Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects
Abstract
1. Introduction
2. Arsenic Contamination in Soil and Water Environments
2.1. Arsenic Content in Soil
2.2. Arsenic Concentration in Water
3. Speciation of Arsenic in Soil
3.1. Effect of Soil Chemical Properties on As Speciation and Bioavailability in Soil
3.2. The Effect of Soil Microbial Activity on As Speciation and Bioavailability in Soil
4. Translocation of Arsenic from Soil to Plant
5. Speciation of Arsenic in Plants
5.1. Uptake and Transport of Inorganic Arsenic Species
5.2. Uptake and Transport of Organic Arsenic Species
6. Arsenic Transporters in Plants
7. Physiological Effects of Arsenic on Plants
7.1. Effect of Arsenic on Plant Growth
7.2. Impact of Arsenic on Photosynthesis of Plants
7.3. Effect of Arsenic on ATP Synthesis
7.4. Effect of Arsenic Toxicity on Membrane Integrity
8. Biochemical and Molecular Effects of Arsenic on Plants
8.1. Arsenic-Induced Reactive Oxygen Species (ROS) Generation
8.2. ROS Homeostasis and Plant Development
8.3. Impact of Arsenic on Carbohydrate Metabolism in Plants
8.4. Arsenic Effect on Lipid Metabolism
8.5. Arsenic Effects on Protein Metabolism
8.6. Arsenic Impact on Changes in DNA Structure
9. Detoxification Mechanisms of Arsenic in Plants
9.1. Arsenic Complexation and Sequestration in Plants
9.2. Role of Antioxidant Enzymes in Arsenic Detoxification in Plants
9.3. Role of Proline in Arsenic Detoxification in Plants
9.4. Role of Nitric Oxide in Arsenic Detoxification Processes in Plants
9.5. Role of Salicylic Acid in Arsenic Detoxification
9.6. Effect of Phosphate (Pi) on Arsenic Toxicity and Detoxification in Plants
10. Conclusions
- (i)
- How does As affect the germination and post-germination phases of plant development at the biochemical and molecular level?
- (ii)
- What are the deleterious consequences (at the gene level) of As toxicity to plants and its organs?
- (iii)
- How can plant toxicity symptoms be minimized without inducing any permanent damage to the plants?
- (iv)
- How and to what extent can the exogenous application of various agents protect plants against As stress under soil conditions?
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shahid, M.; Dumat, C.; Pourrut, B.; Abbas, G.; Shahid, N.; Pinelli, E. Role of metal speciation in lead-induced oxidative stress to Vicia faba roots. Russ. J. Plant Physiol. 2015, 62, 448–454. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic behaviour in soil-plant system: Biogeochemical reactions and chemical speciation influences. In Enhancing Cleanup of Environmental Pollutants; Springer: Berlin, Germany, 2017; pp. 97–140. [Google Scholar]
- Mombo, S.; Foucault, Y.; Deola, F.; Gaillard, I.; Goix, S.; Shahid, M.; Schreck, E.; Pierart, A.; Dumat, C. Management of human health risk in the context of kitchen gardens polluted by lead and cadmium near a lead recycling company. J. Soils Sedim. 2016, 16, 1214–1224. [Google Scholar] [CrossRef]
- Xiong, T.; Dumat, C.; Pierart, A.; Shahid, M.; Kang, Y.; Li, N.; Bertoni, G.; Laplanche, C. Measurement of metal bioaccessibility in vegetables to improve human exposure assessments: Field study of soil-plant-atmosphere transfers in urban areas, South China. Environ. Geochem. Health 2016, 38, 1283–1301. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Springer: Berlin, Germany, 2015; pp. 1–25. [Google Scholar]
- Chandrakar, V.; Naithani, S.C.; Keshavkant, S. Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: A review. Biologia 2016, 71, 367–377. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Fatimah, A.; Shahid, M.; Javed, M.T.; Wang, H.; Ok, Y.S.; Bashir, S.; Murtaza, B.; Saqib, Z.A. Phosphate-assisted phytoremediation of arsenic by Brassica napus and Brassica juncea: Morphological and physiological response. Int. J. Phytoremed. 2017, 19, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Bibi, I.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Wang, H.; Ok, Y.S.; Sarkar, B.; Javed, M.T.; Murtaza, G. Effect of compost addition on arsenic uptake, morphological and physiological attributes of maize plants grown in contrasting soils. J. Geochem. Explor. 2017, 178, 83–91. [Google Scholar] [CrossRef]
- Abid, M.; Niazi, N.K.; Bibi, I.; Farooqi, A.; Ok, Y.S.; Kunhikrishnan, A.; Ali, F.; Ali, S.; Igalavithana, A.D.; Arshad, M. Arsenic(V) biosorption by charred orange peel in aqueous environments. Int. J. Phytoremed. 2016, 18, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Castor, J.; Guzmán-Mar, J.; Hernández-Ramírez, A.; Garza-González, M.; Hinojosa-Reyes, L. Arsenic accumulation in maize crop (Zea mays): A review. Sci. Total Environ. 2014, 488, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef] [PubMed]
- ATSDR, ATSDR (Agency for Toxic Substances and Disease Registry). 2013. Available online: http://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid¼3 (accessed on 23 October 2017).
- Imran, M.A.; Khan, R.M.; Ali, Z.; Mahmood, T. Toxicity of arsenic (As) on seed germination of sunflower (Helianthus annuus L.). Int. J. Phys. Sci. 2013, 8, 840–847. [Google Scholar]
- Rahman, M.A.; Hogan, B.; Duncan, E.; Doyle, C.; Krassoi, R.; Rahman, M.M.; Naidu, R.; Lim, R.P.; Maher, W.; Hassler, C. Toxicity of arsenic species to three freshwater organisms and biotransformation of inorganic arsenic by freshwater phytoplankton (Chlorella sp. CE-35). Ecotoxicol. Environ. Saf. 2014, 106, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H. Arsenic. In Handbook of Geochemistry; Wedepohl, K.H., Ed.; Springer: Berlin, Germany, 1969. [Google Scholar]
- Panda, S.; Upadhyay, R.; Nath, S. Arsenic stress in plants. J. Agron. Crop Sci. 2010, 196, 161–174. [Google Scholar] [CrossRef]
- Rafiq, M.; Shahid, M.; Abbas, G.; Shamshad, S.; Khalid, S.; Niazi, N.K.; Dumat, C. Comparative effect of calcium and EDTA on arsenic uptake and physiological attributes of Pisum sativum. Int. J. Phytoremed. 2017, 19, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.; Shahid, M.; Shamshad, S.; Khalid, S.; Niazi, N.K.; Abbas, G.; Saeed, M.F.; Ali, M.; Murtaza, B. A comparative study to evaluate efficiency of EDTA and calcium in alleviating arsenic toxicity to germinating and young Vicia faba L. seedlings. J. Soils Sedim. 2017, 1–11. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed]
- Armendariz, A.L.; Talano, M.A.; Villasuso, A.L.; Travaglia, C.; Racagni, G.E.; Reinoso, H.; Agostini, E. Arsenic stress induces changes in lipid signalling and evokes the stomata closure in soybean. Plant Physiol. Biochem. 2016, 103, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J. Soil Sci. Plant Nutr. 2016, 16, 662–676. [Google Scholar] [CrossRef]
- Giri, A.; Patel, R. Phytoaccumulation potential and toxicity of arsenic ions by Eichhornia crassipes in hydroponic system. J. Bioremed. Biodegrad 2012, 3, 137. [Google Scholar] [CrossRef]
- Nath, S.; Panda, P.; Mishra, S.; Dey, M.; Choudhury, S.; Sahoo, L.; Panda, S.K. Arsenic stress in rice: Redox consequences and regulation by iron. Plant Physiol. Biochem. 2014, 80, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.; Augustine, R.; Panthri, M.; Zia, I.; Bisht, N.C.; Gupta, M. Arsenic affects the production of glucosinolate, thiol and phytochemical compounds: A comparison of two Brassica cultivars. Plant Physiol. Biochem. 2017, 111, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Kumar, N.; Dixit, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Pandey, V. A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol. Biochem. 2017, 115, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.G.; Maher, W.A.; Foster, S.D.; Krikowa, F.; O’Sullivan, C.A.; Roper, M.M. Dimethylarsenate (DMA) exposure influences germination rates, arsenic uptake and arsenic species formation in wheat. Chemosphere 2017, 181, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, M.; Martínez-López, S.; García-Lorenzo, M.; Martínez-Martínez, L.; Pérez-Sirvent, C. Evaluation of arsenic in soils and plant uptake using various chemical extraction methods in soils affected by old mining activities. Geoderma 2011, 160, 535–541. [Google Scholar] [CrossRef]
- Neidhardt, H.; Kramar, U.; Tang, X.; Guo, H.; Norra, S. Arsenic accumulation in the roots of Helianthus annuus and Zea mays by irrigation with arsenic-rich groundwater: Insights from synchrotron X-ray fluorescence imaging. Chem. Erde-Geochem. 2015, 75, 261–270. [Google Scholar] [CrossRef]
- Ghosh, P.; Rathinasabapathi, B.; Ma, L.Q. Phosphorus solubilization and plant growth enhancement by arsenic-resistant bacteria. Chemosphere 2015, 134, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Niazi, N.K.; Singh, B.; Minasny, B. Mid-infrared spectroscopy and partial least-squares regression to estimate soil arsenic at a highly variable arsenic-contaminated site. Int. J. Environ. Sci. Technol. 2015, 12, 1965–1974. [Google Scholar] [CrossRef]
- Begum, M.C.; Islam, M.S.; Islam, M.; Amin, R.; Parvez, M.S.; Kabir, A.H. Biochemical and molecular responses underlying differential arsenic tolerance in rice (Oryza sativa L.). Plant Physiol. Biochem. 2016, 104, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ashraf, U.; Khan, I.; Wang, L. Alteration in Growth, Leaf Gas Exchange, and Photosynthetic Pigments of Maize Plants under Combined Cadmium and Arsenic Stress. Water Air Soil Pollut. 2017, 228, 13. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, P.; Sharma, Y.K. Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J. Plant Nutr. 2017, 40, 298–306. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev. Environ. Contam. Toxicol. 2017, 241, 73–137. [Google Scholar] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D. Arsenic-induced changes in growth and antioxidant metabolism of fenugreek. Russ. J. Plant Physiol. 2013, 60, 652–660. [Google Scholar] [CrossRef]
