1. Introduction
Burn injuries are a concern worldwide, with approximately 265,000 burn related deaths every year. Burns are a common injury in all societies and may be a result of hot liquid or hot food spills, contact with hot surfaces, exposure to flames and hot gases, and thermal radiation. In Bangladesh, about 173,000 children under 18 suffer burn-related injuries yearly [
1]. In the United States, yearly about half a million patients seek medical treatment at hospital emergency departments in addition to burn injuries treated at clinics, local health centers, and by private institutions [
2]. In Norway, the number of fatalities in fires has been reduced significantly during the last 30–40 years. The number of burn victims is, however, according to Smolle et al. [
3] not decreasing. Severe burn injuries may result in very long hospitalization periods, and in addition to the physical injury result in major psychological consequences such as body image dissatisfaction, self-harm, post-traumatic stress disorder, anxiety, and depression [
4,
5,
6].
Stimulating the healing of severe burns is difficult. Burn injury treatment therefore has received much research interest [
7,
8,
9]. Improved knowledge about thermal skin injury development, the mechanisms involved, and possible injury-limiting measures are therefore much appreciated. Understanding the situations that may result in burn risks, limiting the likelihood of burns and/or consequences (burn severity), and the promotion of healing processes are therefore necessary measures.
Due to the many burn wounds experienced by the soldiers in the World War II, a systematic approach to studying thermal skin burns was initiated following the war. A series of seminal burn studies was then published in The American Journal of Pathology. These studies included heat transport to and through porcine skin [
10], the importance of time and surface temperature in developing cutaneous burns [
11], and the pathology and pathogenesis of cutaneous burns on pigs [
8]. It was revealed that the degree of burns was dependent on both the temperature and exposure time. A threshold temperature for skin injury was suggested. Several researchers refer to temperature >44 °C for causing burns [
12,
13]. For hot liquids, others refer to 43 °C for the onset of skin injury [
14]. Skin models have also been built for simulating “skin” temperatures during controlled heat flux exposure [
15]. It is assumed that the injury develops linearly with time and exponentially with absolute temperature, i.e., an Arrhenius type of injury development.
Human skin pain receptors are located at approximately 0.1 mm depth and the pain temperature threshold is 44.8 °C [
16,
17]. This is above the assumed threshold temperatures for slow injury development. As burns usually involve much higher basal layer temperatures, the pain signal gives a suitable warning about excessive skin surface heating. In flame exposure scenarios, such as the exposure to the flames from burning biofuel, the skin heating is quite instantaneous, i.e., the damage develops even though the victim is warned of the ongoing process. This is especially dangerous when the victim wears very thin clothing, combustible clothing, or is exposed to burning liquids. Injuries caused by flames, from e.g., kerosene-fueled kitchens/stoves/heaters, represent the most common etiology of burns in adults needing treatment at specialized burn intensive care units [
18].
About 20 years ago, bioethanol design fireplaces were introduced in the consumer market. These units typically release combustion products in the indoor air, raising concerns about indoor climate quality [
19,
20,
21]. These units were generally advertised as being ecologically friendly and with a do-it-yourself installation. However, soon after their introduction, patients injured while handling these units showed up at local burn treatment units. One of the first studies regarding these burn injury incidents was an overview of this new recreational fire threat published by Kraemer et al. [
22], who warned about an underestimated fire risk among the uninformed customers. Later on, several other research groups also reported that bioethanol design fireplaces were involved in severe burn injuries [
23,
24,
25,
26,
27]. Many of these incidents were related to refilling operations, and it was reported that burning liquid fuel exposed persons several meters away. Similar incidents have also been reported in dentist offices [
28] as well as in educational demonstrations [
29] alerting the U.S. Chemical Safety Board (CSB, Washington, DC, USA) about accidents similar to the biofuel incidents [
30].
