Arsenic Methylation Capacity and Metabolic Syndrome in the 2013–2014 U.S. National Health and Nutrition Examination Survey (NHANES)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exclusion Factors
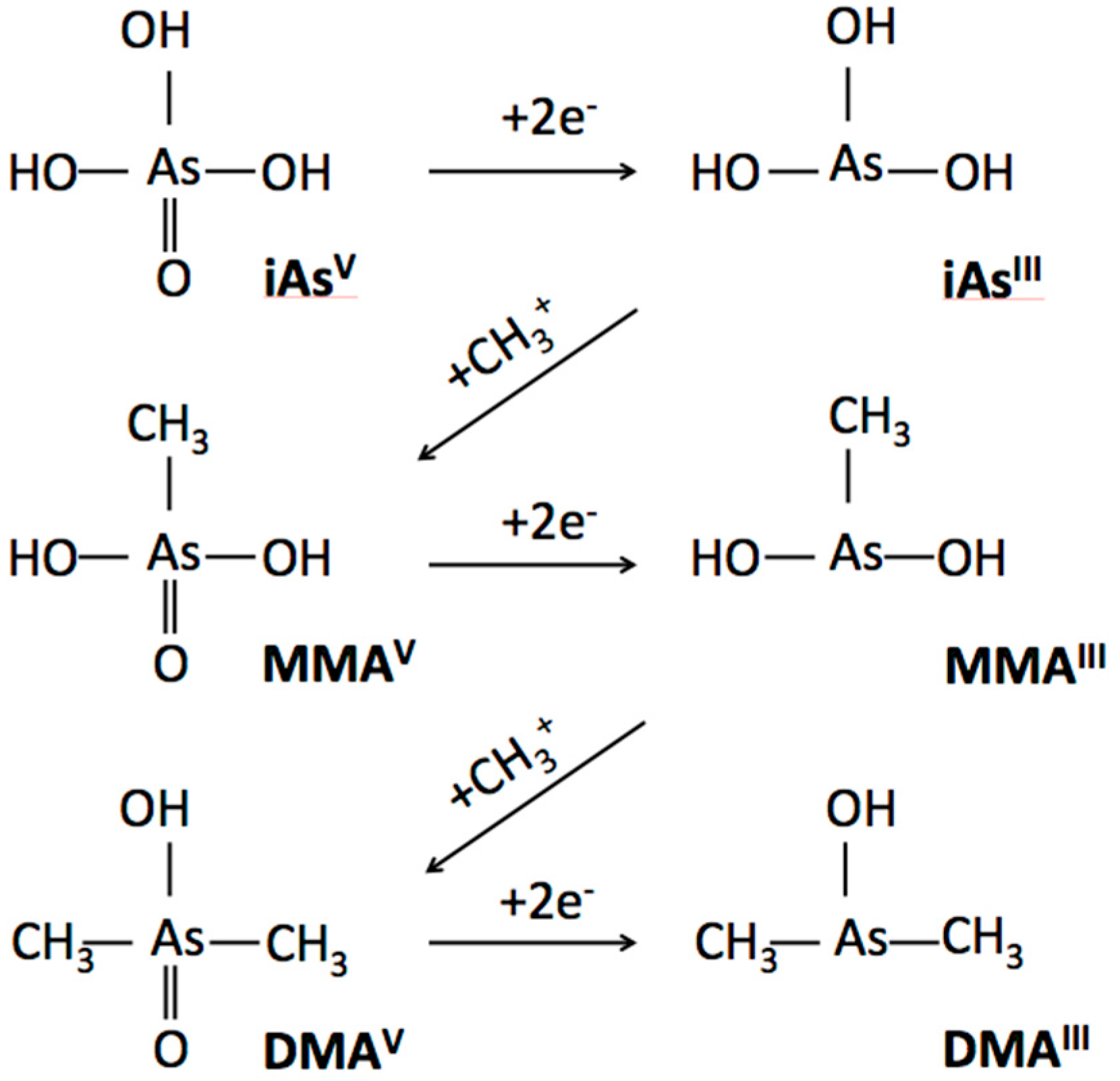
2.3. Speciated Arsenicals
2.4. Metabolic Syndrome Components
2.5. Fasting Glucose
2.6. Hypertension
2.7. Elevated Triglycerides
2.8. Waist Circumference
2.9. Low HDL Cholesterol
2.10. Covariates
2.11. Statistical Analysis
3. Results
3.1. Study Population & Metabolic Syndrome
3.2. Covariate Association with Arsenic Measures & Metabolic Syndrome
3.3. Logistic Regression
4. Discussion
4.1. Oxidative Stress & Inflammation
4.2. Increased Toxicity of Methylated Species
4.3. Arsenic Distribution Patterns
4.4. Gender & BMI
4.5. Selection of Adjustment Factors
4.6. Policy Implications
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Singh, A.P.; Goel, R.K.; Kaur, T. Mechanisms pertaining to arsenic toxicity. Toxicol. Int. 2011, 18, 87–93. [Google Scholar] [PubMed]
- Pace, C.; Banerjee, T.D.; Welch, B.; Khalili, R.; Dagda, R.K.; Angermann, J. Monomethylarsonous acid, but not inorganic arsenic, is a mitochondria-specific toxicant in vascular smooth muscle cells. Toxicol. In Vitro 2016, 35, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Styblo, M.; Del Razo, L.M.; Vega, L.; Germolec, D.R.; LeCluyse, E.L.; Hamilton, G.A.; Reed, W.; Wang, C.; Cullen, W.R.; Thomas, D.J. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000, 74, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Devesa, V.; Vélez, D. Differential toxicity and gene expression in caco-2 cells exposed to arsenic species. Toxicol. Lett. 2013, 218, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Carrus, A.; Sciannamblo, G.; Caputo, F.; Minunni, V.; de Nichilo, G.; Bellotta, M.R.; Gagliardi, T.; Bisceglia, L.; Assennato, G. A study of factors influencing urinary arsenic excretion in exposed workers. Int. J. Environ. Health Res. 2009, 19, 369–377. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Programme on Chemical Safety, Arsenic and Arsenic Compounds, Environmental Health Criteria 224, 2nd ed.; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- American Conference of Governmental Hygienists. Arsenic and Its Inorganic Compounds; American Conference of Governmental Hygienists: Cincinnati, OH, USA, 2007. [Google Scholar]
- Tsuda, T.; Babazono, A.; Yamamoto, E.; Kurumatani, N.; Mino, Y.; Ogawa, T.; Kishi, Y.; Aoyama, H. Ingested arsenic and internal cancer: A historical cohort study followed for 33 years. Am. J. Epidemiol. 1995, 141, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Argos, M.; Kalra, T.; Rathouz, P.J.; Chen, Y.; Pierce, B.; Parvez, F.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; Hasan, R.; et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in bangladesh (heals): A prospective cohort study. Lancet 2010, 376, 252–258. [Google Scholar] [CrossRef]