- Singh, A.P.; Dixit, G.; Mishra, S.; Dwivedi, S.; Tiwari, M.; Mallick, S.; Pandey, V.; Trivedi, P.K.; Chakrabarty, D.; Tripathi, R.D. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 340. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Indoliya, Y.; Chauhan, A.S.; Singh, S.P.; Singh, A.P.; Dwivedi, S.; Tripathi, R.D.; Chakrabarty, D. Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Pourrut, B.; Silvestre, J.; Laplanche, C.; Pinelli, E. Influence of EDTA and citric acid on lead-induced oxidative stress to Vicia faba roots. J. Soils Sedim. 2014, 14, 835–843. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D. Balancing Roles of Reactive Oxygen Species in Plants’ Response to Metalloid Exposure: Boon or Bane—Revisiting the Role of ROS. In Reactive Oxygen Species in Plants; Wiley: Hobokem, NJ, USA, 2017. [Google Scholar]
- Armendariz, A.L.; Talano, M.A.; Travaglia, C.; Reinoso, H.; Oller, A.L.W.; Agostini, E. Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol. Biochem. 2016, 98, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Karimi, N.; de Oliveira, L.M. Antioxidant enzymes responses in shoots of arsenic hyperaccumulator, Isatis cappadocica Desv, under interaction of arsenate and phosphate. Environ. Technol. 2017, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Srivastava, S.; Chauhan, R.; Awasthi, S.; Mishra, S.; Dwivedi, S.; Tripathi, P.; Kalra, A. Arsenic tolerant Trichoderma sp. reduces arsenic induced stress in chickpea (Cicer arietinum). Environ. Pollut. 2017, 223, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar] [PubMed]
- Pandey, C.; Gupta, M. Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. J. Hazard. Mater. 2015, 287, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, F.; Duman, F.; Leblebici, Z.; Temizgul, R. Arsenic accumulation and biological responses of watercress (Nasturtium officinale R. Br.) exposed to arsenite. Environ. Exp. Bot. 2010, 69, 167–174. [Google Scholar] [CrossRef]
- Yadu, B.; Chandrakar, V.; Keshavkant, S. Responses of plants to fluoride: An overview of oxidative stress and defense mechanisms. Fluoride 2016, 49, 293. [Google Scholar]
- Degola, F.; Fattorini, L.; Bona, E.; Sprimuto, C.T.; Argese, E.; Berta, G.; di Toppi, L.S. The symbiosis between Nicotiana tabacum and the endomycorrhizal fungus Funneliformis mosseae increases the plant glutathione level and decreases leaf cadmium and root arsenic contents. Plant Physiol. Biochem. 2015, 92, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Dixit, G.; Singh, A.P.; Kumar, A.; Mishra, S.; Dwivedi, S.; Kumar, S.; Trivedi, P.K.; Pandey, V.; Tripathi, R.D. Reduced arsenic accumulation in rice (Oryza sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol. Biochem. 2016, 99, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Saidi, I.; Yousfi, N.; Borgi, M.A. Salicylic Acid Improves the Antioxidant Ability against Arsenic-Induced Oxidative Stress in Sunflower (Helianthus annuus) Seedling. J. Plant Nutr. 2017, 40, 2326–2335. [Google Scholar] [CrossRef]
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Chakrabarty, D.; Mallick, S.; Pandey, V. Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 1272. [Google Scholar] [CrossRef] [PubMed]
- Bustingorri, C.; Noriega, G.; Lavado, R.S.; Balestrasse, K. Protective effect exerted by soil phosphorus on soybean subjected to arsenic and fluoride. Redox Rep. 2017, 22, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Saha, D.; Saha, R.; Ghosh, T.; Saha, B. A review on sources, toxicity and remediation technologies for removing arsenic from drinking water. Res. Chem. Intermed. 2014, 40, 447–485. [Google Scholar] [CrossRef]
- Karczewska, A.; Bogda, A.; Krysiak, A. Arsenic in soils in the areas of former mining and mineral processing in Lower Silesia, southwestern Poland. Trace Met. Contam. Environ. 2007, 9, 411–440. [Google Scholar]
- Stafilov, T.; Aliu, M.; Sajn, R. Arsenic in surface soils affected by mining and metallurgical processing in K. Mitrovica Region, Kosovo. Int. J. Environ. Res. Public Health 2010, 7, 4050–4061. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.; Kay, P.; Slack, R.; Gong, Y.Y.; Carter, A. Human exposure assessment of different arsenic species in household water sources in a high risk arsenic area. Sci. Total Environ. 2017, 584, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; Gossai, A.; Pierce, B.; Ahsan, H. Drinking water arsenic contamination, skin lesions, and malignancies: A systematic review of the global evidence. Curr. Environ. Health Rep. 2015, 2, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Begum, A.; Akhtar, K. Study on knowledge about arsenic contamination in drinking water among the people living in selected villages of Bangladesh. J. Shaheed Suhrawardy Med. Coll. 2017, 6, 57–59. [Google Scholar] [CrossRef]
- Mondal, P.; Bhowmick, S.; Chatterjee, D.; Figoli, A.; Van der Bruggen, B. Remediation of inorganic arsenic in groundwater for safe water supply: A critical assessment of technological solutions. Chemosphere 2013, 92, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Smith, E.; Owens, G.; Bhattacharya, P. Managing Arsenic in the Environment: From Soil to Human Health; CSIRO Publishing: Clayton, Australia, 2006. [Google Scholar]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.K.; Alamdar, A.; Katsoyiannis, I.; Shen, H.; Ali, N.; Ali, S.M.; Bokhari, H.; Schäfer, R.B.; Eqani, S.A.M.A.S. Mapping human health risks from exposure to trace metal contamination of drinking water sources in Pakistan. Sci. Total Environ. 2015, 538, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Rahman, M.M.; Naidu, R.; Dong, Z.; Shahid, M.; Arshad, M. Unraveling health risk and speciation of arsenic from groundwater in rural areas of Punjab, Pakistan. Int. J. Environ. Res. Public Health 2015, 12, 12371–12390. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Murtaza, G.; Kunhikrishnan, A.; Seshadri, B.; Shahid, M.; Ali, S.; Bolan, N.S.; Ok, Y.S. Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit. Rev. Environ. Sci. Technol. 2016, 46, 467–499. [Google Scholar] [CrossRef]
- Shahid, N.; Zia, Z.; Shahid, M.; Faiq Bakhat, H.; Anwar, S.; Mustafa Shah, G.; Rizwan Ashraf, M. Assessing Drinking Water Quality in Punjab, Pakistan. Polish J. Environ. Stud. 2015, 24, 2597–2606. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Das, B.; Murrill, M.; Dey, S.; Mukherjee, S.C.; Dhar, R.K.; Biswas, B.K.; Chowdhury, U.K.; Roy, S. Status of groundwater arsenic contamination in Bangladesh: A 14-year study report. Water Res. 2010, 44, 5789–5802. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.J.; Cotter-Howells, J.; Meharg, A.A. Arsenic uptake and accumulation in rice (Oryza sativa L.) irrigated with contaminated water. Plant Soil 2002, 240, 311–319. [Google Scholar] [CrossRef]
- Farooqi, A.; Masuda, H.; Firdous, N. Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ. Pollut. 2007, 145, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Khalid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Imran, M.; Bibi, I.; Ahmad, I.; Hammad, H.M.; Tabassum, R.A. Arsenic Level and Risk Assessment of Groundwater in Vehari, Punjab Province, Pakistan. Expos. Health 2017, 1–11. [Google Scholar] [CrossRef]
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 28. [Google Scholar]
- Pio, I.; Scarlino, A.; Bloise, E.; Mele, G.; Santoro, O.; Pastore, T.; Santoro, D. Efficient removal of low-arsenic concentrations from drinking water by combined coagulation and adsorption processes. Sep. Purif. Technol. 2015, 147, 284–291. [Google Scholar] [CrossRef]
- Rahman, M.M.; Owens, G.; Naidu, R. Arsenic levels in rice grain and assessment of daily dietary intake of arsenic from rice in arsenic-contaminated regions of Bangladesh—Implications to groundwater irrigation. Environ. Geochem. Health 2009, 31, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Roberge, J.; Abalos, A.T.; Skinner, J.M.; Kopplin, M.; Harris, R.B. Presence of arsenic in commercial beverages. Am. J. Environ. Sci. 2009, 5, 688–694. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.J.; Pan, W.-C.; Kile, M.L.; Baccarelli, A.A.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Mostofa, G.; Lin, X.; Christiani, D.C. Arsenic reduction in drinking water and improvement in skin lesions: A follow-up study in Bangladesh. Environ. Health Perspect. 2012, 120, 1733. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.; Kinniburgh, D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2012, 17, 517–568. [Google Scholar] [CrossRef]
- Nearing, M.M.; Koch, I.; Reimer, K.J. Complementary arsenic speciation methods: A review. Spectrochim. Acta Part B 2014, 99, 150–162. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Silvestre, J.; Pinelli, E. Effect of fulvic acids on lead-induced oxidative stress to metal sensitive Vicia faba L. plant. Biol. Fertil. Soils 2012, 48, 689–697. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Aslam, M.; Pinelli, E. Assessment of lead speciation by organic ligands using speciation models. Chem. Spec. Bioavailab. 2012, 24, 248–252. [Google Scholar] [CrossRef]
- Shahid, M.; Zia-Ur-Rehman, M.; Sabir, M.; Ahmad, H.R. Chapter 14—Phytoremediation of Pb-Contaminated Soils Using Synthetic Chelates. In Soil Remediation and Plants; Academic Press: San Diego, CA, USA, 2015; pp. 397–414. [Google Scholar]
- Shahid, M.; Pinelli, E.; Pourrut, B.; Silvestre, J.; Dumat, C. Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicol. Environ. Saf. 2011, 74, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Pierart, A.; Shahid, M.; Séjalon-Delmas, N.; Dumat, C. Antimony bioavailability: Knowledge and research perspectives for sustainable agricultures. J. Hazard. Mater. 2015, 289, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Pourrut, B.; Sabir, M.; Pinelli, E. Assessing the effect of metal speciation on lead toxicity to Vicia faba pigment contents. J. Geochem. Explor. 2014, 144, 290–297. [Google Scholar] [CrossRef]
- Shahid, M.; Ferrand, E.; Schreck, E.; Dumat, C. Behavior and impact of zirconium in the soil–plant system: Plant uptake and phytotoxicity. Rev. Environ. Contam. Toxicol. 2013, 221, 107–127. [Google Scholar] [PubMed]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C. EDTA-enhanced phytoremediation of heavy metals: A review. Soil Sedim. Contam. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar]
- Shuman, L.M. Chemical Forms of Micronutrients in Soils. Available online: https://dl.sciencesocieties.org/publications/books/abstracts/sssabookseries/micronutrientsi2/113/preview/pdf?search-result=1 (accessed on 5 November 2017).