The purpose of the present work is to analyze the hazardous conditions associated with biofuel fireplaces (and heaters) that may result in flame and/or burning fuel exposure to persons operating, and in the vicinity of, these units. Cases reported in the literature are reviewed and analyzed based on the physical and chemical properties of the fuels involved, i.e., methanol and ethanol. Similar incidents involving technically produced methylated liquid fuel products, for educational or professional purposes, are also included in the analysis. Fuel characteristics, such as the lower flammability limit (LFL) and upper flammability limit (UFL), ignition temperatures, and minimum ignition energy (MIE), as well as typical fuel container designs and refueling methods, are analyzed. Typical accident situations as well as fire/explosion risk perception regarding environmentally friendly fuels are discussed. The study is unique in analyzing the whole process, including technical issues regarding the heaters and fuel containers, the clothing when being exposed, and the environmentally friendly, but still very dangerous, fuels involved.
The motivation for publishing this work is to provide information about the possible accident mechanisms and the victims’ risk perception prior to the incidents as a background for future work for preventing similar accidents. The paper starts with explaining challenges and research on burns and biofuel burns in particular (
Section 1). Selected cases from the literature are presented in
Section 2. Then the theory of ignition, combustion and heat transfer is presented as well as possible temperature and damage modeling (
Section 3). Then, the results based on the previous sections are presented (
Section 4) before the results are discussed (
Section 5) followed by the conclusions (
Section 6). A full analysis as presented in this paper has not been found elsewhere in the literature.
2. Physical, Chemical and Fire Related Properties of Methanol and Ethanol
2.1. Methanol and Ethanol Vapor Density Relative to Air
The molar masses of methanol and ethanol,
and
, are 0.03204 kg/mol and 0.04607 g/mol, respectively. The molar mass of dry air,
, is 0.02897 g/mol. The density of gases, or gas mixtures, may be calculated by:
where
(101,325 Pa) is the ambient pressure,
(kg/mol) is the molar mass of the actual gas or the average molar mass of the gas mixture, and
(J/molK) is the universal gas constant. Since the density is proportional to the molar mass, Equation (1) demonstrates than the density of methanol, and especially ethanol, is higher than the air. A release of these gases may accumulate at lower levels if there is no mixing by e.g., wind or indoor air circulation.
2.2. Chemical Reaction with Air and Minimum Ignition Energy (MIE)
It is well known that methanol and ethanol are combustible liquids. For simplicity, assuming that the air consists of 21 mol% O
2 and 79 mol% N
2 (3.76 mol N
2 per mol O
2), the stoichiometric combustion reaction for methanol in air is given by:
and for ethanol it is given by:
with heat of combustion,
, 635 kJ/mol (19.83 MJ/kg) and 1232 kJ/mol (26.78 MJ/kg), respectively [
31].
Generally, gas–air mixtures ignite most easily at, or close to, the stoichiometric concentrations. For methanol, it may be seen from Equation (1) that this corresponds to 1/8.14 = 12.29% and for ethanol this corresponds to 1/15.28 = 6.54%.
The heat required for igniting an air methanol or an air ethanol gas mixture at just above stoichiometric conditions, i.e., minimum ignition energy (MIE), is respectively 0.14 mJ and 0.28 mJ. This number is recorded for electrical sparks, but indicates that any burning flame will easily ignite such fuel–air mixtures. It is on the same level as the MIE for familiar flammable hydrocarbons such as propane, gasoline, etc.
Vapors of combustible liquids may also ignite when in contact with hot surfaces. The auto ignition temperature (AIT) is recorded for stoichiometric concentrations. The AITs for methanol and ethanol are 470 °C and 365 °C, respectively [
31]. These liquids are therefore not as easily ignited by hot surfaces as long chained hydrocarbon, e.g., diesel with an AIT of 210 °C.