- Moon, K.A.; Guallar, E.; Umans, J.G.; Devereux, R.B.; Best, L.G.; Francesconi, K.A.; Goessler, W.; Pollak, J.; Silbergeld, E.K.; Howard, B.V.; et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann. Intern. Med. 2013, 159, 649–659. [Google Scholar] [PubMed]
- Chiou, H.Y.; Huang, W.I.; Su, C.L.; Chang, S.F.; Hsu, Y.H.; Chen, C.J. Dose-Response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke 1997, 28, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Jeng, J.S.; Yip, P.K.; Chen, C.L.; Hsu, L.I.; Hsueh, Y.M.; Chiou, H.Y.; Wu, M.M.; Chen, C.J. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation 2002, 105, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Abhyankar, L.N.; Jones, M.R.; Guallar, E.; Navas-Acien, A. Arsenic exposure and hypertension: A systematic review. Environ. Health Perspect. 2012, 120, 494–500. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Wang, S.L.; Chang, F.H.; Liou, S.H.; Wang, H.J.; Li, W.F.; Hsieh, D.P. Inorganic arsenic exposure and its relation to metabolic syndrome in an industrial area of Taiwan. Environ. Int. 2007, 33, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Connell, J.M. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Yamauchi, H.; Kumagai, Y.; Sun, G.; Yoshida, T.; Aikawa, H.; Hopenhayn-Rich, C.; Shimojo, N. Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environ. Health Perspect. 2002, 110, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Barchowsky, A.; Dudek, E.J.; Treadwell, M.D.; Wetterhahn, K.E. Arsenic induces oxidant stress and nf-kappa b activation in cultured aortic endothelial cells. Free Radic. Biol. Med. 1996, 21, 783–790. [Google Scholar] [CrossRef]
- Hopenhayn-Rich, C.; Biggs, M.L.; Smith, A.H.; Kalman, D.A.; Moore, L.E. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ. Health Perspect. 1996, 104, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, A.L.; Ekström, E.C.; Nermell, B.; Rahman, M.; Lönnerdal, B.; Persson, L.A.; Vahter, M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ. Res. 2008, 106, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, A.L.; Kumar, R.; Goessler, W.; Thirumaran, R.; Gurzau, E.; Koppova, K.; Rudnai, P.; Leonardi, G.; Fletcher, T.; Vahter, M. Metabolism of low-dose inorganic arsenic in a central european population: Influence of sex and genetic polymorphisms. Environ. Health Perspect. 2007, 115, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Wang, S.L.; Wang, Y.H.; Sun, C.W.; Huang, Y.L.; Chen, C.J.; Li, W.F. Arsenic methylation, gsto1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere 2012, 88, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.L.; Jones, R.L.; Verdon, C.P.; Jarrett, J.M.; Caudill, S.P.; Osterloh, J.D. Levels of urinary total and speciated arsenic in the US population: National health and nutrition examination survey 2003–2004. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Shiue, I. Higher urinary heavy metal, phthalate, and arsenic but not parabens concentrations in people with high blood pressure, U.S. NHANES, 2011–2012. Int J. Environ. Res. Public Health 2014, 11, 5989–5999. [Google Scholar] [CrossRef] [PubMed]