- Niazi, N.K.; Singh, B.; Shah, P. Arsenic speciation and phytoavailability in contaminated soils using a sequential extraction procedure and XANES spectroscopy. Environ. Sci. Technol. 2011, 45, 7135–7142. [Google Scholar] [CrossRef] [PubMed]
- Belogolova, G.; Sokolova, M.; Gordeeva, О.; Vaishlya, О. Speciation of arsenic and its accumulation by plants from rhizosphere soils under the influence of Azotobacter and Bacillus bacteria. J. Geochem. Explor. 2015, 149, 52–58. [Google Scholar] [CrossRef]
- Newman, M.; Jagoe, C. Ligands and the bioavailability of metals in aquatic environments. In Bioavailability: Physical, Chemical and Biological Interactions; CRC Press, Inc.: Boca Raton, FL, USA, 1994. [Google Scholar]
- Li, H.-B.; Li, J.; Zhu, Y.-G.; Juhasz, A.L.; Ma, L.Q. Comparison of arsenic bioaccessibility in housedust and contaminated soils based on four in vitro assays. Sci. Total Environ. 2015, 532, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Xiong, T.; Castrec-Rouelle, M.; Leveque, T.; Dumat, C. Water extraction kinetics of metals, arsenic and dissolved organic carbon from industrial contaminated poplar leaves. J. Environ. Sci. 2013, 25, 2451–2459. [Google Scholar] [CrossRef]
- Joseph, T.; Dubey, B.; McBean, E.A. Human health risk assessment from arsenic exposures in Bangladesh. Sci. Total Environ. 2015, 527, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M. Arsenic chemistry in soils: An overview of thermodynamic predictions and field observations. Water Air Soil Pollut. 1997, 93, 117–136. [Google Scholar] [CrossRef]
- Warren, G.; Alloway, B. Reduction of arsenic uptake by lettuce with ferrous sulfate applied to contaminated soil. J. Environ. Q. 2003, 32, 767–772. [Google Scholar] [CrossRef]
- Abbas, G.; Saqib, M.; Akhtar, J.; Murtaza, G.; Shahid, M.; Hussain, A. Relationship between rhizosphere acidification and phytoremediation in two acacia species. J. Soils Sedim. 2016, 16, 1392–1399. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Dumat, C.; Niazi, N.K.; Bibi, I.; Gul Bakhat, H.F.S.; Abbas, G.; Murtaza, B.; Javeed, H.M.R. Influence of groundwater and wastewater irrigation on lead accumulation in soil and vegetables: Implications for health risk assessment and phytoremediation. Int. J. Phytoremed. 2017, 19, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Pinelli, E.; Dumat, C. Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J. Hazard. Mater. 2012, 219, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Signes-Pastor, A.; Burló, F.; Mitra, K.; Carbonell-Barrachina, A. Arsenic biogeochemistry as affected by phosphorus fertilizer addition, redox potential and pH in a west Bengal (India) soil. Geoderma 2007, 137, 504–510. [Google Scholar] [CrossRef]
- Adra, A.; Morin, G.; Ona-Nguema, G.; Brest, J. Arsenate and arsenite adsorption onto Al-containing ferrihydrites. Implications for arsenic immobilization after neutralization of acid mine drainage. Appl. Geochem. 2016, 64, 2–9. [Google Scholar] [CrossRef]
- Gorny, J.; Billon, G.; Lesven, L.; Dumoulin, D.; Madé, B.; Noiriel, C. Arsenic behavior in river sediments under redox gradient: A review. Sci. Total Environ. 2015, 505, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, R. Interactions between Metals, Microbes and Plants-Bioremediation of Arsenic and Lead Contaminated Soils. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2002. [Google Scholar]
- Ahmad, I.; Akhtar, M.J.; Asghar, H.N.; Ghafoor, U.; Shahid, M. Differential effects of plant growth-promoting rhizobacteria on maize growth and cadmium uptake. J. Plant Growth Regul. 2016, 35, 303–315. [Google Scholar] [CrossRef]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front. Microbial. 2017, 8, 1706. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Suhadolnik, M.L.; Salgado, A.P.; Scholte, L.L.; Bleicher, L.; Costa, P.S.; Reis, M.P.; Dias, M.F.; Ávila, M.P.; Barbosa, F.A.; Chartone-Souza, E. Novel arsenic-transforming bacteria and the diversity of their arsenic-related genes and enzymes arising from arsenic-polluted freshwater sediment. Sci. Rep. 2017, 7, 11231. [Google Scholar] [CrossRef] [PubMed]
- Anguita, J.M.; Rojas, C.; Pastén, P.A.; Vargas, I.T. A new aerobic chemolithoautotrophic arsenic oxidizing microorganism isolated from a high Andean watershed. Biodegradation 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pongratz, R.; Heumann, K.G. Production of methylated mercury, lead, and cadmium by marine bacteria as a significant natural source for atmospheric heavy metals in polar regions. Chemosphere 1999, 39, 89–102. [Google Scholar] [CrossRef]
- Yamamura, S.; Amachi, S. Microbiology of inorganic arsenic: From metabolism to bioremediation. J. Biosci. Bioeng. 2014, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Diorio, C.; Cai, J.; Marmor, J.; Shinder, R.; DuBow, M.S. An Escherichia coli chromosomal ars operon homolog is functional in arsenic detoxification and is conserved in gram-negative bacteria. J. Bacteriol. 1995, 177, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Vaxevanidou, K.; Giannikou, S.; Papassiopi, N. Microbial arsenic reduction in polluted and unpolluted soils from Attica, Greece. J. Hazard. Mater. 2012, 241, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Stazi, S.R.; Marabottini, R.; Papp, R.; Moscatelli, M.C. Arsenic in Soil: Availability and Interactions with Soil Microorganisms. In Heavy Metal Contamination of Soils; Springer: Berlin, Germany, 2015; pp. 113–126. [Google Scholar]
- Hua, J.; Jiang, Q.; Bai, J.; Ding, F.; Lin, X.; Yin, Y. Interactions between arbuscular mycorrhizal fungi and fungivorous nematodes on the growth and arsenic uptake of tobacco in arsenic-contaminated soils. Appl. Soil Ecol. 2014, 84, 176–184. [Google Scholar] [CrossRef]
- Gadd, G. Microbial formation and transformation of organometallic and organometalloid compounds. FEMS Microbiol. Rev. 1993, 11, 297–316. [Google Scholar] [CrossRef]
- Gao, S.; Burau, R.G. Environmental factors affecting rates of arsine evolution from and mineralization of arsenicals in soil. J. Environ. Q. 1997, 26, 753–763. [Google Scholar] [CrossRef]
- Gulz, P.A.; Gupta, S.-K.; Schulin, R. Arsenic accumulation of common plants from contaminated soils. Plant Soil 2005, 272, 337–347. [Google Scholar] [CrossRef]
- Adriano, D.C. Arsenic. In Trace Elements in Terrestrial Environments; Springer: Berlin, Germany, 2001; pp. 219–261. [Google Scholar]
- Austruy, A.; Wanat, N.; Moussard, C.; Vernay, P.; Joussein, E.; Ledoigt, G.; Hitmi, A. Physiological impacts of soil pollution and arsenic uptake in three plant species: Agrostis capillaris, Solanum nigrum and Vicia faba. Ecotoxicol. Environ. Saf. 2013, 90, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wan, X.-M.; Huang, Z.-C.; Chen, T.-B.; Li, X.-W.; Liu, Y.-R. First evidence on different transportation modes of arsenic and phosphorus in arsenic hyperaccumulator Pteris vittata. Environ. Pollut. 2012, 161, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Pochon, N.; Ayadi, A.; Nakanishi, T.M.; Thibaud, M.-C. Phosphate import in plants: Focus on the PHT1 transporters. Front. Plant Sci. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.S.; McKinney, E.C.; Meagher, R.B.; Smith, A.P. Hijacking membrane transporters for arsenic phytoextraction. J. Biotechnol. 2013, 163, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Bakhat, H.F.; Zia, Z.; Fahad, S.; Abbas, S.; Hammad, H.M.; Shahzad, A.N.; Abbas, F.; Alharby, H.; Shahid, M. Arsenic uptake, accumulation and toxicity in rice plants: Possible remedies for its detoxification: A review. Environ. Sci. Pollut. Res. 2017, 24, 9142–9158. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Tamai, K.; Mitani, N. Genotypic difference in silicon uptake and expression of silicon transporter genes in rice. Plant Physiol. 2007, 145, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [PubMed]
- Gomes, M.; Carneiro, M.; Nogueira, C.; Soares, A.; Garcia, Q. The system modulating ROS content in germinating seeds of two Brazilian savanna tree species exposed to As and Zn. Acta Physiol. Plant. 2013, 35, 1011–1022. [Google Scholar] [CrossRef]
