H.A.; Sterud, T.; Härmä, M.; Rugulies, R.; Seitsamo, J.; Borg, V. The contribution from psychological, social, and organizational work factors to risk of disability retirement: A systematic review with meta-analyses. BMC Public Health 2017, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Chandola, T.; Heraclides, A.; Kumari, M. Psychophysiological biomarkers of workplace stressors. Neurosci. Biobehav. Rev. 2010, 35, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, J.; Li, J. Work Stress and Altered Biomarkers: A Synthesis of Findings Based on the Effort-Reward Imbalance Model. Int. J. Environ. Res. Public Health 2017, 14, 1373. [Google Scholar] [CrossRef] [PubMed]
- Wahrendorf, M.; Chandola, T. A Life Course Perspective on Work Stress and Health. In Work Stress and Health in a Globalized Economy. Aligning Perspectives on Health, Safety and Well-Being; Siegrist, J., Wahrendorf, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 43–66. ISBN 978-3-319-32935-2. [Google Scholar]
- Kivimäki, M.; Nyberg, S.T.; Batty, G.D.; Fransson, E.I.; Heikkilä, K.; Alfredsson, L.; Bjorner, J.B.; Borritz, M.; Burr, H.; Casini, A.; et al. Job strain as a risk factor for coronary heart disease: A collaborative meta-analysis of individual participant data. Lancet 2012, 380, 1491–1497. [Google Scholar] [CrossRef]
- Chandola, T.; Brunner, E.; Marmot, M. Chronic stress at work and the metabolic syndrome: Prospective study. BMJ 2006, 332, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Wahrendorf, M.; Chandola, T. A Life Course Perspective on Work Stress and Health. In Work Stress and Health in a Globalized Economy, 1st ed.; Siegrist, J., Wahrendorf, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 43–66. ISBN 978-3-319-32935-2. [Google Scholar]
- Karasek, R.A. Job Demands, Job Decision Latitude, and Mental Strain: Implications for Job Redesign. Adm. Sci. Q. 1979, 24, 285. [Google Scholar] [CrossRef]
- Siegrist, J.; Wahrendorf, M. (Eds.) Failed Social Reciprocity beyond the Work Role. In Work Stress and Health in a Globalized Economy, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 275–291. ISBN 978-3-319-32935-2. [Google Scholar]
- Siegrist, J. Adverse health effects of high-effort/low-reward conditions. J. Occup. Health Psychol. 1996, 1, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H. Perturbing the Organism: The Biology of Stressful Experience, 1st ed.; University of Chicago Press: Chicago, IL, USA, 1992; ISBN 978-0226890418. [Google Scholar]
- Eddy, P.; Wertheim, E.H.; Hale, M.W.; Wright, B.J. A Systematic Review and Meta-analysis of the Effort-reward Imbalance Model of Workplace Stress and HPA Axis Measures of Stress. Psychosom. Med. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Jarczok, M.N.; Jarczok, M.; Mauss, D.; Koenig, J.; Li, J.; Herr, R.M.; Thayer, J.F. Autonomic nervous system activity and workplace stressors—A systematic review. Neurosci. Biobehav. Rev. 2013, 37, 1810–1823. [Google Scholar] [CrossRef] [PubMed]
- Eddy, P.; Heckenberg, R.; Wertheim, E.H.; Kent, S.; Wright, B.J. A systematic review and meta-analysis of the effort-reward imbalance model of workplace stress with indicators of immune function. J. Psychosom. Res. 2016, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hansen, Š.A.M.; Larsen, A.D.; Rugulies, R.; Garde, A.H.; Knudsen, L.E. A Review of the Effect of the Psychosocial Working Environment on Physiological Changes in Blood and Urine. Basic Clin. Pharmacol. Toxicol. 2009, 105, 73–83. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Seeman, T. Protective and Damaging Effects of Mediators of Stress: Elaborating and Testing the Concepts of Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1999, 896, 30–47. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef]