- Shiue, I.; Hristova, K. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3–19% of the population attributable risk for high blood pressure: U.S. NHANES, 2009–2012. Hypertens. Res. 2014, 37, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Shiue, I. Higher urinary heavy metal, arsenic, and phthalate concentrations in people with high blood pressure: U.S. NHANES, 2009–2010. Blood Press 2014, 23, 363–369. [Google Scholar] [CrossRef] [PubMed]
- NHANES 2013–2014 Questionnaire Data. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Questionnaire&CycleBeginYear=2013 (accessed on 4 December 2017).
- NHANES 2013–2014 Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2013 (accessed on 4 December 2017).
- Concha, G.; Vogler, G.; Lezcano, D.; Nermell, B.; Vahter, M. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci. 1998, 44, 185–190. [Google Scholar] [CrossRef] [PubMed]
- NHANES 2013–2014 Lab Methods. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2013 (accessed on 4 December 2017).
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J.; et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Hypertension 2005, 45, 142–161. [Google Scholar] [PubMed]
- NHANES 2013–2014 Survey Operations Manuals. Available online: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/manuals.aspx?BeginYear=2013 (accessed on 4 December 2017).
- Gamble, M.V.; Liu, X. Urinary creatinine and arsenic metabolism. Environ. Health Perspect. 2005, 113, A442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trasande, L.; Spanier, A.J.; Sathyanarayana, S.; Attina, T.M.; Blustein, J. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics 2013, 132, e646–e655. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol. Lett. 2000, 112–113, 209–217. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Aljada, A.; Mohanty, P.; Ghanim, H.; Abdo, T.; Tripathy, D.; Chaudhuri, A.; Dandona, P. Increase in intranuclear nuclear factor kappab and decrease in inhibitor kappab in mononuclear cells after a mixed meal: Evidence for a proinflammatory effect. Am. J. Clin. Nutr. 2004, 79, 682–690. [Google Scholar] [PubMed]
- Diamant, M.; Lamb, H.J.; van de Ree, M.A.; Endert, E.L.; Groeneveld, Y.; Bots, M.L.; Kostense, P.J.; Radder, J.K. The association between abdominal visceral fat and carotid stiffness is mediated by circulating inflammatory markers in uncomplicated type 2 diabetes. J. Clin. Endocrinol. Metab. 2005, 90, 1495–1501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Andreeva-Gateva, P.; Popova, D.; Orbetsova, V. Antioxidant parameters in metabolic syndrome—A dynamic evaluation during oral glucose tolerance test. Vutr Boles 2001, 33, 48–53. [Google Scholar] [PubMed]