- Mirza, N.; Mahmood, Q.; Maroof Shah, M.; Pervez, A.; Sultan, S. Plants as useful vectors to reduce environmental toxic arsenic content. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dubey, R.S.; Tripathi, R.D.; Chakrabarty, D.; Trivedi, P.K. Omics and biotechnology of arsenic stress and detoxification in plants: Current updates and prospective. Environ. Int. 2015, 74, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Macnair, M.R. An altered phosphate uptake system in arsenate-tolerant Holcus lanatus L. New Phytol. 1990, 116, 29–35. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Hakvoort, H.W.; Bliek, M.; Souer, E.; Schat, H. Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant J. 2006, 45, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Wood, B.A.; Raab, A.; McGrath, S.P.; Zhao, F.-J.; Feldmann, J. Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol. 2010, 152, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Raab, A.; Feldmann, J.; Meharg, A.A. The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol. 2004, 134, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Indriolo, E.; Na, G.; Ellis, D.; Salt, D.E.; Banks, J.A. A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell 2010, 22, 2045–2057. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Logoteta, B.; Xu, X.; Macnair, M.; McGrath, S.; Zhao, F. Arsenite efflux is not enhanced in the arsenate-tolerant phenotype of Holcus lanatus. New Phytol. 2009, 183, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Mosa, K.A.; Kumar, K.; Chhikara, S.; Mcdermott, J.; Liu, Z.; Musante, C.; White, J.C.; Dhankher, O.P. Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgen. Res. 2012, 21, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.-L.; Wood, B.A.; Stroud, J.L.; Andralojc, P.J.; Raab, A.; McGrath, S.P.; Feldmann, J.; Zhao, F.-J. Arsenic speciation in phloem and xylem exudates of castor bean. Plant Physiol. 2010, 154, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Y.; Ago, Y.; Liu, W.-J.; Mitani, N.; Feldmann, J.; McGrath, S.P.; Ma, J.F.; Zhao, F.-J. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009, 150, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Raab, A.; Schat, H.; Meharg, A.A.; Feldmann, J. Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): Formation of arsenic–phytochelatin complexes during exposure to high arsenic concentrations. New Phytol. 2005, 168, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Mitani-Ueno, N.; Yamaji, N.; Zhao, F.-J.; Ma, J.F. The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J. Exp. Bot. 2011, 62, 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Raab, A.; Wright, S.H.; Jaspars, M.; Meharg, A.A.; Feldmann, J. Pentavalent arsenic can bind to biomolecules. Angew. Chem. Int. Ed. 2007, 46, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.H.; Creed, P.A.; Parks, A.N.; Fricke, M.W.; Schwegel, C.A.; Creed, J.T.; Heitkemper, D.T.; Vela, N.P. Comparison of a chemical and enzymatic extraction of arsenic from rice and an assessment of the arsenic absorption from contaminated water by cooked rice. Environ. Sci. Technol. 2005, 39, 5241–5246. [Google Scholar] [CrossRef] [PubMed]
- Irtelli, B.; Navari-Izzo, F. Uptake kinetics of different arsenic species by Brassica carinata. Plant Soil 2008, 303, 105. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.-Y.; Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.-T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.S.; Choi, W.-G.; Roberts, D.M. The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim. Biophys. Acta 2006, 1758, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ma, J.; Meharg, A.; McGrath, S. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Ma, L.; Luongo, T. Root exudates and arsenic accumulation in arsenic hyperaccumulating Pteris vittata and non-hyperaccumulating Nephrolepis exaltata. Plant Soil 2004, 258, 9–19. [Google Scholar] [CrossRef]
- Ullrich-Eberius, C.; Sanz, A.; Novacky, A. Evaluation of arsenate-and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba G1. J. Exp. Bot. 1989, 40, 119–128. [Google Scholar] [CrossRef]
- Palmieri, L.; Picault, N.; Arrigoni, R.; Besin, E.; Palmieri, F.; Hodges, M. Molecular identification of three Arabidopsis thaliana mitochondrial dicarboxylate carrier isoforms: Organ distribution, bacterial expression, reconstitution into liposomes and functional characterization. Biochem. J. 2008, 410, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, H.; Smith, S.; Pope, S.; Smith, F. No evidence for competition between arsenate and phosphate for uptake from soil by medic or barley. Environ. Int. 2009, 35, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Shaheen, S.M.; Rinklebe, J.; Wang, H.; Murtaza, B.; Islam, E.; Nawaz, M.F. Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: Investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci. Total Environ. 2017. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.M.; Gousul, G. Arsenic Removal from Contaminated Waters by Fe-Based (hydr) Oxides and Its Phytoavailability in Soil-Plant System. Ph.D. Thesis, Università degli Studi di Napoli Federico II, Neapel, Italy, 2015. [Google Scholar]
- Monteiro, C.; Santos, C.A.O.; Pinho, S.N.; Oliveira, H.; Pedrosa, T.; Dias, M.C. Cadmium-induced cyto-and genotoxicity are organ-dependent in lettuce. Chem. Res. Toxicol. 2012, 25, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Goel, S.; Sandhir, R.; Nayyar, H. Uptake and distribution of arsenic in chickpea: Effects on seed yield and seed composition. Commun. Soil Sci. Plant Anal. 2011, 42, 1728–1738. [Google Scholar] [CrossRef]
- Vromman, D.; Lutts, S.; Lefèvre, I.; Somer, L.; De Vreese, O.; Šlejkovec, Z.; Quinet, M. Effects of simultaneous arsenic and iron toxicities on rice (Oryza sativa L.) development, yield-related parameters and As and Fe accumulation in relation to As speciation in the grains. Plant Soil 2013, 371, 199–217. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Bagci, E.G.; Kadioglu, Y.K. Combined effect of arsenic and phosphorus on mineral element concentrations of sunflower. Commun. Soil Sci. Plant Anal. 2010, 41, 361–372. [Google Scholar] [CrossRef]
- Duman, F.; Ozturk, F.; Aydin, Z. Biological responses of duckweed (Lemna minor L.) exposed to the inorganic arsenic species As(III) and As(V): Effects of concentration and duration of exposure. Ecotoxicology 2010, 19, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, H.; Mirza, N.; Chai, L.-Y.; Yang, Z.-H.; Yong, W.; Tang, C.-J.; Mahmood, Q.; Pervez, A.; Farooq, U.; Fahad, S. Biochemical and Metabolic Changes in Arsenic Contaminated Boehmeria nivea L. BioMed Res. Int. 2016, 2016, 1423828. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, L.M.; Detmann, K.C.; Martins, S.C.; Teixeira, R.A.; Pereira, L.F.; Sanglard, M.L.; Fernie, A.R.; Araújo, W.L.; DaMatta, F.M. The role of silicon in metabolic acclimation of rice plants challenged with arsenic. Environ. Exp. Bot. 2016, 123, 22–36. [Google Scholar] [CrossRef]
- Upadhyay, A.; Singh, N.; Singh, R.; Rai, U. Amelioration of arsenic toxicity in rice: Comparative effect of inoculation of Chlorella vulgaris and Nannochloropsis sp. on growth, biochemical changes and arsenic uptake. Ecotoxicol. Environ. Saf. 2016, 124, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Iriel, A.; Dundas, G.; Cirelli, A.F.; Lagorio, M.G. Effect of arsenic on reflectance spectra and chlorophyll fluorescence of aquatic plants. Chemosphere 2015, 119, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Bhardwaj, R. Arsenic induced modulation of antioxidative defense system and brassinosteroids in Brassica juncea L. Ecotoxicol. Environ. Saf. 2015, 115, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate–glutathione cycle: Possible involvement of nitric oxide. J. Plant Physiol. 2015, 181, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, A.; Singh, B.; Suprasanna, P.; D’souza, S. The effect of arsenic on pigment composition and photosynthesis in Hydrilla verticillata. Biol. Plant. 2013, 57, 385–389. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Gaur, R.; Gupta, M. Comparative biochemical and RAPD analysis in two varieties of rice (Oryza sativa) under arsenic stress by using various biomarkers. J. Hazard. Mater. 2012, 217, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Mishra, A.; Tripathi, P.; Dave, R.; Kumar, A.; Srivastava, S.; Chakrabarty, D.; Trivedi, P.K.; Adhikari, B.; Norton, G.J. Arsenic affects essential and non-essential amino acids differentially in rice grains: Inadequacy of amino acids in rice based diet. Environ. Int. 2012, 46, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Gouri, S.S.; De, D.; Das, B.K.; Mondal, K.C.; Pati, B.R. Effect of arsenic on nodulation and nitrogen fixation of blackgram (Vigna mungo). Indian J. Microbiol. 2011, 51, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-W.; Xu, Y.-F.; Huang, Y.-F. Protective effect of nitric oxide against arsenic-induced oxidative damage in tall fescue leaves. Afr. J. Biotechnol. 2010, 9, 1619–1627. [Google Scholar]
- Gunes, A.; Pilbeam, D.J.; Inal, A. Effect of arsenic–phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant Soil 2009, 314, 211–220. [Google Scholar] [CrossRef]
- Mascher, R.; Lippmann, B.; Holzinger, S.; Bergmann, H. Arsenate toxicity: Effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci. 2002, 163, 961–969. [Google Scholar] [CrossRef]
- Nagajyoti, P.; Lee, K.; Sreekanth, T. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Gusman, G.S.; Oliveira, J.A.; Farnese, F.S.; Cambraia, J. Arsenate and arsenite: The toxic effects on photosynthesis and growth of lettuce plants. Acta Physiol. Plant. 2013, 35, 1201–1209. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.-Y.; Wang, L.-C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Al Mahmud, J.; Hossain, S.; Alam, K.; Oku, H.; Fujita, M. Actions of Biological Trace Elements in Plant Abiotic Stress Tolerance. In Essential Plant Nutrients; Springer: Berlin, Germany, 2017; pp. 213–274. [Google Scholar]