- McEwen, B.S. Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiol. Aging 2002, 23, 921–939. [Google Scholar] [CrossRef]
- Mauss, D.; Li, J.; Schmidt, B.; Angerer, P.; Jarczok, M.N. Measuring allostatic load in the workforce: A systematic review. Ind. Health 2015, 53, 5–20. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004, 1032, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stressed or stressed out: What is the difference? J. Psychiatry Neurosci. 2005, 30, 315–318. [Google Scholar] [PubMed]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Juster, R.P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Wingfield, J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010, 57, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mauss, D.; Jarczok, M.N.; Fischer, J.E. A streamlined approach for assessing the Allostatic Load Index in industrial employees. Stress 2015, 18, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Mauss, D.; Jarczok, M.N.; Fischer, J.E. The streamlined Allostatic Load Index: A replication of study results. Stress 2016, 19, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Juster, R.P.; Sindi, S.; Marin, M.F.; Perna, A.; Hashemi, A.; Pruessner, J.C.; Lupien, S.J. A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology 2011, 36, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Bellingrath, S.; Weigl, T.; Kudielka, B.M. Chronic work stress and exhaustion is associated with higher allostastic load in female school teachers. Stress 2009, 12, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Allisey, A.; Rodwell, J.; Noblet, A. Personality and the effort-reward imbalance model of stress: Individual differences in reward sensitivity. Work Stress 2012, 26, 230–251. [Google Scholar] [CrossRef]
- Van Vegchel, N.; De Jonge, J.; Bosma, H.; Schaufeli, W. Reviewing the effort-reward imbalance model: Drawing up the balance of 45 empirical studies. Soc. Sci. Med. 2005, 60, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Landolt, K.; O’Donnell, E.; Hazi, A.; Dragano, N.; Wright, B.J. An experimental examination of the effort-reward imbalance model of occupational stress: Increased financial reward is related to reduced stress physiology. Biol. Psychol. 2017, 125, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kudielka, B.M.; Von Känel, R.; Gander, M.-L.; Fischer, J.E. Effort-reward imbalance, overcommitment and sleep in a working population. Work Stress 2004, 18, 167–178. [Google Scholar] [CrossRef]
- Calnan, M.; Wadsworth, E.; May, M.; Smith, A.; Wainwright, D. Job strain, effort—Reward imbalance, and stress at work: Competing or complementary models? Scand. J. Public Health 2004, 32, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, J.; Starke, D.; Chandola, T.; Godin, I.; Marmot, M.; Niedhammer, I.; Peter, R. The measurement of effort-reward imbalance at work: European comparisons. Soc. Sci. Med. 2004, 58, 1483–1499. [Google Scholar] [CrossRef]