- Maeda, K.; Yasunari, K.; Sato, E.F.; Inoue, M. Enhanced oxidative stress in neutrophils from hyperlipidemic guinea pig. Atherosclerosis 2005, 181, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Barnard, R.J.; Sindhu, R.K.; Jurczak, M.; Ehdaie, A.; Vaziri, N.D. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism 2006, 55, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Huang, Y.K.; Huang, Y.L.; Chung, C.J.; Yang, M.H.; Chen, C.J.; Hsueh, Y.M. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol. Appl. Pharmacol. 2005, 206, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, B.; Xi, S.; Zheng, Q.; Lv, X.; Sun, G. Prolonged environmental exposure of arsenic through drinking water on the risk of hypertension and type 2 diabetes. Environ. Sci. Pollut. Res. Int. 2013, 20, 8151–8161. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Vega, L.; Styblo, M.; Patterson, R.; Cullen, W.; Wang, C.; Germolec, D. Differential effects of trivalent and pentavalent arsenicals on cell proliferation and cytokine secretion in normal human epidermal keratinocytes. Toxicol. Appl. Pharmacol. 2001, 172, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.K.; Tseng, C.H.; Huang, Y.L.; Yang, M.H.; Chen, C.J.; Hsueh, Y.M. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol. Appl. Pharmacol. 2007, 218, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M.; Concha, G. Role of metabolism in arsenic toxicity. Pharmacol. Toxicol. 2001, 89, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rubio, P.; Roberge, J.; Arendell, L.; Harris, R.B.; O’Rourke, M.K.; Chen, Z.; Cantu-Soto, E.; Meza-Montenegro, M.M.; Billheimer, D.; Lu, Z.; et al. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol. Appl. Pharmacol. 2011, 252, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Zhu, S.; Palaniappan, L.; Heshka, S.; Carnethon, M.R.; Heymsfield, S.B. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the third national health and nutrition examination survey, 1988–1994. Arch. Intern. Med. 2003, 163, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Francesconi, K.A.; Silbergeld, E.K.; Guallar, E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ. Res. 2011, 111, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.; Smit, E.; Cardenas, A.; Hystad, P.; Kile, M.L. Trends in urinary arsenic among the U.S. Population by drinking water source: Results from the national health and nutritional examinations survey 2003–2014. Environ. Res. 2017, 162, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Adak, P.; Gurian, P.L.; Lockwood, J.R. Arsenic exposure in US public and domestic drinking water supplies: A comparative risk assessment. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 245–254. [Google Scholar] [CrossRef] [PubMed]
- George, C.M.; Smith, A.H.; Kalman, D.A.; Steinmaus, C.M. Reverse osmosis filter use and high arsenic levels in private well water. Arch. Environ. Occup. Health 2006, 61, 171–175. [Google Scholar] [CrossRef] [PubMed]
- National Public Water Systems Compliance Report. Available online: https://www.epa.gov/sites/production/files/2014-04/documents/sdwacom2011.pdf (accessed on 21 January 2018).
