The Association between Low Body Weight and Scoliosis among Korean Elementary School Students
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Tools
2.2.1. Body Measurements
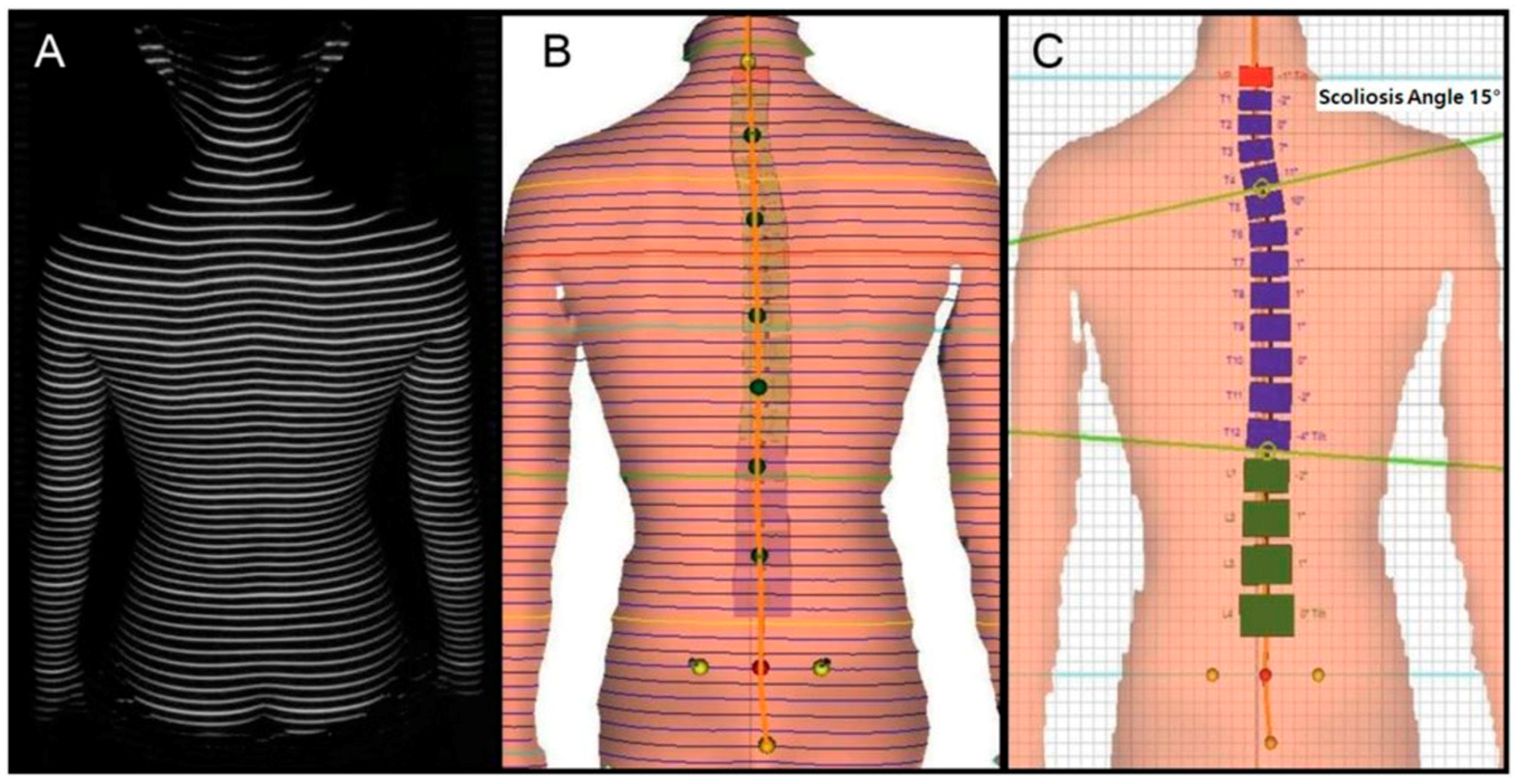
2.2.2. Identifying Scoliosis
2.3. Statistical Analysis
3. Results
3.1. Correlation between BMI, Body Composition, and Scoliosis Risk Factors
3.2. Correlation between BMI and Risk of Scoliosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suh, S.-W.; Modi, H.N.; Yang, J.-H.; Hong, J.-Y. Idiopathic scoliosis in Korean schoolchildren: A prospective screening study of over 1 million children. Eur. Spine J. 2011, 20, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.A.; Izatt, M.T.; Adam, C.J.; Labrom, R.D.; Askin, G.N. Postoperative pain relief using intermittent intrapleural analgesia following thoracoscopic anterior correction for progressive adolescent idiopathic scoliosis. Scoliosis 2013, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Wick, J.M.; Konze, J.; Alexander, K.; Sweeney, C. Infantile and juvenile scoliosis: The crooked path to diagnosis and treatment. AORN J. 2009, 90, 347–380. [Google Scholar] [CrossRef] [PubMed]
- Nault, M.L.; Allard, P.; Hinse, S.; Le Blanc, R.; Caron, O.; Labelle, H.; Sadeghi, H. Relations between standing stability and body posture parameters in adolescent idiopathic scoliosis. Spine 2002, 27, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Michikawa, T.; Yonezawa, I.; Takaso, M.; Minami, S.; Soshi, S.; Tsuji, T.; Okada, E.; Abe, K.; Takahashi, M.; et al. Physical activities and lifestyle factors related to adolescent idiopathic scoliosis. JBJS 2017, 99, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Hockenberry, M.J.; Wilson, D. Wong’s Nursing Care of Infants and Children; Elsevier Health Sciences: Philadelphia, PA, USA, 2014. [Google Scholar]
- Sapountzi-Krepia, D.S.; Valavanis, J.; Panteleakis, G.P.; Zangana, D.T.; Vlachojiannis, P.C.; Sapkas, G.S. Perceptions of body image, happiness and satisfaction in adolescents wearing a Boston brace for scoliosis treatment. J. Adv. Nurs. 2001, 35, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.M.; Latchford, G.J.; Hall, R.M.; Dickson, R.A. Do chronic medical conditions increase the risk of eating disorder? A cross-sectional investigation of eating pathology in adolescent females with scoliosis and diabetes. J. Adolesc. Health 2008, 42, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.S.K.; Lee, W.T.K.; Tse, Y.K.; Tang, S.P.; Lee, K.M.; Guo, X.; Cheng, J.C.Y. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: A study of 598 patients. Spine 2003, 28, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Grieken, A.; Renders, C.; Wijtzes, A.; Hirasing, R.; Raat, H. Overweight, obesity and underweight is associated with adverse psychosocial and physical health outcomes among 7-year-old children: The ‘be active, eat right’ study. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Goodbody, C.M.; Sankar, W.N.; Flynn, J.M. Presentation of adolescent idiopathic scoliosis: The bigger the kid, the bigger the curve. J. Pediatr. Orthop. 2017, 37, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yong, F.; Wong, H.-K.; Chow, K.-Y. Prevalence of adolescent idiopathic scoliosis among female school children in Singapore. Ann. Acad. Med. Singapore 2009, 38, 1056–1063. [Google Scholar] [PubMed]
- Glattes, R.C.; Burton, D.C.; Lai, S.M.; Frasier, E.; Asher, M.A. The reliability and concurrent validity of the scoliosis research society-22r patient questionnaire compared with the child health questionnaire-cf87 patient questionnaire for adolescent spinal deformity. Spine 2007, 32, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Drerup, B.; Hierholzer, E. Back shape measurement using video rasterstereography and three-dimensional reconstruction of spinal shape. Clin. Biomech. 1994, 9, 28–36. [Google Scholar] [CrossRef]
- Schroder, J. In Posture analysis: Variations and reliability of biomechnical parameters in bepedal standing by means of formetric-system. In Proceedings of the 14th Annual Congress of the European College of Sports Science, Oslo, Norway, 24–27 June 2009. [Google Scholar]
- Dommisse, G. The management of scoliosis. S. Afr. Med. J. 1970, 44, 1331–1335. [Google Scholar] [PubMed]
- Lonstein, J.E.; Bjorklund, S.; Wanninger, M.H.; Nelson, R.P. Voluntary school screening for scoliosis in Minnesota. J. Bone Joint Surg. Am. 1982, 64, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Yamagata, M.; Arai, S.; Kitahara, H.; Minami, S. School screening for scoliosis by the Chiba University medical school screening program: Results of 1.24 million students over an 8-year period. Spine 1988, 13, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Willner, S.; Udén, A. A prospective prevalence study of scoliosis in southern Sweden. Acta Orthop. Scand. 1982, 53, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Janicki, J.A.; Alman, B. Scoliosis: Review of diagnosis and treatment. Paediatr. Child Health 2007, 12, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Goldbloom, R.B. Screening for Idiopathic Adolescent Scoliosi; Canadian Task Force on the Periodic Health Examination: Ottawa, ON, Canada, 1994; pp. 346–354. [Google Scholar]