- Kouvonen, A.; Kivimäki, M.; Elovainio, M.; Pentti, J.; Linna, A.; Virtanen, M.; Vahtera, J. Effort/reward imbalance and sedentary lifestyle: An observational study in a large occupational cohort. Occup. Environ. Med. 2006, 63, 422–427. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, J.; Bosma, H.; Peter, R.; Siegrist, J. Job strain, effort-reward imbalance and employee well-being: A large-scale cross-sectional study. Soc. Sci. Med. 2000, 50, 1317–1327. [Google Scholar] [CrossRef]
- Söderberg, M.; Rosengren, A.; Hillström, J.; Lissner, L.; Torén, K. A cross-sectional study of the relationship between job demand-control, effort-reward imbalance and cardiovascular heart disease risk factors. BMC Public Health 2012, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Bathman, L.M.; Almond, J.; Hazi, A.; Wright, B.J. Effort-reward imbalance at work and pre-clinical biological indices of ill-health: The case for salivary immunoglobulin A. Brain Behav. Immun. 2013, 33, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Sibille, K.T.; McBeth, J.; Smith, D.; Wilkie, R. Allostatic load and pain severity in older adults: Results from the English Longitudinal Study of Ageing. Exp. Gerontol. 2017, 88, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jarczok, M.N.; Koenig, J.; Li, J.; Mauss, D.; Hoffmann, K.; Schmidt, B.; Fischer, J.E.; Thayer, J.F. The association of work stress and glycemic status is partially mediated by autonomic nervous system function: Cross-sectional results from the Mannheim Industrial Cohort Study (MICS). PLoS ONE 2016, 11, e0160743. [Google Scholar] [CrossRef] [PubMed]
- Beckie, T.M. A Systematic Review of Allostatic Load, Health, and Health Disparities. Biol. Res. Nurs. 2012, 14, 311–346. [Google Scholar] [CrossRef] [PubMed]
- Chandola, T.; Rouxel, P.; Marmot, M.G.; Kumari, M. Retirement and Socioeconomic Differences in Diurnal Cortisol: Longitudinal Evidence From a Cohort of British Civil Servants. J. Gerontol. Ser. B 2017, 341, c6149. [Google Scholar] [CrossRef] [PubMed]
- Wahrendorf, M.; Dragano, N.; Siegrist, J. Social position, work stress, and retirement intentions: A study with older employees from 11 European countries. Eur. Sociol. Rev. 2013, 29, 792–802. [Google Scholar] [CrossRef]
- Read, S.; Grundy, E. Allostatic load and health in the older population of England: A crossed-lagged analysis. Psychosom. Med. 2014, 76, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.E.; McEwen, B.S.; Rowe, J.W.; Singer, B.H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl. Acad. Sci. USA 2001, 98, 4770–4775. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Breeze, E.; Banks, J.; Nazroo, J. Cohort profile: The English Longitudinal Study of Ageing. Int. J. Epidemiol. 2013, 42, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N.; Garbarino, S.; Siegrist, J. The Use of Parsimonious Questionnaires in Occupational Health Surveillance: Psychometric Properties of the Short Italian Version of the Effort/Reward Imbalance Questionnaire. Sci. World J. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siegrist, J.; Li, J.; Montano, D. Psychometric Properties of the Effort-Reward Imbalance Questionnaire; Duesseldorf University: Duesseldorf, Germany, 2014. [Google Scholar]