- Balazs, C.L.; Morello-Frosch, R.; Hubbard, A.E.; Ray, I. Environmental justice implications of arsenic contamination in California’s San Joaquin Valley: A cross-sectional, cluster-design examining exposure and compliance in community drinking water systems. Environ. Health 2012, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.V.; Johnston, R.B.; Zheng, Y. Arsenic in tube well water in bangladesh: Health and economic impacts and implications for arsenic mitigation. Bull. World Health Organ. 2012, 90, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Gatti, M.F.; Gagliardi, T.; Cuccaro, F.; De Maria, L.; Caputi, A.; Quarato, M.; Baldassarre, A. Environmental exposure to arsenic and chromium in an industrial area. Environ. Sci. Pollut. Res. Int. 2017, 24, 11528–11535. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Mean (±SD) or N (%) | |
|---|---|---|
| Age (years) | 47.44 | (±15.55) |
| Sex | ||
| Male | 491 | (51.30%) |
| Race/Ethnicity | ||
| Non-Hispanic White | 442 | (46.2%) |
| Mexican American | 120 | (12.5%) |
| Non-Hispanic Black | 204 | (21.3%) |
| Non-Hispanic Asian | 87 | (9.1%) |
| Other Race | 104 | (10.8%) |
| PIR a | 2.2 | (±1.61) |
| PIR < 1 | 278 | (29.0%) |
| Smoker | 196 | (20.5%) |
| BMI b (kg/m2) | 29.01 | (±7.34) |
| Normal | 313 | (32.7%) |
| Overweight | 290 | (30.3%) |
| Obese | 354 | (36.9%) |
| Metabolic syndrome components | ||
| Fasting glucose (mg/dL) | 102.21 | (±34.94) |
| Systolic blood pressure (mmHg) | 122.09 | (±17.53) |
| Diastolic blood pressure (mmHg) | 68.99 | (±11.55) |
| Serum triglycerides (mg/dL) | 139.93 | (±235.32) |
| Waist circumference (cm) | 99.12 | (±16.91) |
| HDL cholesterol (mg/dL) | 52.63 | (±15.28) |
| Urinary Arsenic Species | ||
| iAs III c (μg/L) | 0.51 | (±0.45) |
| MMA III + V d (μg/L) | 0.63 | (±0.55) |
| DMA III + V e (μg/L) | 4.68 | (±5.38) |
| %iAs f | 9.68 | (±6.70) |
| %MMA g | 11.69 | (±5.95) |
| %DMA h | 78.63 | (±9.94) |
| PMI i | 1.89 | (±1.89) |
| SMI j | 10.44 | (±20.72) |
| Number of Metabolic syndrome components | ||
| 0 | 179 (18.7%) | |
| 1 | 233 (24.3%) | |
| 2 | 214 (22.4%) | |
| 3 | 193 (20.2%) | |
| 4 | 104 (10.9%) | |
| 5 | 34 (3.6%) | |
| Less than 2 | 626 (65.4%) | |
| 3 or more | 331 (34.6%) | |
| Covariate | Arsenic Measures | ||||
|---|---|---|---|---|---|
| Mean (SD) | |||||
| %iAs a | %MMA b | %DMA c | PMI d | SMI e | |
| p-Value f | p-Value | p-Value | p-Value | p-Value | |
| Total (n = 957) | 9.68 (6.69) | 11.69 (5.95) | 78.63 (9.94) | 1.89 (1.89) | 10.44 (20.72) |
| Gender | <0.001 * | <0.001 * | <0.001 * | 0.003 * | <0.001 * |
| Male (n = 491) | 10.73 (6.92) | 12.41 (6.02) | 76.85 (10.13) | 1.73 (1.67) | 10.13 (27.32) |
| Female (n = 466) | 8.56 (6.27) | 10.93 (5.79) | 80.51 (9.41) | 2.05 (2.08) | 10.76 (9.81) |
| Race/Ethnicity g | 0.041 * | <0.001 * | 0.098 | <0.001 * | 0.001 * |
| White (n = 442) | 9.35 (7.08) | 12.53 (6.07) | 78.11 (10.41) | 2.18 (2.05) | 9.00 (9.40) |
| MexAmer (n = 120) | 10.50 (6.86) | 11.30 (6.02) | 78.20 (10.28) | 1.38 (1.50) h | 9.71 (7.13) |
| Black (n = 204) | 10.17 (6.52) | 10.61 (5.52) h | 79.22 (9.24) | 1.64 (1.83) h | 13.24 (40.59) h |
| Asian (n = 87) | 8.14 (4.60) | 10.17 (5.64) h | 81.69 (8.02) h | 1.64 (1.42) | 13.58 (16.07) h |