- Suneja, Y. Physio-Biochemical Responses and Allelic Diversity for Water Deficit Tolerance Related Traits in Aegilops tauschii and Triticum dicoccoides. Ph.D. Thesis, Punjab Agricultural University, Ludhiana, India, 2014. [Google Scholar]
- Pandey, S.; Rai, R.; Rai, L.C. Biochemical and Molecular Basis of Arsenic Toxicity and Tolerance in Microbes and Plants. In Handbook of Arsenic Toxicology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Stoeva, N.; Bineva, T. Oxidative changes and photosynthesis in oat plants grown in As-contaminated soil. Bulg. J. Plant Physiol. 2003, 29, 87–95. [Google Scholar]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Abiotic and Biotic Plant Stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Musil, S.; Matoušek, T.S.; Currier, J.M.; Stýblo, M.; Dědina, J.Í. Speciation analysis of arsenic by selective hydride generation-cryotrapping-atomic fluorescence spectrometry with flame-in-gas-shield atomizer: Achieving extremely low detection limits with inexpensive instrumentation. Anal. Chem. 2014, 86, 10422–10428. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P. Toxic Metals and Environmental Issues; Sarup & Sons: New Delhi, India, 2005. [Google Scholar]
- Tawfik, D.S.; Viola, R.E. Arsenate replacing phosphate: Alternative life chemistries and ion promiscuity. Biochemistry 2011, 50, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; De Sarkar, N.; Banerjee, P.; Banerjee, S.; Mukherjee, S.; Chattopadhyay, D.; Mukhopadhyay, A. Effects of arsenic toxicity on germination, seedling growth and peroxidase activity in Cicer arietinum. Int. J. Agric. Food Sci. 2012, 2, 131–137. [Google Scholar]
- Gresser, M. ADP-arsenate. Formation by submitochondrial particles under phosphorylating conditions. J. Biol. Chem. 1981, 256, 5981–5983. [Google Scholar] [PubMed]
- Moore, S.A.; Moennich, D.; Gresser, M. Synthesis and hydrolysis of ADP-arsenate by beef heart submitochondrial particles. J. Biol. Chem. 1983, 258, 6266–6271. [Google Scholar] [PubMed]
- Watling-Payne, A.S.; Selwyn, M.J. Inhibition and uncoupling of photophosphorylation in isolated chloroplasts by organotin, organomercury and diphenyleneiodonium compounds. Biochem. J. 1974, 142, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.B.; Alencar, N.L.M.; Gomes-Filho, E. Comparison between the water and salt stress effects on plant growth and development. In Responses of Organisms to Water Stress; Intech: Rijeka, Croatia, 2013. [Google Scholar]
- Evangelou, M.W.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ma, L.Q.; Srivastava, M.; Rathinasabapathi, B. Metabolic adaptations to arsenic-induced oxidative stress in Pterisvittata L. and Pterisensiformis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K.; Arora, K. Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul. 2007, 53, 65–73. [Google Scholar] [CrossRef]
- Flora, S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011, 51, 257–281. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Napolitano, G.; Di Meo, S. Role of mitochondria and other ROS sources in hyperthyroidism-linked oxidative stress. Immunol. Endocr. Metab. Agents Med. Chem. 2015, 15, 5–36. [Google Scholar] [CrossRef]
- Singh, G. Antibacterial Activity Testing of Cotton Medical Textiles Sonochemically Impregnated with Metal Oxide Nanoparticles. Ph.D. Thesis, Coventry University, Coventry, UK, 2014. [Google Scholar]
- Pourrut, B.; Shahid, M.; Douay, F.; Dumat, C.; Pinelli, E. Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. In Heavy Metal Stress in Plants; Springer: Berlin, Germany, 2013; pp. 121–147. [Google Scholar]
- Keshavkant, S.; Naithani, S. Chilling-induced oxidative stress in young sal (Shorea robusta) seedlings. Acta Physiol. Plant. 2001, 23, 457–466. [Google Scholar] [CrossRef]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Karuppanapandian, T.; Moon, J.-C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709. [Google Scholar]
- Huang, W.; Yang, X.; Yao, S.; LwinOo, T.; He, H.; Wang, A.; Li, C.; He, L. Reactive oxygen species burst induced by aluminum stress triggers mitochondria-dependent programmed cell death in peanut root tip cells. Plant Physiol. Biochem. 2014, 82, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A.; Corpas, F.J.; Sandalio, L.M.; Palma, J.M.; Gómez, M.; Barroso, J.B. Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J. Exp. Bot. 2002, 53, 1255–1272. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I. Arsenic induced oxidative stress in plants. Biologia 2012, 67, 447–453. [Google Scholar] [CrossRef]
- Keshavkant, S.; Padhan, J.; Parkhey, S.; Naithani, S. Physiological and antioxidant responses of germinating Cicer arietinum seeds to salt stress. Russ. J. Plant Physiol. 2012, 59, 206–211. [Google Scholar] [CrossRef]
- Li, C.-X.; Feng, S.-L.; Yun, S.; Jiang, L.-N.; Lu, X.-Y.; Hou, X.-L. Effects of arsenic on seed germination and physiological activities of wheat seedlings. J. Environ. Sci. 2007, 19, 725–732. [Google Scholar] [CrossRef]
- Mylona, P.V.; Polidoros, A.N.; Scandalios, J.G. Modulation of antioxidant responses by arsenic in maize. Free Radic. Biol. Med. 1998, 25, 576–585. [Google Scholar] [CrossRef]
- Ghosh, S.; Saha, J.; Biswas, A.K. Interactive influence of arsenate and selenate on growth and nitrogen metabolism in wheat (Triticum aestivum L.) seedlings. Acta Physiol. Plant. 2013, 35, 1873–1885. [Google Scholar] [CrossRef]
- Choudhury, B.; Chowdhury, S.; Biswas, A.K. Regulation of growth and metabolism in rice (Oryza sativa L.) by arsenic and its possible reversal by phosphate. J. Plant Interact. 2011, 6, 15–24. [Google Scholar] [CrossRef]
- Shahid, M.; Rafiq, M.; Niazi, N.K.; Dumat, C.; Shamshad, S.; Khalid, S.; Bibi, I. Arsenic accumulation and physiological attributes of spinach in the presence of amendments: An implication to reduce health risk. Environ. Sci. Pollut. Res. 2017, 24, 16097–16106. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Negi, A.; Mahajan, P.; Rana, S.; Kohli, R.K. Arsenic (As) Inhibits radicle emergence and elongation in Phaseolus aureus by altering starch-metabolizing enzymes vis-à-vis disruption of oxidative metabolism. Biol. Trace Elem. Res. 2012, 146, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Pinelli, E.; Pourrut, B.; Dumat, C. Effect of organic ligands on lead-induced oxidative damage and enhanced antioxidant defense in the leaves of Vicia faba plants. J. Geochem. Explor. 2014, 144, 282–289. [Google Scholar] [CrossRef]
- Livanos, P.; Apostolakos, P.; Galatis, B. Plant cell division: ROS homeostasis is required. Plant Signal. Behav. 2012, 7, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Livanos, P.; Galatis, B.; Quader, H.; Apostolakos, P. Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cytoskeleton 2012, 69, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G. Potential contributions of oxidoreductases from alfalfa plants to soil enzymology and biotechnology: A review. J. Nat. Sci. Sustain. Technol. 2012, 6, 169. [Google Scholar]
- Jha, A.; Dubey, R. Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J. Plant Physiol. 2004, 161, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T.; González, M.-C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Baud, S.; Lepiniec, L. Regulation of de novo fatty acid synthesis in maturing oilseeds of Arabidopsis. Plant Physiol. Biochem. 2009, 47, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Parkhey, S.; Naithani, S.; Keshavkant, S. ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol. Biochem. 2012, 57, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Redox Homeost. Manag. Plants Environ. Stress. 2014. [Google Scholar] [CrossRef]
- Srivastava, M.; Ma, L.Q.; Singh, N.; Singh, S. Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J. Exp. Bot. 2005, 56, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, S.L.; Esteban, E.; Carpena, R.N.O. Evolution of arsenate toxicity in nodulated white lupine in a long-term culture. J. Agric. Food Chem. 2008, 56, 8580–8587. [Google Scholar] [CrossRef] [PubMed]
- Montillet, J.-L.; Chamnongpol, S.; Rustérucci, C.; Dat, J.; Van De Cotte, B.; Agnel, J.-P.; Battesti, C.; Inzé, D.; Van Breusegem, F.; Triantaphylidès, C. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 2005, 138, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, C.; Ji, G.; Ramirez, J.; Silver, S. Resistance to arsenic compounds in microorganisms. FEMS Microbiol. Rev. 1994, 15, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dubey, R.S. Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: Role of proline as enzyme protectant. J. Plant Physiol. 2006, 163, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Hartley-Whitaker, J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002, 154, 29–43. [Google Scholar] [CrossRef]