- Seeman, T.E.; Singer, B.H.; Rowe, J.W.; Horwitz, R.I.; McEwen, B.S. Price of adaptation—Allostatic load and its health consequences. MacArthur studies of successful aging. Arch. Intern. Med. 1997, 157, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Kirschbaum, C.; Steptoe, A. Hair cortisol and adiposity in a population-based sample of 2527 men and women aged 54 to 87 years. Obesity 2017, 25, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Kirschbaum, C. Analysis of cortisol in hair—State of the art and future directions. Brain Behav. Immun. 2012, 26, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.D.; Yoshinaga, H. Abdominal fat distribution and coronary heart disease risk factors in men-waist/height ratio as a simple and useful predictor. Int. Obes. 1995, 19, 589. [Google Scholar]
- Almadi, T.; Cathers, I.; Chow, C.M. Associations among work-related stress, cortisol, inflammation, and metabolic syndrome. Psychophysiology 2013, 50, 821–830. [Google Scholar] [CrossRef] [PubMed]
- NatCen Social Research. English Longitudinal Study of Ageing (ELSA). Waves 2, 4 and 6. User Guide to the Nurse Datasets; Natcen Social Research: London, UK, 2014. [Google Scholar]
- StataCorp. StataCorp Stata Statistical Software 2015; StataCorp.: College Station, TX, USA, 2015. [Google Scholar]
- Hintsa, T.; Kouvonen, A.; McCann, M.; Jokela, M.; Elovainio, M.; Demakakos, P. Higher effort–reward imbalance and lower job control predict exit from the labour market at the age of 61 years or younger: Evidence from the English Longitudinal Study of Ageing. J. Epidemiol. Community Health 2015, 69, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N.; Garbarino, S. Sleep, health and wellness at work: A scoping review. Int. J. Environ. Res. Public Health 2017, 14, 1347. [Google Scholar] [CrossRef] [PubMed]
| Cut off for AL Index | |||||
|---|---|---|---|---|---|
| Wave 6 Allostatic Load Biomarkers | Mean/% | SD | n | Women | Men |
| Neuroendocrine system | |||||
| Cortisol (pg/mL) | 46.9 | 201.9 | 947 | >21.9 | >18.5 |
| Cortisone (pg/mg) | 8.5 | 9.0 | 956 | >7.6 | >11.5 |
| Insulin growth factor 1 (nmol/L) | 17.1 | 4.9 | 2640 | <13 | <15 |
| Pulse rate (beats per minute) | 54.7 | 12.2 | 2638 | >67.5 | >66.5 |
| Immune system | |||||
| White blood cell (×109 cells/L) | 6.3 | 1.8 | 2614 | >7.42 | >7.63 |
| C-reactive protein (mg/L) | 2.0 | 1.9 | 2539 | >3 | >2.6 |
| Fibrinogen (g/L) | 2.9 | 0.5 | 2561 | >3.3 | >3.2 |
| Metabolic system | |||||
| Total cholesterol to HDL ratio | 3.6 | 1.1 | 2648 | >3.89 | >4.46 |
| Triglycerides (mmol/L) | 1.5 | 0.9 | 2650 | >1.7 | >1.9 |
| Hba1c (%) | 40.0% | 7.1 | 2613 | >42 | >43 |
| Cardiovascular system | |||||
| Systolic blood pressure (mmHg) | 132.6 | 17.2 | 2638 | >145.5 | >147 |
| Diastolic blood pressure (mmHg) | 76.9 | 10.2 | 2638 | >81.5 | >82.5 |
| Anti-hypertensive medication | 21.0% | - | 2663 | ||
| Anthropometric system | |||||
| Waist to height ratio | 0.6 | 0.1 | 2641 | >0.6325 | >0.6327 |
| Underweight (%) | 0.7% | - | 2628 | <18.5 | <18.5 |
| Wave 6 allostatic load index | 2.6 | 2.0 | 2663 | ||
| Wave 2 allostatic load biomarkers | |||||
| Neuroendocrine system | |||||
| Pulse rate (beats per minute) | 54.1 | 11.5 | 1020 | >69 | >68 |
| Immune system | |||||
| C-reactive protein (mg/L) | 2.1 | 2.1 | 968 | >3.7 | >3.2 |
| Fibrinogen (g/L) | 3.0 | 0.6 | 999 | >3.7 | >3.5 |
| Metabolic system | |||||
| Total cholesterol to HDL ratio | 4.0 | 1.0 | 1013 | >4.44 | >4.85 |
| Triglycerides (mmol/L) | 1.8 | 1.2 | 1014 | >2.1 | >2.3 |
| Hba1c (mmol/mol) | 36.1 | 6.2 | 1007 | >38.8 | >39.9 |
| Cardiovascular system | |||||
| Systolic blood pressure (mmHg) | 132.2 | 17.3 | 1020 | >149 | >148 |
| Diastolic blood pressure (mmHg) | 77.7 | 10.7 | 1020 | >82.5 | >84 |
| Anti-hypertensive medication | 8.2% | - | 1020 | ||