| Other (n = 104) | 10.44 (6.39) | 11.96 (5.96) | 77.60 (9.98) | 1.71 (1.89) | 9.19 (7.23) |
| Smoker | 0.143 | 0.141 | 0.962 | 0.009 * | 0.268 |
| No (n = 761) | 9.84 (6.83) | 11.54 (5.90) | 78.61 (10.01) | 1.84 (1.87) | 10.78 (22.95) |
| Yes (n = 196) | 9.054 (6.11) | 12.27 (6.14) | 78.677 (9.72) | 2.09 (1.95) | 9.08 (7.13) |
| PIR i | 0.524 | 0.053 | 0.194 | 0.988 | 0.049 * |
| <1 (n = 278) | 9.89 (6.57) | 12.24 (5.97) | 77.86 (10.16) | 1.90 (1.92) | 10.89 (35.18) |
| >1 (n = 679) | 9.59 (6.75) | 11.46 (5.93) | 78.94 (9.85) | 1.88 (1.88) | 10.25 (9.98) |
| BMI | 0.001 * | <0.001 * | <0.001 * | 0.780 | <0.001 * |
| Normal (n = 313) j | 10.60 (7.26) | 12.72 (6.44) | 76.67 (10.81) | 1.85 (1.76) | 9.29 (11.56) |
| Overwt (n = 290) k | 9.84 (6.62) | 12.00 (5.51) | 78.16 (9.50) | 1.89 (1.73) | 8.98 (7.31) |
| Obese (n = 354) l | 8.73 (6.10) | 10.52 (5.67) | 80.75 (9.09) | 1.91 (2.11) | 12.66 (31.52) |
| [B(Se) Beta m p-Value n] | |||||
| Age | −0.11 (0.01) 0.268 | −0.04 (0.01) −0.116 | 0.15 (0.02) 0.250 | 0.00 (0.00) 0.182 | 0.00 (0.00) 0.159 |
| 0.001 * | 0.002 * | 0.001 * | 0.001 * | 0.001 * | |
| Creatinine o | 0.003 (0.002) 00.03 | 1.59 (0.59) 0.078 | −2.74 (0.97) −0.018 | −0.04 (0.04) −0.037 | −0.07 (0.03) −0.072 |
| 0.233 | 0.011 * | 0.002 * | 0.220 | 0.008 * | |
| Arsenobetaine p | −0.026 (0.01) −0.10 | −2.60 (0.29) −0.252 | 4.61 (0.51) 0.267 | 0.00 (0.02) 0.000 | 0.15 (0.02) 0.292 |
| 0.003 * | 0.001 * | 0.001 * | 0.995 | 0.001 * | |
| Covariate | Metabolic Syndrome Status | |||||
|---|---|---|---|---|---|---|
| (No) n = 334 | (Yes) n = 157 | p-Value a | (No) n = 292 | (Yes) n = 174 | p-Value | |
| Men | Women | |||||
| Mean (±SD) or n | Mean (±SD) or n | |||||
| BMI | <0.001 * | <0.001 * | ||||
| Normal b | 148 | 17 | 132 | 16 | ||
| Overweight c | 132 | 55 | 67 | 36 | ||
| Obese d | 54 | 85 | 93 | 122 | ||
| Smoker | <0.001 * | <0.001 * | ||||
| Yes | 87 | 42 | 41 | 26 | ||
| Non | 247 | 115 | 251 | 148 | ||
| PIR e | <0.001 * | <0.001 * | ||||
| <1 | 38 | 96 | 85 | 59 | ||
| >1 | 238 | 119 | 207 | 148 | ||
| Race/Ethnicity | 0.472 | 0.049 * | ||||
| White | 150 | 72 | 133 | 87 | ||
| MexAmer f | 38 | 22 | 41 | 19 | ||
| Black | 73 | 38 | 50 | 43 | ||
| Asian | 30 | 13 | 28 | 13 | ||
| Other | 43 | 12 | 40 | 12 | ||
| Age | 44.4 (±16.7) | 53.6 (±15.0) | <0.001 * | 44.2 (±16.3) | 53.3 (±15.0) | <0.001 * |
| Creatinine g | 133.1 (±76.7) | 134.1 (±73.4) | 0.767 | 98.0 (±69.2) | 109.4 (±70.1) | 0.036 * |
| Arsenobetaine h | 7.9 (19.4) | 6.7 (19.9) | 0.266 | 10.4 (37.2) | 6.6 (18.8) | 0.477 |
| Variable | Men | Women | ||||
|---|---|---|---|---|---|---|
| R2 | OR [95% CI] | p-Value b | R2 | OR [95% CI] | p-Value | |
| %iAs c | 0.069 | 0.999 [0.970, 1.029] | 0.950 | 0.085 | 0.977 [0.944, 1.012] | 0.977 |
| %MMA d | 0.079 | 0.960 [0.927, 0.994] | 0.021 * | 0.085 | 0.974 [0.940, 1.010] | 0.153 |
| %DMA e | 0.073 | 1.015 [0.995, 1.037] | 0.150 | 0.087 | 1.020 [0.998, 1.044] | 0.078 |
| PMI f | 0.073 | 0.656 [0.358, 1.203] | 0.173 | 0.081 | 0.956 [0.543, 1.683] | 0.876 |