- Singh, H.P.; Kaur, S.; Batish, D.R.; Sharma, V.P.; Sharma, N.; Kohli, R.K. Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide 2009, 20, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ismail, G.S.M. Protective role of nitric oxide against arsenic-induced damages in germinating mung bean seeds. Acta Physiol. Plant. 2012, 34, 1303–1311. [Google Scholar] [CrossRef]
- Ranki, H.; Sopanen, T.; Voutilainen, R. Localization of carboxypeptidase I in germinating barley grain. Plant Physiol. 1990, 93, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Shan, X.; Zhu, Y.-G. Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere 2005, 61, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Parkhey, S.; Naithani, S.; Keshavkant, S. Protein metabolism during natural ageing in desiccating recalcitrant seeds of Shorea robusta. Acta Physiol. Plant. 2014, 36, 1649–1659. [Google Scholar] [CrossRef]
- Job, C.; Rajjou, L.; Lovigny, Y.; Belghazi, M.; Job, D. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol. 2005, 138, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.; Belghazi, M.; Job, C.; Job, D. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Naikoo, M.I.; Khan, F.A.; Rehman, F.; Green, I.D.; Naushin, F.; Ansari, A.A. An Introduction to Reactive Oxygen Species Metabolism under Changing Climate in Plants. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Berlin, Germany, 2017; pp. 25–52. [Google Scholar]
- Oracz, K.; Bailly, C.; Gniazdowska, A.; Côme, D.; Corbineau, F.; Bogatek, R. Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds. J. Chem. Ecol. 2007, 33, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.; Desikan, R.; Harrison, J.; Bright, J.; Hooley, R.; Neill, S. Doing the unexpected: Proteins involved in hydrogen peroxide perception. J. Exp. Bot. 2006, 57, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Karuppanapandian, T.; Kim, W. Cobalt-induced oxidative stress causes growth inhibition associated with enhanced lipid peroxidation and activates antioxidant responses in Indian mustard (Brassica juncea L.) leaves. Acta Physiol. Plant. 2013, 35, 2429–2443. [Google Scholar] [CrossRef]
- Ramakrishna, B.; Rao, S.S.R. 24-Epibrassinolide maintains elevated redox state of AsA and GSH in radish (Raphanus sativus L.) seedlings under zinc stress. Acta Physiol. Plant. 2013, 35, 1291–1302. [Google Scholar] [CrossRef]
- Mustafa, G.; Komatsu, S. Toxicity of heavy metals and metal-containing nanoparticles on plants. Biochim. Biophys. Acta 2016, 1864, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Naliwajski, M.; Skłodowska, M. The oxidative stress and antioxidant systems in cucumber cells during acclimation to salinity. Biol. Plant. 2014, 58, 47–54. [Google Scholar] [CrossRef]
- Patra, M.; Bhowmik, N.; Bandopadhyay, B.; Sharma, A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004, 52, 199–223. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Faita, F.; Cori, L.; Bianchi, F.; Andreassi, M.G. Arsenic-induced genotoxicity and genetic susceptibility to arsenic-related pathologies. Int. J. Environ. Res. Public Health 2013, 10, 1527–1546. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, K.; Blasiak, J. In vitro genotoxicity of lead acetate: Induction of single and double DNA strand breaks and DNA–protein cross-links. Mutat. Res. 2003, 535, 127–139. [Google Scholar] [CrossRef]
- Kitchin, K.T.; Wallace, K. Evidence against the nuclear in situ binding of arsenicals–Oxidative stress theory of arsenic carcinogenesis. Toxicol. Appl. Pharmacol. 2008, 232, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Ziech, D.; Franco, R.; Georgakilas, A.G.; Georgakila, S.; Malamou-Mitsi, V.; Schoneveld, O.; Pappa, A.; Panayiotidis, M.I. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem. Biol. Interact. 2010, 188, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Chou, P.-H.; Kim, S.Y.; Suzuki, N.; Laxmi, Y.S.; Okamoto, K.; Liu, X.; Matsuda, T.; Shibutani, S. Oxidative DNA damage in XPC-knockout and its wild mice treated with equine estrogen. Chem. Res. Toxicol. 2008, 21, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Crohns, M. Antioxidants, Cytokines and Markers of Oxidative Stress in Lung Cancer: Associations with Adverse Events, Response and Survival; Tampere University Press: University of Tampere, Tampere, Finland, 2010. [Google Scholar]
- De Vizcaya-Ruiz, A.; Barbier, O.; Ruiz-Ramos, R.; Cebrian, M.E. Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat. Res. 2009, 674, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, A.D.; Malik, S.I.; Campbell, J.A. Cytogenetic insights into DNA damage and repair of lesions induced by a monomethylated trivalent arsenical. Mutat. Res. 2010, 695, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Shipton, W.A. The Biology of Fungi Impacting Human Health; PartridgeIndia: Gurgaon, India, 2014. [Google Scholar]
- Tofan-Lazar, J.; Al-Abadleh, H.A. ATR-FTIR studies on the adsorption/desorption kinetics of dimethylarsinic acid on iron–(oxyhydr) oxides. J. Phys. Chem. A 2012, 116, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Colognato, R.; Coppede, F.; Ponti, J.; Sabbioni, E.; Migliore, L. Genotoxicity induced by arsenic compounds in peripheral human lymphocytes analysed by cytokinesis-block micronucleus assay. Mutagenesis 2007, 22, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Duquesnoy, I.; Champeau, G.M.; Evray, G.; Ledoigt, G.; Piquet-Pissaloux, A. Enzymatic adaptations to arsenic-induced oxidative stress in Zea mays and genotoxic effect of arsenic in root tips of Vicia faba and Zea mays. Comptes Rendus Biol. 2010, 333, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Atienzar, F.A.; Conradi, M.; Evenden, A.J.; Jha, A.N.; Depledge, M.H. Qualitative assessment of genotoxicity using random amplified polymorphic DNA: Comparison of genomic template stability with key fitness parameters in Daphnia magna exposed to benzo [a] pyrene. Environ. Toxicol. Chem. 1999, 18, 2275–2282. [Google Scholar] [CrossRef]
- Atienzar, F.A.; Cordi, B.; Donkin, M.E.; Evenden, A.J.; Jha, A.N.; Depledge, M.H. Comparison of ultraviolet-induced genotoxicity detected by random amplified polymorphic DNA with chlorophyll fluorescence and growth in a marine macroalgae, Palmariapalmata. Aquat. Toxicol. 2000, 50, 1–12. [Google Scholar] [CrossRef]
- Cenkci, S.; Ciğerci, İ.H.; Yıldız, M.; Özay, C.; Bozdağ, A.; Terzi, H. Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environ. Exp. Bot. 2010, 67, 467–473. [Google Scholar] [CrossRef]
- Adhikari, D.; Pal, S. Molecular Markers in Plant-Based Bioassays for the Detection of Molecular Endpoints to Probe of Aquatic Genotoxicity—An Overview. J. Environ. Sociobiol. 2015, 12, 143–162. [Google Scholar]
- Körpe, D.A.; Aras, S. Evaluation of copper-induced stress on eggplant (Solanum melongena L.) seedlings at the molecular and population levels by use of various biomarkers. Mutat. Res. 2011, 719, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Härtling, S.; Tanneberg, H. The identification and quantification of arsenic-induced phytochelatins—Comparison between plants with varying As sensitivities. Plant Soil 2008, 303, 275–287. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, N. Mitigation approach of arsenic toxicity in chickpea grown in arsenic amended soil with arsenic tolerant plant growth promoting Acinetobacter sp. Ecol. Eng. 2014, 70, 146–153. [Google Scholar] [CrossRef]
- Silveira, N.M.; de Oliveira, J.A.; Ribeiro, C.; Canatto, R.A.; Siman, L.; Cambraia, J.; Farnese, F. Nitric oxide attenuates oxidative stress induced by arsenic in lettuce (Lactuca sativa) leaves. Water Air Soil Pollut. 2015, 226, 379. [Google Scholar] [CrossRef]
- Tiwari, S.; Sarangi, B.K. Comparative analysis of antioxidant response by Pteris vittata and Vetiveria zizanioides towards arsenic stress. Ecol. Eng. 2017, 100, 211–218. [Google Scholar] [CrossRef]
- Garg, N.; Singla, P. Arsenic toxicity in crop plants: Physiological effects and tolerance mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
- Matysik, J.; Alia; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Maheshwari, R.; Dubey, R. Nickel toxicity inhibits ribonuclease and protease activities in rice seedlings: Protective effects of proline. Plant Growth Regul. 2007, 51, 231–243. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Dubey, G.; Prasad, S.M. Exogenous proline application ameliorates toxic effects of arsenate in Solanum melongena L. seedlings. Ecotoxicol. Environ. Saf. 2015, 117, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Namdjoyan, S.; Kermanian, H. Exogenous nitric oxide (as sodium nitroprusside) ameliorates arsenic-induced oxidative stress in watercress (Nasturtium officinale R. Br.) plants. Sci. Horticult. 2013, 161, 350–356. [Google Scholar] [CrossRef]
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Nitric oxide alleviates arsenic-induced toxic effects in ridged Luffa seedlings. Plant Physiol. Biochem. 2013, 71, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Odjegba, V.J. Exogenous salicylic acid alleviates arsenic toxicity in Arabidopsis thaliana. Indian J. Innov. Dev. 2012, 1, 515–522. [Google Scholar]