- Soucacos, P.N.; Zacharis, K.; Soultanis, K.; Gelalis, J.; Xenakis, T.; Beris, A.E. Risk factors for idiopathic scoliosis: Review of a 6-year prospective study. Orthopedics 2000, 23, 833–838. [Google Scholar] [PubMed]
- Nissinen, M.; Heliövaara, M.; Seitsamo, J.; Poussa, M. Trunk asymmetry, posture, growth, and risk of scoliosis: A three-year follow-up of Finnish prepubertal school children. Spine 1993, 18, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Pearsall, D.; Reid, J.; Hedden, D. Comparison of three noninvasive methods for measuring scoliosis. Phys. Ther. 1992, 72, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-K.; Hui, J.H.; Rajan, U.; Chia, H.-P. Idiopathic scoliosis in Singapore schoolchildren: A prevalence study 15 years into the screening program. Spine 2005, 30, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Roberts, R.; Towell, T. Factors predictive of bone mineral density in eating-disordered women: A longitudinal study. Int. J. Eat. Disord. 2000, 27, 29–35. [Google Scholar] [CrossRef]
- Mirtz, T.A.; Thompson, M.A.; Greene, L.; Wyatt, L.A.; Akagi, C.G. Adolescent idiopathic scoliosis screening for school, community, and clinical health promotion practice utilizing the precede-proceed model. Chiropr. Osteop. 2005, 13, 25. [Google Scholar] [CrossRef] [PubMed]
| Variables | Male (n = 504) | Female (n = 461) | p-Value |
|---|---|---|---|
| Age (years) | 11.15 ± 1.21 | 11.14 ± 1.22 | 0.985 |
| Weight (kg) | 38.82 ± 8.99 | 38.17 ± 17.94 | 0.467 |
| Height (cm) | 143.97 ± 9.86 | 143.80 ± 11.32 | 0.810 |
| Lean mass (kg) | 28.13 ± 6.12 | 26.17 ± 5.24 | <0.001 |
| Percent body fat (%) | 22.38 ± 7.94 | 24.87 ± 7.11 | <0.001 |
| BMI (kg/m2) | 18.51 ± 2.59 | 17.85 ± 2.50 | <0.001 |
| Trunk Length (mm) | 369.39 ± 34.69 | 375.79 ± 36.80 | 0.006 |
| Trunk Inclination (mm) | 13.42 ± 19.75 | 12.71 ± 18.43 | 0.565 |
| Trunk Imbalance (mm) | −2.07 ± 8.86 | −1.42 ± 9.40 | 0.265 |
| Trunk Torsion (°) | −0.38 ± 5.94 | 0.34 ± 5.50 | 0.047 |
| Pelvic Inclination (°) | 0.18 ± 2.85 | 0.23 ± 2.72 | 0.812 |
| Pelvic Torsion (°) | 0.78 ± 2.46 | 0.87 ± 2.18 | 0.535 |
| Pelvic Rotation (°) | −1.32 ± 4.23 | −0.91 ± 4.63 | 0.148 |