2.3. Vapor Pressures and Flammability Limits in Air
The lower explosion limits (LEL) for methanol and ethanol in air are 6.7% and 3.3%, respectively [
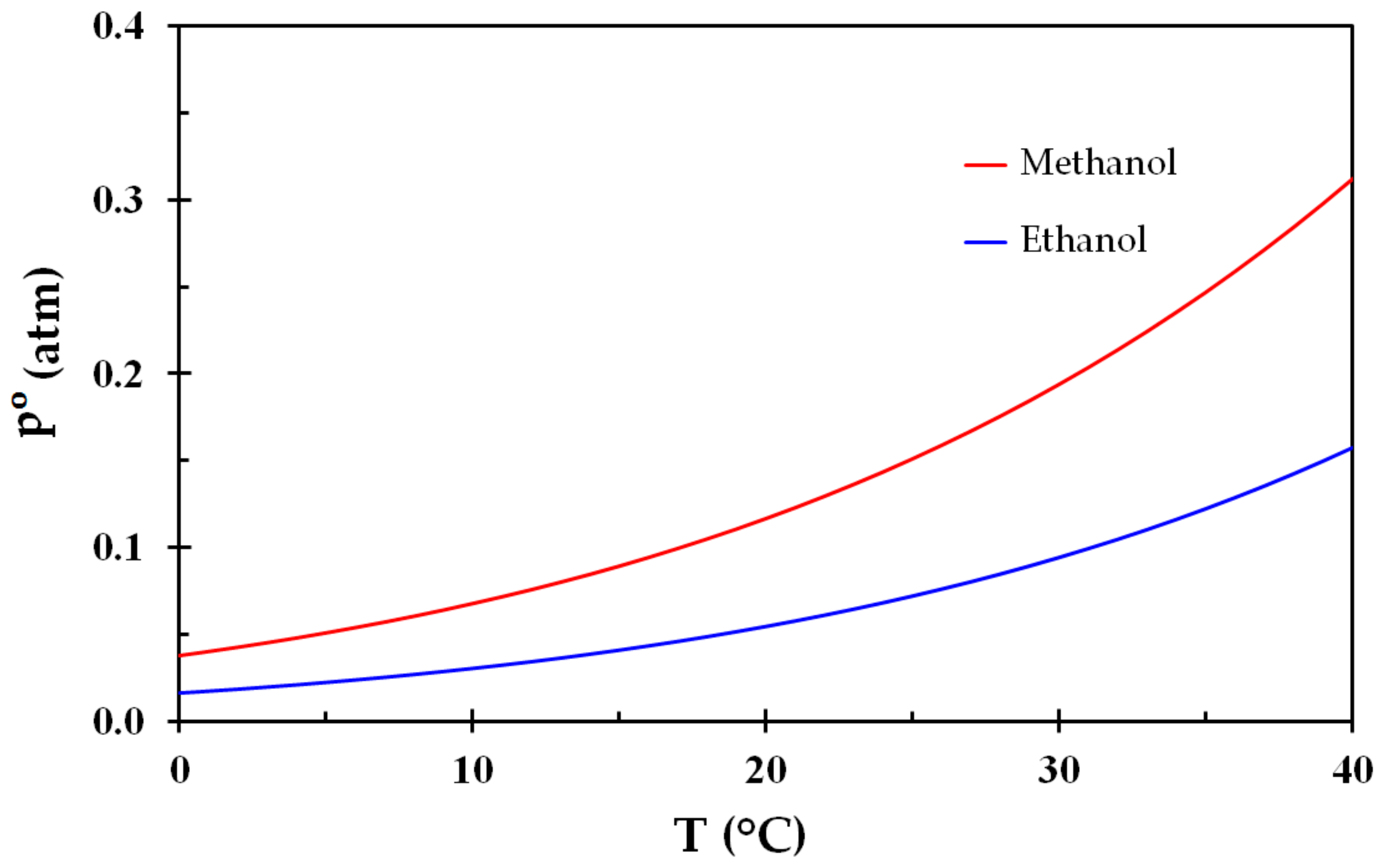
31]. The respective upper explosion limits are 36% and 19%. The hydrogen bonds between the molecule OH-groups makes these liquids harder to vaporize than their equivalent alkanes, giving heat of vaporization 1.1 MJ/kg and 0.84 MJ/kg, respectively. The vapor pressure of methanol and ethanol as a function of temperature may be expressed by [
31]:
where
(torr) is the equilibrium vapor pressure. For methanol, the values of the constants are:
E = 8978.8 K and
= 8.6398, while for ethanol the constants are:
= 9673.9 K and
= 8.8274. The vapor pressure for these liquids is shown in
Figure 1.