| SMI g | 0.073 | 1.636 [0.834, 3.209] | 0.152 | 0.090 | 2.148 [1.048, 4.402] | 0.037 * |
| IV | Normal BMI b | Overweight BMI c | Obese BMI d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | OR [95% CI] | p-Value e | R2 | OR [95% CI] | p-Value | R2 | OR [95% CI] | p-Value | |
| %iAs f | 0.073 | 0.979 [0.879, 1.069] | 0.639 | 0.101 | 1.017 [0.966, 1.071] | 0.512 | 0.110 | 1.000 [0.946, 1.058] | 0.994 |
| %MMA g | 0.073 | 0.974 [0.888, 1.068] | 0.577 | 0.101 | 1.019 [0.958, 1.083] | 0.554 | 0.112 | 0.979 [0.913, 1.049] | 0.549 |
| %DMA h | 0.074 | 1.019 [0.963, 1.078] | 0.519 | 0.102 | 0.986 [0.951 1.022] | 0.429 | 0.110 | 1.007 [0.966 1.050] | 0.728 |
| PMI i | 0.072 | 1.179 [0.230, 6.043] | 0.843 | 0.099 | 0.926 [0.312, 2.748] | 0.890 | 0.110 | 0.995 [0.333, 2.979] | 0.993 |
| SMI j | 0.072 | 1.470 [0.238, 9.081] | 0.678 | 0.106 | 0.477 [0.129, 1.757] | 0.266 | 0.110 | 0.929 [0.275, 3.144] | 0.906 |
| IV | Normal BMI b | Overweight BMI c | Obese BMI d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | OR [95% CI] | p-Value e | R2 | OR [95% CI] | p-Value | R2 | OR [95% CI] | p-Value | |
| %iAs f | 0.145 | 0.955 [0.854, 1.069] | 0.426 | 0.179 | 1.026 [0.950, 1.109] | 0.509 | 0.050 | 0.992 [0.942, 1.044] | 0.746 |
| %MMA g | 0.182 | 0.826 [0.701, 0.973] | 0.022 * | 0.219 | 1.113 [1.016, 1.218] | 0.021 * | 0.057 | 0.966 [0.919, 1.015] | 0.174 |
| %DMA h | 0.167 | 1.085 [0.999, 1.178] | 0.053 | 0.203 | 0.951 [0.903 1.003] | 0.063 | 0.054 | 1.018 [0.986 1.052] | 0.273 |
| PMI i | 0.160 | 0.644 [0.365, 1.135] | 0.128 | 0.187 | 1.149 [0.912, 1.446] | 0.239 | 0.049 | 0.992 [0.879 1.120] | 0.896 |
| SMI j | 0.175 | 11.485 [1.371, 96.196] | 0.024 * | 0.220 | 0.089 [0.011, 0.704] | 0.022 * | 0.064 | 2.590 [0.926, 7.243] | 0.070 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pace, C.; Smith-Gagen, J.; Angermann, J. Arsenic Methylation Capacity and Metabolic Syndrome in the 2013–2014 U.S. National Health and Nutrition Examination Survey (NHANES). Int. J. Environ. Res. Public Health 2018, 15, 168. https://doi.org/10.3390/ijerph15010168
Pace C, Smith-Gagen J, Angermann J. Arsenic Methylation Capacity and Metabolic Syndrome in the 2013–2014 U.S. National Health and Nutrition Examination Survey (NHANES). International Journal of Environmental Research and Public Health. 2018; 15(1):168. https://doi.org/10.3390/ijerph15010168
Chicago/Turabian StylePace, Clare, Julie Smith-Gagen, and Jeff Angermann. 2018. "Arsenic Methylation Capacity and Metabolic Syndrome in the 2013–2014 U.S. National Health and Nutrition Examination Survey (NHANES)" International Journal of Environmental Research and Public Health 15, no. 1: 168. https://doi.org/10.3390/ijerph15010168
APA StylePace, C., Smith-Gagen, J., & Angermann, J. (2018). Arsenic Methylation Capacity and Metabolic Syndrome in the 2013–2014 U.S. National Health and Nutrition Examination Survey (NHANES). International Journal of Environmental Research and Public Health, 15(1), 168. https://doi.org/10.3390/ijerph15010168