| Anthropometric system | |||||
| Waist to height ratio | 0.6 | 0.1 | 1009 | >0.6214 | >0.6289 |
| Underweight (%) | 0.5% | - | 1003 | <18.5 | <18.5 |
| Wave 2 allostatic load index | 1.7 | 1.6 | 1020 | ||
| Wave 6 Variables | Cumulative Effort-Reward Imbalance | ||
|---|---|---|---|
| No Report | One Report | Two or More Reports | |
| Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | |
| n | 1403 | 717 | 543 |
| AL index mean (SD) | 2.45 (1.90) | 2.63 (2.07) | 2.91 (1.97) |
| Gender | |||
| Men | 53% | 52% | 47% |
| Women | 47% | 48% | 53% |
| Age groups | |||
| 50–54 | 10% | 17% | 1% |
| 55–59 | 21% | 26% | 36% |
| 60–64 | 28% | 29% | 40% |
| 65–69 | 26% | 20% | 19% |
| 70–74 | 11% | 6% | 3% |
| 75+ | 4% | 2% | 1% |
| Ethnicity | |||
| White British | 97% | 98% | 97% |
| Non-white | 3% | 2% | 3% |
| Social class | |||
| Professional | 44% | 40% | 32% |
| Intermediate | 15% | 11% | 11% |
| Small employers | 14% | 14% | 10% |
| Lower & technical | 5% | 10% | 13% |
| Semi-routine & routine | 21% | 25% | 34% |
| Employment status | |||
| Employed | 52% | 60% | 71% |
| Retired | 44% | 35% | 28% |
| Disabled/looking after family | 4% | 5% | 2% |
| Current smoker | |||
| No | 91% | 88% | 88% |
| Yes | 9% | 12% | 12% |
| Self-reported health | |||
| Excellent/good | 90% | 85% | 78% |
| Fair/poor | 10% | 15% | 22% |
| Number of medications | |||
| 0 meds. | 39% | 35% | 32% |
| 1–2 meds. | 30% | 32% | 33% |
| 3–5 meds. | 22% | 22% | 25% |
| ≥6 meds. | 9% | 10% | 10% |
| Depressive symptoms | |||
| CESD score < 4 | 95% | 91% | 87% |
| CESD score ≥ 4 | 5% | 9% | 13% |
| Vigorous physical activity | |||
| <once a week | 30% | 27% | 28% |
| Once a week | 12% | 10% | 13% |
| 1–3 times a month | 12% | 12% | 11% |
| Never | 47% | 51% | 48% |
| Moderate physical activity | |||
| <once a week | 73% | 73% | 74% |
| Once a week | 16% | 14% | 13% |
| 1–3 times a month | 5% | 6% | 6% |
| Never | 5% | 8% | 7% |
| Alcohol consumption | |||
| Almost every day | 16% | 14% | 14% |
| 5–6 days a week | 8% | 6% | 6% |
| 3–4 days a week | 20% | 18% | 13% |
| 1–2 a week | 24% | 29% | 28% |
| 1–2 a month | 12% | 13% | 14% |
| Once in 2 months | 7% | 6% | 6% |
| 1–2 times a year | 6% | 8% | 9% |
| Never | 7% | 5% | 9% |
| Wave 6 Allostatic Load (AL) System | Cumulative Effort-Reward Imbalance (Ref.: No Report) | |
|---|---|---|
| One Report | Two or More Reports | |
| Coeff. (95% CI) | Coeff. (95% CI) | |
| Sympathoadrenal 1,2 | 0.09 (−0.04, 0.22) | 0.11 (−0.02, 0.24) |
| p-value | 0.187 | 0.105 |
| Immune 1,2 | 0.11 (−0.02, 0.24) | 0.16 (0.02, 0.29) |
| p-value | 0.107 | 0.025 |
| Metabolic 1,2 | 0.08 (−0.05, 0.21) | 0.10 (−0.04, 0.23) |
| p-value | 0.206 | 0.152 |
| Cardiovascular 1,2 | 0.01 (−0.11, 0.13) | 0.11 (−0.01, 0.23) |
| p-value | 0.859 | 0.081 |
| Anthropometric 1,3 | −0.05 (−0.30, 0.21) | 0.06 (−0.21, 0.33) |
| p-value | 0.727 | 0.670 |
| Overall AL index 1,4 | 0.08 (0.003, 0.16) | 0.13 (0.05, 0.21) |
| p-value | 0.042 | 0.001 |
| ERI Measured at | Coeff. (95% CI) | p-Value |
|---|---|---|
| Wave 2 | 0.03 (−0.06, 0.12) | 0.476 |
| Wave 3 | −0.03 (−0.12, 0.06) | 0.487 |
| Wave 4 | 0.04 (−0.05, 0.12) | 0.368 |
| Wave 5 | 0.09 (−0.002, 0.17) | 0.055 |
| Wave 6 | 0.13 (0.03, 0.22) | 0.008 |
| Coeff. (95% CI) | p-Value | Coeff. (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Cumulative ERI (Ref.: No report of ERI) | Vigorous physical activity (Ref.: <once a week) | ||||