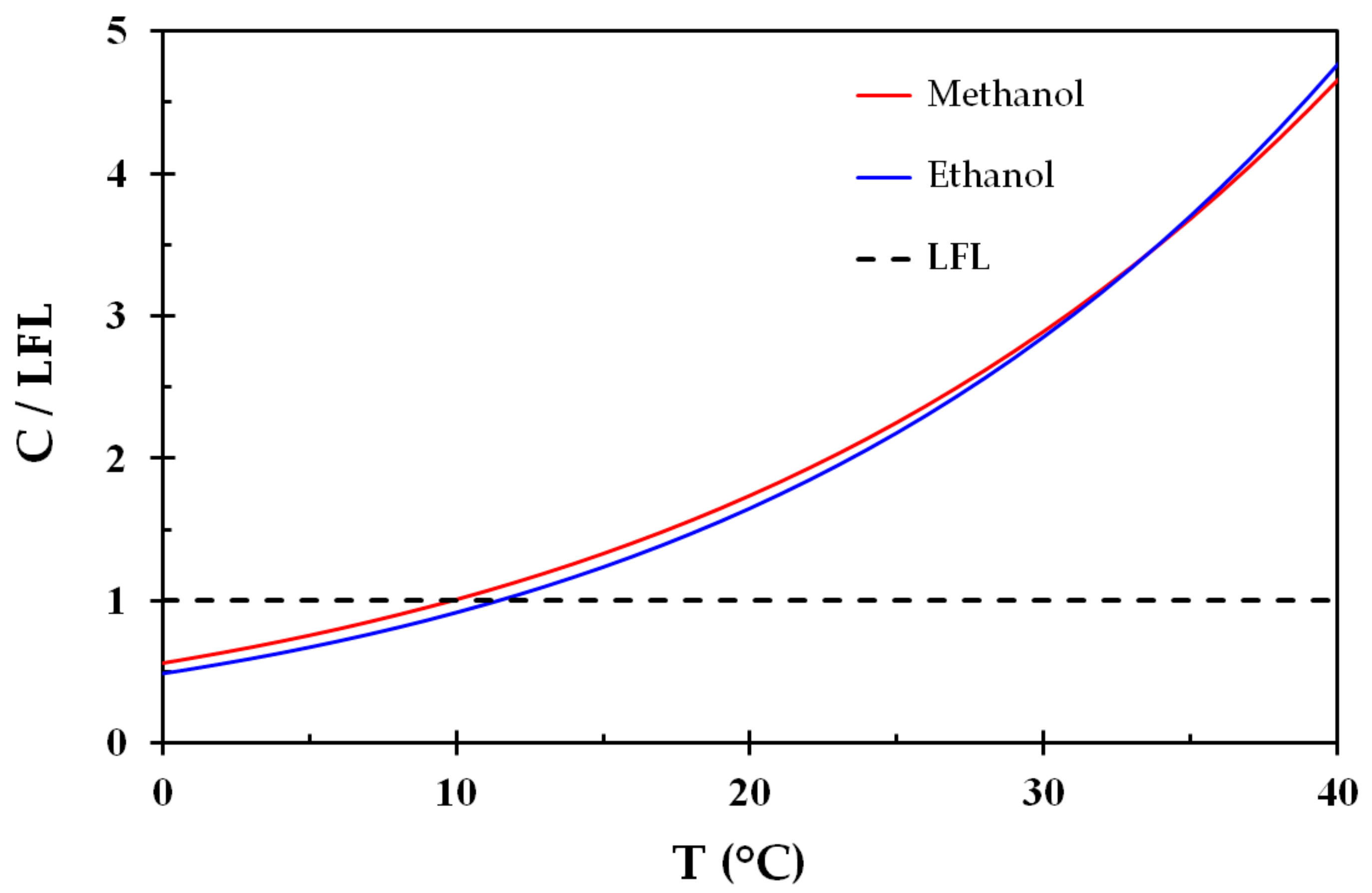
The vapor pressure normalized by the LEL is shown in
Figure 2, i.e., where the LEL is represented by the dashed black line. Despite the differences in vapor pressure (
Figure 1), these liquids display very similar behavior regarding flammability as a function of temperature. It may be seen from
Figure 2 that an air vapor phase at temperatures from about 10–12 °C in equilibrium with any one of these liquids is highly flammable. This is reflected by flash points at around 10–12 °C.
It should be noted that bioethanol products sold to the consumers also contains 5–10% isopropanol and 1–5% butanone to prevent inadvertent consumption. Isopropanol, i.e., CH3CH(OH)CH3, has a flash point of 10 °C, i.e., very similar to methanol. Butanone, also known as methyl ethyl ketone (MEK) CH3C(O)CH2CH3, has a flash point of −9 °C. There is also some water content. These “impurities” do not significantly change the flash point or the ignition properties of the liquid or vapor, but may give some luminosity to the flames compared to the pure alcohols. The main conclusion is that these liquids produce highly flammable vapors at normal room temperatures.