- Tripathi, R.D.; Tripathi, P.; Dwivedi, S.; Dubey, S.; Chatterjee, S.; Chakrabarty, D.; Trivedi, P.K. Arsenomics: Omics of arsenic metabolism in plants. Front. Physiol. 2012, 3, 275. [Google Scholar] [CrossRef] [PubMed]
- Delnomdedieu, M.; Basti, M.M.; Otvos, J.D.; Thomas, D.J. Reduction and binding of arsenate and dimethylarsinate by glutathione: A magnetic resonance study. Chem. Biol. Interact. 1994, 90, 139–155. [Google Scholar] [CrossRef]
- Hartley-Whitaker, J.; Woods, C.; Meharg, A.A. Is differential phytochelatin production related to decreased arsenate influx in arsenate tolerant Holcus lanatus? New Phytol. 2002, 155, 219–225. [Google Scholar] [CrossRef]
- Dwivedi, S.; Tripathi, R.; Tripathi, P.; Kumar, A.; Dave, R.; Mishra, S.; Singh, R.; Sharma, D.; Rai, U.; Chakrabarty, D. Arsenate exposure affects amino acids, mineral nutrient status and antioxidants in rice (Oryza sativa L.) genotypes. Environ. Sci. Technol. 2010, 44, 9542–9549. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mishra, S.; Tripathi, R.; Dwivedi, S.; Trivedi, P.; Tandon, P. Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (Lf) Royle. Environ. Sci. Technol. 2007, 41, 2930–2936. [Google Scholar] [CrossRef] [PubMed]
- Schat, H.; Llugany, M.; Vooijs, R.; Hartley-Whitaker, J.; Bleeker, P.M. The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J. Exp. Bot. 2002, 53, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-B.; Smith, A.P.; Howden, R.; Dietrich, W.M.; Bugg, S.; O’Connell, M.J.; Goldsbrough, P.B.; Cobbett, C.S. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 1999, 11, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Hartley-Whitaker, J.; Ainsworth, G.; Meharg, A. Copper-and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ. 2001, 24, 713–722. [Google Scholar] [CrossRef]
- Dave, R.; Tripathi, R.D.; Dwivedi, S.; Tripathi, P.; Dixit, G.; Sharma, Y.K.; Trivedi, P.K.; Corpas, F.J.; Barroso, J.B.; Chakrabarty, D. Arsenate and arsenite exposure modulate antioxidants and amino acids in contrasting arsenic accumulating rice (Oryza sativa L.) genotypes. J. Hazard. Mater. 2013, 262, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Al-Huqail, A.A.; AL-Rashed, S.A.; Ibrahim, M.M.; El-Gaaly, G.A.; Qureshi, M.I. Arsenic induced eco-physiological changes in Chickpea (Cicer arietinum) and protection by gypsum, a source of sulphur and calcium. Sci. Horticult. 2017, 217, 226–233. [Google Scholar] [CrossRef]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Trivedi, P.K. Thiol metabolism and antioxidant systems complement each other during arsenate detoxification in Ceratophyllum demersum L. Aquat. Toxicol. 2008, 86, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Sayantan, D. Phosphate Amendments Moderate the Arsenate Accumulation and Its Subsequent Oxidative and Physiological Toxicities in Amaranthus viridis L. Proc. Natl. Acad. Sci. India Sect. B 2017, 87, 1343–1353. [Google Scholar] [CrossRef]
- Khan, I.; Ahmad, A.; Iqbal, M. Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotoxicol. Environ. Saf. 2009, 72, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Niazi, N.K.; Bashir, S.; Bibi, I.; Murtaza, B.; Shahid, M.; Javed, M.T.; Shakoor, M.B.; Saqib, Z.A.; Nawaz, M.F.; Aslam, Z.; et al. Phytoremediation of Arsenic-Contaminated Soils Using Arsenic Hyperaccumulating Ferns. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 3, pp. 521–545. [Google Scholar]
- Ribera-Fonseca, A.; Inostroza-Blancheteau, C.; Cartes, P.; Rengel, Z.; Mora, M. Early induction of Fe-SOD gene expression is involved in tolerance to Mn toxicity in perennial ryegrass. Plant Physiol. Biochem. 2013, 73, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Saqib, M.; Akhtar, J.; Murtaza, G.; Shahid, M. Effect of salinity on rhizosphere acidification and antioxidant activity of two acacia species. Can. J. For. Res. 2014, 45, 124–129. [Google Scholar] [CrossRef]
- Gusman, G.S.; Oliveira, J.A.; Farnese, F.S.; Cambraia, J. Mineral nutrition and enzymatic adaptation induced by arsenate and arsenite exposure in lettuce plants. Plant Physiol. Biochem. 2013, 71, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Yang, G.-M.; Fu, J.-W.; Guan, D.-X.; Chen, Y.; Ma, L.Q. Arsenic-induced plant growth of arsenic-hyperaccumulator Pteris vittata: Impact of arsenic and phosphate rock. Chemosphere 2016, 149, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Han, Y.-S.; Seong, H.J.; Ahn, J.S.; Nam, I.-H. Arsenic uptake and speciation in Arabidopsis thaliana under hydroponic conditions. Chemosphere 2016, 154, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Dixit, G.; Singh, A.P.; Kumar, A.; Singh, P.K.; Kumar, S.; Dwivedi, S.; Trivedi, P.K.; Pandey, V.; Norton, G.J.; Dhankher, O.P. Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J. Hazard. Mater. 2015, 298, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide dismutases. An adaptation to a paramagnetic gas. J. Biol. Chem. 1989, 264, 7761–7764. [Google Scholar] [PubMed]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Chongpraditnun, P.; Mori, S.; Chino, M. Excess copper induces a cytosolic Cu, Zn-superoxide dismutase in soybean root. Plant Cell Physiol. 1992, 33, 239–244. [Google Scholar] [CrossRef]
- Requejo, R.; Tena, M. Maize response to acute arsenic toxicity as revealed by proteome analysis of plant shoots. Proteomics 2006, 6, S156–S162. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, N.; Khavari-Nejad, R.A.; Fahimi, H.; Saadatmand, S.; Nejad-Sattari, T. Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci. Horticult. 2010, 126, 402–407. [Google Scholar] [CrossRef]
- Mallick, N.; Mohn, F.H. Reactive oxygen species: Response of algal cells. J. Plant Physiol. 2000, 157, 183–193. [Google Scholar] [CrossRef]
- Rai, U.; Singh, N.; Upadhyay, A.; Verma, S. Chromate tolerance and accumulation in Chlorella vulgaris L.: Role of antioxidant enzymes and biochemical changes in detoxification of metals. Bioresour. Technol. 2013, 136, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zilinskas, B. Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J. Biol. Chem. 1992, 267, 21802–21807. [Google Scholar] [PubMed]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Ghaderian, S.M.; Raab, A.; Feldmann, J.; Meharg, A.A. An arsenic-accumulating, hypertolerant brassica, Isatis capadocica. New Phytol. 2009, 184, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A. Variation in arsenic accumulation–hyperaccumulation in ferns and their allies. New Phytol. 2003, 157, 25–31. [Google Scholar] [CrossRef]
- Singh, N.; Ma, L.Q. Arsenic speciation, and arsenic and phosphate distribution in arsenic hyperaccumulator Pteris vittata L. and non-hyperaccumulator Pteris ensiformis L. Environ. Pollut. 2006, 141, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.S.; Sayre, R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qi, M.; Mei, C. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004, 40, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Coitiño, E.L.; Borsani, O.; Monza, J. Molecular mechanisms for the reaction between (·)OH radicals and proline: Insights on the role as reactive oxygen species scavenger in plant stress. J. Phys. Chem. B 2013, 118, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-F.; Gong, M.; Liu, Y.; Hu, J.-L.; Deng, M.-H. Effect of hydrogen peroxide on growth and activity of some enzymes involved in proline metabolism of sweet corn seedlings under copper stress. Sci. Horticult. 2013, 164, 366–371. [Google Scholar] [CrossRef]
- De Campos, M.K.F.; de Carvalho, K.; de Souza, F.S.; Marur, C.J.; Pereira, L.F.P.; Bespalhok Filho, J.C.; Vieira, L.G.E. Drought tolerance and antioxidant enzymatic activity in transgenic ‘Swingle’ citrumelo plants over-accumulating proline. Environ. Exp. Bot. 2011, 72, 242–250. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Siddiqui, F.; Tandon, P.; Srivastava, S. Analysis of arsenic induced physiological and biochemical responses in a medicinal plant, Withania somnifera. Physiol. Mol. Biol. Plants 2015, 21, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chandrakar, V.; Yadu, B.; Meena, R.K.; Dubey, A.; Keshavkant, S. Arsenic-induced genotoxic responses and their amelioration by diphenylene iodonium, 24-epibrassinolide and proline in Glycine max L. Plant Physiol. Biochem. 2017, 112, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Mushtaq, Z.; Andaz, F.; Masood, A. Does proline application ameliorate adverse effects of salt stress on growth, ions and photosynthetic ability of eggplant (Solanum melongena L.)? Sci. Horticult. 2013, 164, 507–511. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; de Sousa, A.; Pinto, A.; Azenha, M.; Teixeira, J.; Azevedo, R.A.; Fidalgo, F. Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ. Exp. Bot. 2016, 122, 115–125. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; Liu, Z.; Zhen, A.; Wang, W. Protective role of proline against salt stress is partially related to the improvement of water status and peroxidase enzyme activity in cucumber. Soil Sci. Plant Nutr. 2009, 55, 698–704. [Google Scholar] [CrossRef]