| One report of ERI | 0.04 (−0.06, 0.13) | 0.434 | Once a week | 0.06 (−0.08, 0.19) | 0.423 |
| Two or more reports of ERI | 0.11 (0.01, 0.22) | 0.037 | 1–3 times a month | 0.06 (−0.09, 0.22) | 0.417 |
| AL index at W2 | 0.18 (0.16, 0.21) | <0.001 | Never | 0.06 (−0.05, 0.17) | 0.289 |
| Socio-economic classification (Ref.: Professional) | Moderate physical activity (Ref.: <once a week) | ||||
| Intermediate | 0.04 (−0.09, 0.17) | 0.577 | Once a week | 0.08 (−0.03, 0.19) | 0.141 |
| Small employers | 0.09 (−0.04, 0.22) | 0.160 | 1–3 times a month | 0.15 (−0.01, 0.30) | 0.067 |
| Lower & technical | 0.05 (−0.10, 0.20) | 0.529 | Never | 0.07 (−0.08, 0.23) | 0.340 |
| Semi-routine & routine | 0.08 (−0.02, 0.19) | 0.132 | Alcohol consumption (Ref.: Almost every day) | ||
| Employment status (Ref.: Employed) | 5–6 days a week | −0.11 (−0.29, 0.08) | 0.271 | ||
| Retired | −0.02 (−0.11, 0.08) | 0.721 | 3–4 days a week | −0.07 (−0.21, 0.08) | 0.371 |
| Sick-Disable/family carer | 0.04 (−0.13, 0.21) | 0.659 | 1–2 a week | −0.09 (−0.22, 0.04) | 0.170 |
| Gender (Ref.: Women) | 1–2 a month | 0.03 (−0.12, 0.18) | 0.682 | ||
| Men | −0.13 (−0.26, −0.01) | 0.040 | Once in 2 months | −0.03 (−0.22, 0.16) | 0.742 |
| Age (Model 1: Ref.: 50–54) (Model 2: Ref.: Men * 60–64) | 1–2 times a year | 0.11 (−0.05, 0.27) | 0.176 | ||
| 55–59 | Never | 0.03 (−0.12, 0.18) | 0.690 | ||
| 60–64 | 0.06 (−0.06, 0.18) | 0.316 | Depressive symptoms (Ref.: CESD score < 4) | ||
| 65–69 | −0.12 (−0.30, 0.06) | 0.204 | CESD score ≥ 4 | −0.05 (−0.21, 0.12) | 0.590 |
| 70–74 | 0.01 (−0.27, 0.29) | 0.957 | Intercept | 0.42 (0.23, 0.62) | <0.001 |
| 75+ | |||||
| Gender * Age (Model 1: Ref.: 50–54) (Model 2: Ref.: Men * 60–64) | |||||
| Men aged 55–59 | |||||
| Men aged 60–64 | |||||
| Men aged 65–69 | 0.04 (−0.14, 0.22) | 0.636 | |||
| Men aged 70–74 | 0.39 (0.15, 0.63) | 0.001 | |||
| Men aged 75+ | 0.30 (−0.05, 0.66) | 0.091 | |||
| Ethnicity (Ref.: White British) | |||||
| Non-White ethnic group | 0.10 (−0.17, 0.37) | 0.487 | |||
| Current smoker (Ref.: No) | |||||
| Yes | 0.07 (−0.06, 0.21) | 0.279 | |||
| Self-reported Health (Ref.: Excellent/good) | |||||
| Fair/poor | 0.07 (−0.04, 0.18) | 0.243 | |||
| Number of medications (Ref.: 0 meds.) | |||||
| 1–2 meds. | 0.11 (−0.01, 0.22) | 0.079 | |||
| 3–5 meds. | 0.16 (0.04, 0.29) | 0.012 | |||
| ≥6 meds. | 0.19 (0.04, 0.34) | 0.012 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuitún Coronado, J.I.; Chandola, T.; Steptoe, A. Allostatic Load and Effort-Reward Imbalance: Associations over the Working-Career. Int. J. Environ. Res. Public Health 2018, 15, 191. https://doi.org/10.3390/ijerph15020191
Cuitún Coronado JI, Chandola T, Steptoe A. Allostatic Load and Effort-Reward Imbalance: Associations over the Working-Career. International Journal of Environmental Research and Public Health. 2018; 15(2):191. https://doi.org/10.3390/ijerph15020191
Chicago/Turabian StyleCuitún Coronado, José Ignacio, Tarani Chandola, and Andrew Steptoe. 2018. "Allostatic Load and Effort-Reward Imbalance: Associations over the Working-Career" International Journal of Environmental Research and Public Health 15, no. 2: 191. https://doi.org/10.3390/ijerph15020191
APA StyleCuitún Coronado, J. I., Chandola, T., & Steptoe, A. (2018). Allostatic Load and Effort-Reward Imbalance: Associations over the Working-Career. International Journal of Environmental Research and Public Health, 15(2), 191. https://doi.org/10.3390/ijerph15020191