Dividing the stoichiometric concentrations for methanol and ethanol, i.e., 12.29% and 6.54%, by the LEL concentrations, i.e., 6.7% and 3.3%, gives 1.83 and 1.98, respectively. These values are marked in
Figure 1 and it is seen that the stoichiometric compositions, i.e., where the combustion is most severe, corresponds to normal room temperatures.
The molar mass of a mixture of air dry air and methanol at e.g., C/LEL = 2, i.e., 13.4%, is 0.02938, which is 1.4% more than dry air. The molar mass of a mixture of air dry air and ethanol at e.g., C/LEL = 2, i.e., 6.6%, is 0.03010 which is 3.9% more than dry air. This means that these gas-air mixtures are a few % heavier than the air and may therefore drain from a container and create a combustible atmosphere close to the unit being refilled.
2.4. Thermal Expansion When Ignited, Density and Fluid Dynamics
In Equations (1) and (2), the number of gas molecules after combustion to the number of gas molecules prior to combustion is close to unity. Ignoring this minor change in number of molecules, the resulting immediate volume after ignition may then, in accordance to the ideal gas law, be estimated by the temperature change:
where
(m
3) is the flammable vapor volume at ambient temperature, and
(K) and
(K) are the resulting flame temperatures after combustion, i.e., before any heat losses take place. Usually, the LFL corresponds to an adiabatic limiting temperature of 1500–1600 K [
31]. At room temperature of 23 °C (295 K), this corresponds to a 5–6 fold volume expansion.
Assuming now that a half-full methanol or ethanol fuel container has been kept at a this temperature, the gas air mixture inside the container will soon approach a concentration close to C/LFL ≈ 2, i.e., well within the flammable region and close to the stoichiometric worst case conditions. If liquid is then poured onto an ignition source, e.g., a burning flame, the poured heavy gas mixture and the liquid will catch fire. It is highly probable that the flame will propagate into the container and ignite the internal combustible air fuel gas mixture. As the internal gas volume ignites, the resulting volume expansion displaces the liquid in the bottom of the container which is then violently released through the container opening. The reaction force is acting below the hands of the person pouring liquid. The container will then be accelerated and rotate around the horizontal axis releasing even more burning liquid. Flames and burning liquid propelled out from the container may expose persons in the vicinity and inflict severe burns. This sequence, as demonstrated by a fire fighter, is shown in
Figure 3. A video showing a chemical tornado in a real-life demonstration going wrong at a museum in Reno, Nevada, injuring 13 children, reveals the hazards involved [
30].
It should be noted that also with bottle shaped containers, the reaction force usually works below the hand wrist. Given a similar ignition as demonstrated in
Figure 3, it is most likely that a 1-L bottle will release its content in a similar way, i.e., propelling the liquid fuel several meters and/or rotating onto the person holding the bottle. The ignition is fast and the resulting reaction forces are probably so strong that it is not possible for an unaware person to compensate the involved reaction forces. Victims exposed to this danger neither have time, nor possibility, to direct the dangerous burning liquid in a safe direction.
2.5. The Fire Plume Behavior
While a flame as shown in
Figure 3f develops, it is generally known that the hot flames and combustion gases will rise as a result of buoyancy forces due to their low density. Since the average molar mass of the gas mixture does not change much during the combustion, i.e., nitrogen is the dominating gas species, the relative change in density may be estimated by the temperature change. Based on Equation (1), the density after combustion may be calculated by:
where
and
are the ambient temperature and the flame temperature, respectively. The hot flames of low density start rising relative to the ambient cold and heavy air. The rising hot gas plume is associated with lower internal pressures resulting in air entrainment [
33]. If, however, there is a solid object, i.e., a person, close to this rising plume, the plume cannot entrain the object and is instead pulling itself toward the object, i.e., toward the person [
31]. A person in the proximity to an ignited gas cloud at low levels may therefore tend to cling to a person while the flames rise, resulting in extended heat exposure.