| Scoliosis Angle (°) | 10.52 ± 4.07 | 10.98 ± 4.35 | 0.088 |
| Total (n = 965) | BMI | ||
|---|---|---|---|
| 1st (n = 391) Normal Weight (18.5 ≤ BMI < 25) | 2nd (n = 375) Underweight (16 ≤ BMI < 18.5) | 3rd (n = 199) Severely Underweight (BMI < 16) | |
| Multivariable-adjusted Ұ | |||
| Lean mass (kg) | 30.21 ± 5.48 | 26.69 ± 5.20 * | 22.19 ± 3.12 * ^ |
| Percent body fat (%) | 29.08 ± 6.67 | 20.77 ± 6.08 * | 18.01 ± 4.68 * ^ |
| Trunk Length (mm) | 380.72 ± 35.08 | 371.27 ± 37.28 * | 358.43 ± 29.47 * |
| Trunk Inclination (mm) | 16.70 ± 19.09 | 12.31 ± 18.74 * | 7.43 ± 18.45 * ^ |
| Trunk Imbalance (mm) | −1.57 ± 9.36 | −2.00 ± 8.93 | −1.68 ± 9.03 |
| Trunk Torsion (°) | −0.24 ± 5.37 | −0.09 ± 5.59 | 0.47 ± 6.68 |
| Pelvic Inclination (°) | 0.05 ± 2.73 | 0.35 ± 2.72 | 0.24 ± 3.01 |
| Pelvic Torsion (°) | 0.48 ± 2.41 | 0.98 ± 2.24 * | 1.21 ± 2.26 * |
| Pelvic Rotation (°) | −1.03 ± 4.20 | −1.28 ± 4.45 | −1.02 ± 4.83 |
| Scoliosis Angle (°) | 10.18 ± 3.79 | 11.06 ± 4.29 * | 11.26 ± 4.71 * |
| Total (n = 965) | BMI | ||
|---|---|---|---|
| 1st (n = 391) Normal Weight (18.5 ≤ BMI < 25) | 2nd (n = 375) Underweight (16 ≤ BMI < 18.5) | 3rd (n = 199) Severely Underweight (BMI < 16) | |
| OR (95% CI) | |||
| Scoliosis | 1 | 1.43 (1.07–1.90) | 1.45 (1.02–2.05) |
| Age-adjusted OR (95% CI) | |||
| Scoliosis | 1 | 1.44 (1.08–1.92) | 1.48 (1.04–2.12) |
| Age and gender-adjusted OR (95% CI) | |||
| Scoliosis | 1 | 1.44 (1.08–1.92) | 1.46 (1.01–2.09) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, K.; Kim, D.-i. The Association between Low Body Weight and Scoliosis among Korean Elementary School Students. Int. J. Environ. Res. Public Health 2018, 15, 2613. https://doi.org/10.3390/ijerph15122613
Jeon K, Kim D-i. The Association between Low Body Weight and Scoliosis among Korean Elementary School Students. International Journal of Environmental Research and Public Health. 2018; 15(12):2613. https://doi.org/10.3390/ijerph15122613
Chicago/Turabian StyleJeon, Kyoungkyu, and Dong-il Kim. 2018. "The Association between Low Body Weight and Scoliosis among Korean Elementary School Students" International Journal of Environmental Research and Public Health 15, no. 12: 2613. https://doi.org/10.3390/ijerph15122613
APA StyleJeon, K., & Kim, D.-i. (2018). The Association between Low Body Weight and Scoliosis among Korean Elementary School Students. International Journal of Environmental Research and Public Health, 15(12), 2613. https://doi.org/10.3390/ijerph15122613