2.6. Burning Characteristics and Heat Flux to Exposed Objects
Liquid fuels, such as propane, butane, naphtha, petrol, kerosene, etc. all burn with a luminous flame resulting from glowing soot particles. Pure methanol and ethanol generally burn cleanly with a bluish flame color and very limited luminosity. In daylight, it may be difficult to spot such flames. Since water has a lower vapor pressure than methanol and ethanol, the liquid left in the burner unit will be enriched in water especially during the terminal phase of the combustion. This may result in very small and invisible flames during the last minutes before flame out. It may therefore be anticipated that tiny flames may persist in a seemingly extinguished bioethanol heater or fire place. The user may be unaware of these flames representing an ignition source during refueling.
The heat flux to an object exposed to hot flames may be expressed by:
where
(W/m K) is the convective heat transfer coefficient,
(K) is the temperature of the flame,
(K) is the temperature of the exposed surface,
is the view factor,
is the flame emissivity, and
(5.67 × 10
−8 W/m
2 K
4) is the Stefan–Boltzmann constant. The emissivity of the flames is given by:
where
(1/m) is the extinction coefficient and
(m) is the optical flame thickness.
Methanol and ethanol burns very clean and is associated with very low extinction coefficients, typically about 0.37 [
31]. This is an order of magnitude less than for other hydrocarbons. For small flame thicknesses, i.e., less than a foot, the emissivity as given by Equation (8) will be low and the heat transfer (Equation (7)) will be dominated by convection. Due to the lower radiant heat losses, i.e., low emissivity, the flame temperature is generally higher for the clean burning methanol and ethanol than for other hydrocarbons. Estimating the heat transfer coefficient may be difficult. However, the range of 20–30 W/m
2K may be appropriate for this type of natural convection [
34]. Assuming a flame temperature of 1500 K and a skin temperature of 310 K, this typically results in a convective heat flux of ≈30 kW/m
2. Exposing naked skin to this heat flux quickly heats the skin surface, and the basal layer, to temperatures associated with burn injuries [
35]. If combustible clothing textiles are exposed to flames of this heat flux or hit by burning liquid, the fabric is pilot-ignited almost instantaneously. Burning liquid in direct contact with the body may prolong the period of high heat fluxes and result in very severe burns.
Clean burning, with slightly bluish low-emissivity methanol and ethanol flames, makes small flames appear quite invisible (especially in daylight), representing a very characteristic threat. Being generally accustomed to luminous yellow and red flames, the users may be unaware of this invisible ignition source, which is characteristic of the ethanol and methanol flames.
5. Discussion
In the present study, the physical and chemical characteristics of methanol and ethanol (bioethanol) have been outlined. It has been demonstrated that the vapor pressures of these liquids at normal room temperatures create highly combustible gas-air mixtures when in equilibrium with the liquid phase. At 22–25 °C, the fuel-to-air ratio is very close to the stoichiometric composition. This is also the most easily ignited composition, either by static electricity or an open flame, and results in the worst case scenarios regarding explosion strength.
The ethanol-air mixture is heavier than air and may pour out of a container and result in a combustible gas air pillow which may ignite when trying to light a biofuel fireplace or heater unit. The gas is invisible, and the operator may probably not be aware of this situation. It has been shown that the container or bottle gas phase may likely become ignited when refilling a biofuel fireplace or biofuel heater which is still burning.
It has been outlined why such fuels may burn with a flame that is quite invisible in day light, especially the last period before the flame self-extinguishes. This persistent flame, thought by the user to be extinguished, represents a very dangerous ignition source, especially when refilling the unit. It may quite likely ignite the vapors being poured out of the refilling container or bottle. During this process, it is quite likely that explosive container gas volume will be ignited, resulting in violent explosion propelling burning liquid out of the container or bottle. The operator, as well as people several meters away, may then be engulfed in flames and soaked with burning liquid exposing naked skin or setting the clothing fabric on fire.
A number of severe bioethanol burn incidents are presented in the literature [
22,
24,
26,
27,
28] and two group studies [
23,
25] were analyzed based on the outlined possible accident mechanisms. Three cases involving very similar, and severe burn, incidents with methanol were also included [
28,
29,
30]. Two of the methanol burn incidents injured several children at science demonstrations. For all these burn incidents, it turned out that the most severe accidents involved ignition of the container gas phase resulting in a violent explosion expelling burning liquid from the container. Since the auto ignition temperatures (AITs) of methanol and ethanol are quite high, i.e., respectively 470 °C and 365 °C, respectively, it is not likely that hot surfaces without a flame represented the ignition source. A persistent flame, unnoticed by the operator, was likely the ignition source in most, if not all, of these incidents. A minority of the incidents represented ignition of the vapor cloud that had been poured from the liquid container or bottle without involving the container or the bottle.
Based on the knowledge that flames cannot travel though small openings, it is suggested to develop bottles and containers with openings less than the maximum experimental safe gap (MESG). Openings less than about 0.9 mm would ensure that no flame could propagate into the container to involve the internal gas phase. Such a restriction may also limit the amount of heavy gas air mixture being poured out of the container while filling or refilling a bio heater or fireplace unit. It is suggested that research is performed on this issue to develop safer containers and bottles, and safer refilling possibilities preventing extensive evaporation if refilling a still warm unit.
Van Zoonen et al. [
23] discovered through interviews with burn victims that they a poor understanding of the hazards involved. The fuel is easily available to a low cost and it does have an odor associated with typically highly flammable long-chained non-oxidized hydrocarbons, e.g., gasoline or naphtha. In may therefore not be associated with any recognizable danger. In order to mitigate this, safety courses may be updated with videos [
29,
32] and information brochures explaining the risks associated with bioethanol and methanol. It is also necessary to focus on mitigating measures, such as prompt and cooling if a burn incident has happened. In order to develop efficient safety courses, it is therefore recommended to analyze the present level of risk understanding in the public in order to find at which level the teaching should start.
The heat exposure may also be a result of hot liquid scalding in combination with flame exposure, which has been expensively researched for hot beverage and hot food spills. The injury mechanisms may therefore be quite complicated as both flame exposure and hot liquid scalding due to wetted fabric contribute to the resulting heat injury. Promptly removing clothing is therefore vital to get the overheated skin cooled by tempered water [
8,
34,
39,
40,
41].
The bottles and containers are marked as flammable and with a health risk. The pictograms indicating the hazards of methanol and bioethanol fuels do not, however, give any indication of an explosion risk. This risk may be understood by professionals. It may, however, be questioned whether communicating flammability and health risk is sufficient for the public to gain the proper understanding of the risks involved when handling these liquids.
Recently, infrared (IR) cameras have been developed for finding gas leakages in e.g., the oil and gas industry [
42]. For future research on methanol and ethanol burn incidents, such equipment, which detects the C-H bonds in the methane and long-chained hydrocarbon molecules, may be tested for methanol and ethanol. It is quite likely that doing research with this equipment may reveal and make visible some of the mechanisms outlined in the present work. It is suggested that such research be initiated to gain understanding as well as to produce material for better public safety courses.
It is important to be aware of accidents related to dislocation of the units, i.e., units detaching from wall mounts, being interfered with, or falling from tables. These fireplaces/heaters can be dangerous for children who may experience large-TBSA burns even when exposed to comparably small fires.
6. Conclusions
In the present study, the possible mechanisms leading to accidental fires involving methanol and ethanol/bioethanol liquid and vapor phase have been outlined. Burn accidents previously described in the literature have been analyzed based on the outlined accident mechanisms. It turns out that equilibrium vapor pressure close to the stoichiometric fuel–air composition represents a very severe risk when handling these liquids. Some accidents have been related to the ethanol/bioethanol fuel–air mixture being poured out of the container, resulting in a combustible cloud accumulating close to the unit to be filled. Similar clouds have also been accumulated when refilling a warm unit. When approaching the unit for ignition, this vapor cloud ignites and engulfs the operator in flames, typically on the chest, face, and arms.
Ignition of the vapor phase resulting in an internal explosion expelling burning liquid resulted in the most of the accidents and generally resulted in the most severe burns. Not only the operators but also people several meters away were exposed to flames and soaked in burning fuel. The ignition source was in most, if not all these burn incidents, most likely a small invisible flame from previous use of the unit.
By understanding the mechanisms involved and analyzing accidents, several risk-reducing measures are suggested for further research in order to reduce this very severe burn risk.