- Aggarwal, M.; Sharma, S.; Kaur, N.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Kaur, R.; Singh, K.; Srivastava, A.; Nayyar, H. Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol. Trace Elem. Res. 2011, 140, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Srivastava, P.K.; Singh, V.P.; Prasad, S.M. Light intensity alters the extent of arsenic toxicity in Helianthus annuus L. seedlings. Biol. Trace Elem. Res. 2014, 158, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013, 63, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Fu, G.; Tao, L.; Zhu, C. Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch. Biochem. Biophys. 2010, 497, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S.; Gupta, M. An update on nitric oxide and its benign role in plant responses under metal stress. Nitric Oxide 2017, 67, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Farnese, F.S.; de Oliveira, J.A.; Gusman, G.S.; Leão, G.A.; Ribeiro, C.; Siman, L.I.; Cambraia, J. Plant responses to arsenic: The role of nitric oxide. Water Air Soil Pollut. 2013, 224, 1660. [Google Scholar] [CrossRef]
- Arasimowicz, M.; Floryszak-Wieczorek, J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci. 2007, 172, 876–887. [Google Scholar] [CrossRef]
- Zhang, X.; Uroic, M.K.; Xie, W.-Y.; Zhu, Y.-G.; Chen, B.-D.; McGrath, S.P.; Feldmann, J.; Zhao, F.-J. Phytochelatins play a key role in arsenic accumulation and tolerance in the aquatic macrophyte Wolffia globosa. Environ. Pollut. 2012, 165, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.N.; Vladkova, R.; Singh, R.; Misra, M.; Dobrikova, A.G.; Apostolova, E.L. Action and target sites of nitric oxide in chloroplasts. Nitric Oxide 2014, 39, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; Latif, H.H.; Hanafy, R.S. Influence of nitric oxide application on some biochemical aspects, endogenous hormones, minerals and phenolic compounds of Vicia faba plant grown under arsenic stress. Gesunde Pflanzen 2016, 68, 99–107. [Google Scholar] [CrossRef]
- Radi, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 2012, 46, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.; Fujita, M. Physiological and biochemical mechanisms of nitric oxide induced abiotic stress tolerance in plants. J. Plant Physiol. 2010, 5, 295–324. [Google Scholar]
- Saxena, I.; Shekhawat, G. Nitric oxide (NO) in alleviation of heavy metal induced phytotoxicity and its role in protein nitration. Nitric Oxide 2013, 32, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Ghasemi, H.; Rostampour, F. The role of oxidative stress in metals toxicity; mitochondrial dysfunction as a key player. Galen Med. J. 2014, 3, 2–13. [Google Scholar]
- He, H.; He, L.; Gu, M. The diversity of nitric oxide function in plant responses to metal stress. Biometals 2014, 27, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Singh, S.; Dubey, P.; Singh, A.; Singh, A. Nitric oxide mediated amelioration of arsenic toxicity which alters the alternative oxidase (Aox1) gene expression in Hordeum vulgare L. Ecotoxicol. Environ. Saf. 2015, 120, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Santa Cruz, D.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 2012, 25, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Jan, N.; Wani, T.A.; Ahmad, M.; Masoodi, F.; Gani, A. Optimization of antioxidant activity and total polyphenols of dried apricot fruit extracts (Prunus armeniaca L.) using response surface methodology. J. Saudi Soc. Agric. Sci. 2017, 16, 119–126. [Google Scholar] [CrossRef]
- Pandey, P.; Srivastava, R.K.; Dubey, R. Salicylic acid alleviates aluminum toxicity in rice seedlings better than magnesium and calcium by reducing aluminum uptake, suppressing oxidative damage and increasing antioxidative defense. Ecotoxicology 2013, 22, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Dražić, G.; Mihailović, N. Salicylic acid modulates accumulation of Cd in seedlings of Cd-tolerant and Cd-susceptible soybean genotypes. Arch. Biol. Sci. 2009, 61, 431–439. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Yang, S.-L.; Lan, S.-S.; Gong, M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J. Plant Physiol. 2009, 166, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Zengin, F. Effects of exogenous salicylic acid on growth characteristics and biochemical content of wheat seeds under arsenic stress. J. Environ. Biol. 2015, 36, 249. [Google Scholar] [PubMed]
- Niazi, N.K.; Burton, E.D. Arsenic sorption to nanoparticulate mackinawite (FeS): An examination of phosphate competition. Environ. Pollut. 2016, 218, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Catarecha, P.; Segura, M.D.; Franco-Zorrilla, J.M.; García-Ponce, B.; Lanza, M.; Solano, R.; Paz-Ares, J.; Leyva, A. A mutant of the Arabidopsis phosphate transporter PHT1; 1 displays enhanced arsenic accumulation. Plant Cell 2007, 19, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yeh, M.-C.; Cheng, K.-T.; Avidan, S. Fast Human Detection Using a Cascade of Histograms of Oriented Gradients. In Proceedings of the 2006 IEEE Computer Society Conference on Computer Vision and Pattern Recognition, New York, NY, USA, 17–22 June 2006; pp. 1491–1498. [Google Scholar]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1; 1 and Pht1; 4 play a major role in phosphate acquisition from both low-and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Remy, E.; Cabrito, T.; Batista, R.; Teixeira, M.; Sá-Correia, I.; Duque, P. The Pht1; 9 and Pht1; 8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytol. 2012, 195, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.K.; Jain, A.; Poling, M.D.; Lewis, A.J.; Raghothama, K.G.; Smith, A.P. Arabidopsis Pht1; 5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol. 2011, 156, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- DiTusa, S.F.; Fontenot, E.B.; Wallace, R.W.; Silvers, M.A.; Steele, T.N.; Elnagar, A.H.; Dearman, K.M.; Smith, A.P. A member of the Phosphate transporter 1 (Pht1) family from the arsenic-hyperaccumulating fern Pteris vittata is a high-affinity arsenate transporter. New Phytol. 2016, 209, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sharma, Y.K. Impact of arsenic toxicity on black gram and its amelioration using phosphate. ISRN Toxicol. 2013, 2013, 340925. [Google Scholar] [CrossRef] [PubMed]
- Pigna, M.; Cozzolino, V.; Violante, A.; Meharg, A.A. Influence of phosphate on the arsenic uptake by wheat (Triticum durum L.) irrigated with arsenic solutions at three different concentrations. Water Air Soil Pollut. 2009, 197, 371–380. [Google Scholar] [CrossRef]
- Fu, J.-W.; Liu, X.; Han, Y.-H.; Mei, H.; Cao, Y.; de Oliveira, L.M.; Liu, Y.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arsenic-hyperaccumulator Pteris vittata efficiently solubilized phosphate rock to sustain plant growth and As uptake. J. Hazard. Mater. 2017, 330, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gong, Y.; Fu, K.K.; He, X.; Hitz, G.T.; Dai, J.; Pearse, A.; Liu, B.; Wang, H.; Rubloff, G. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 2017, 16, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, J.W.; Da Silva, E.; Shi, X.X.; Cao, Y.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Microbial siderophores and root exudates enhanced goethite dissolution and Fe/As uptake by As-hyperaccumulator Pteris vittata. Environ. Pollut. 2017, 223, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, J.W.; Tang, N.; da Silva, E.; Cao, Y.; Turner, B.L.; Chen, Y.; Ma, L.Q. Phytate induced arsenic uptake and plant growth in arsenic-hyperaccumulator Pteris vittata. Environ. Pollut. 2017, 226, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Niazi, N.K.; Singh, B.; Van Zwieten, L.; Kachenko, A.G. Phytoremediation of an arsenic-contaminated site using Pteris vittata L. and Pityrogramma calomelanos var. austroamericana: A long-term study. Environ. Sci. Pollut. Res. 2012, 19, 3506–3515. [Google Scholar] [CrossRef] [PubMed]
- Mead, M.N. Arsenic: In search of an antidote to a global poison. Environ. Health Perspect. 2005, 113, A378. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Ye, Z.; Lin, A.; Wong, M. Interaction of arsenic and phosphate on their uptake and accumulation in Chinese brake fern. Int. J. Phytoremed. 2010, 12, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Souri, Z. Effect of phosphorus on arsenic accumulation and detoxification in Arsenic Hyperaccumulator, Isatis cappadocica. J. Plant Growth Regul. 2015, 34, 88–95. [Google Scholar] [CrossRef]
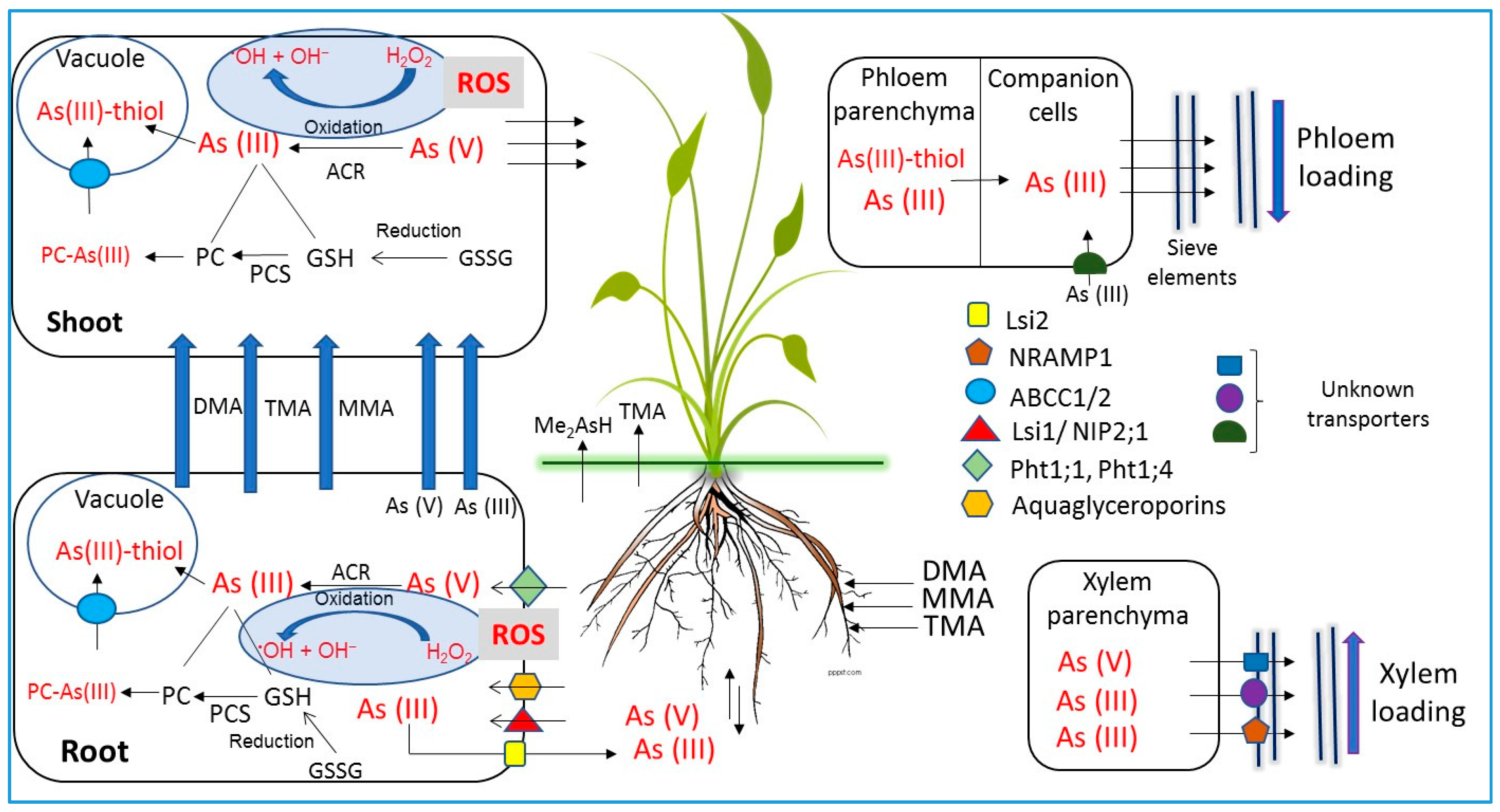
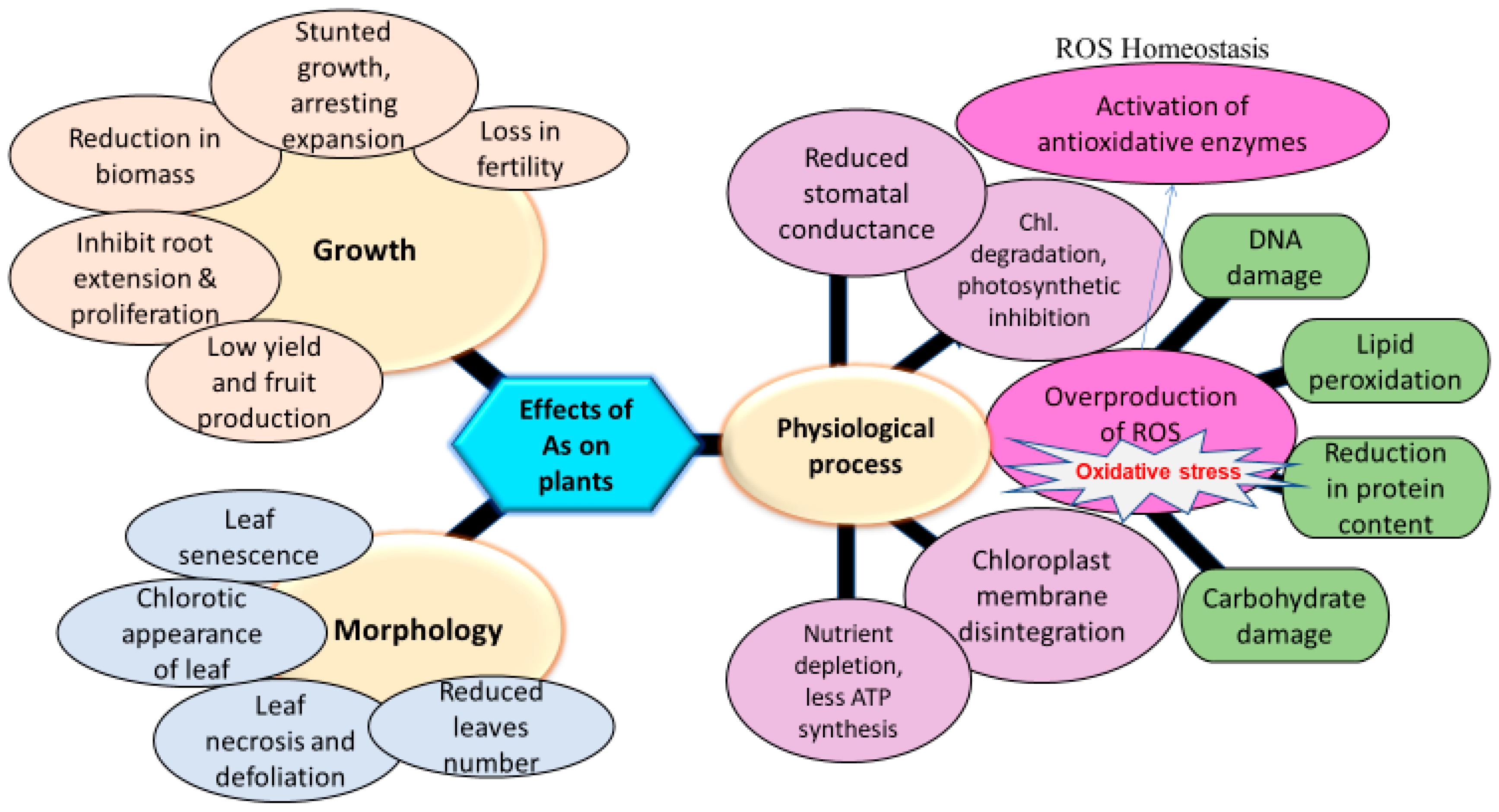
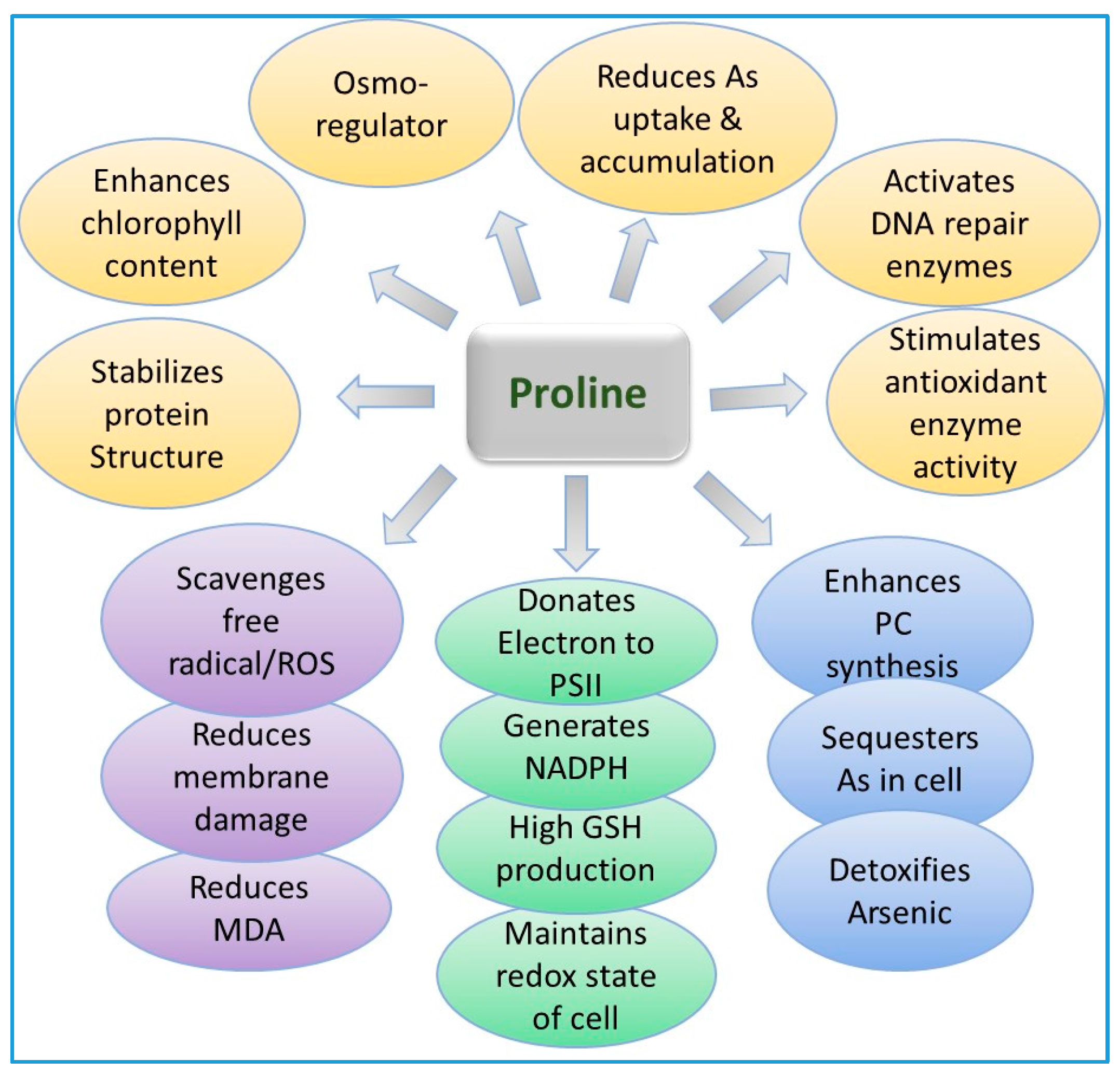
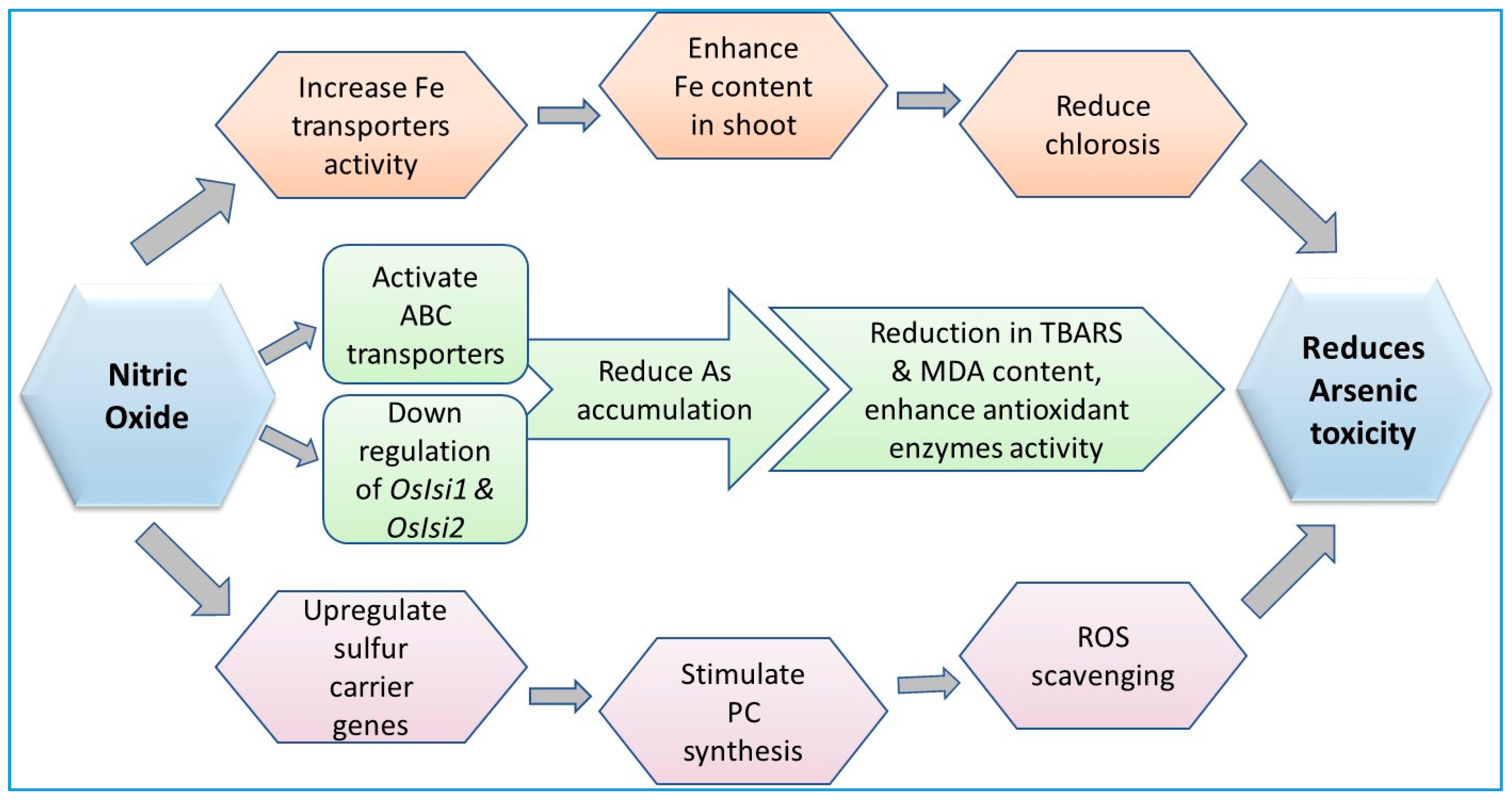
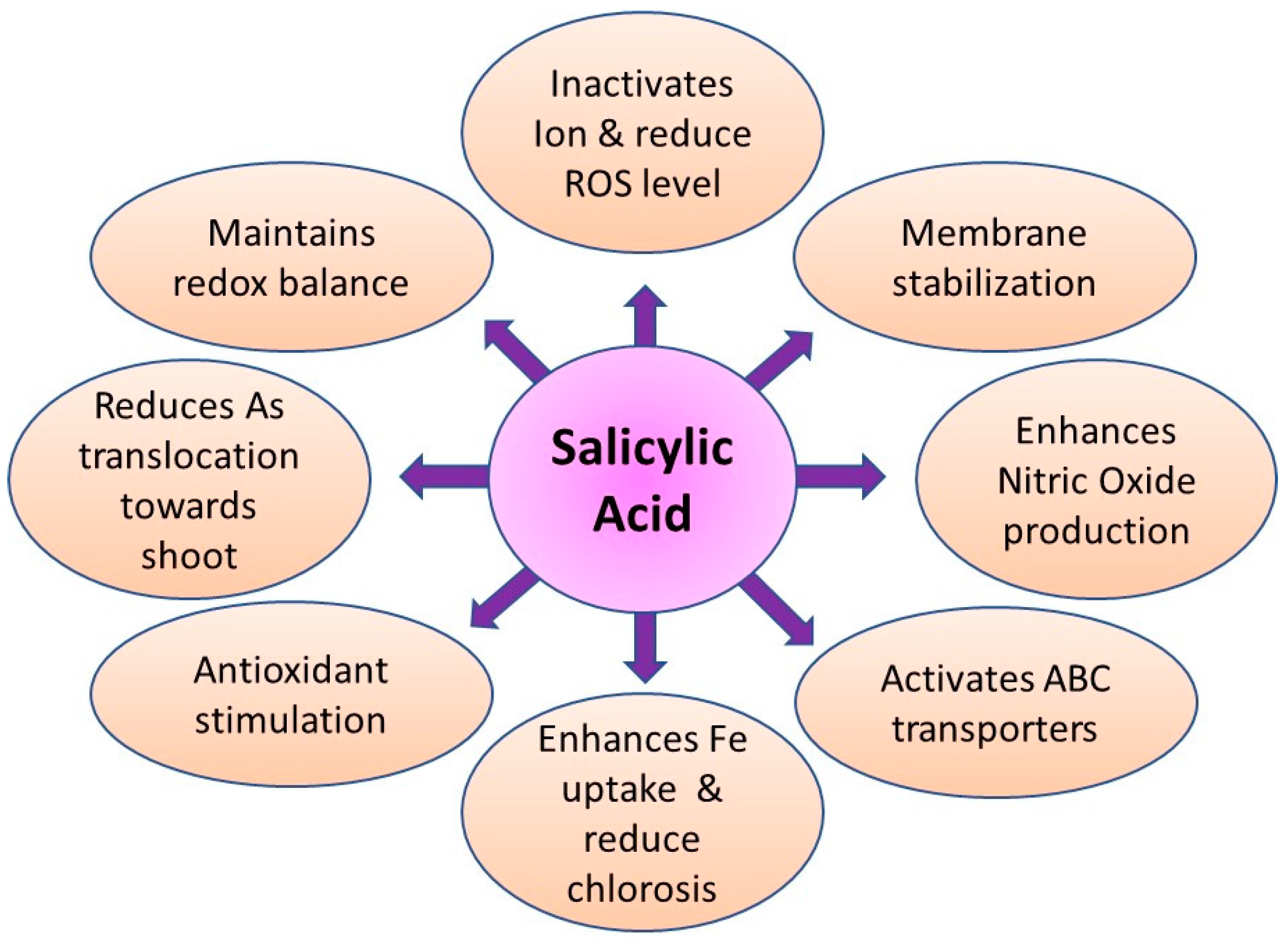
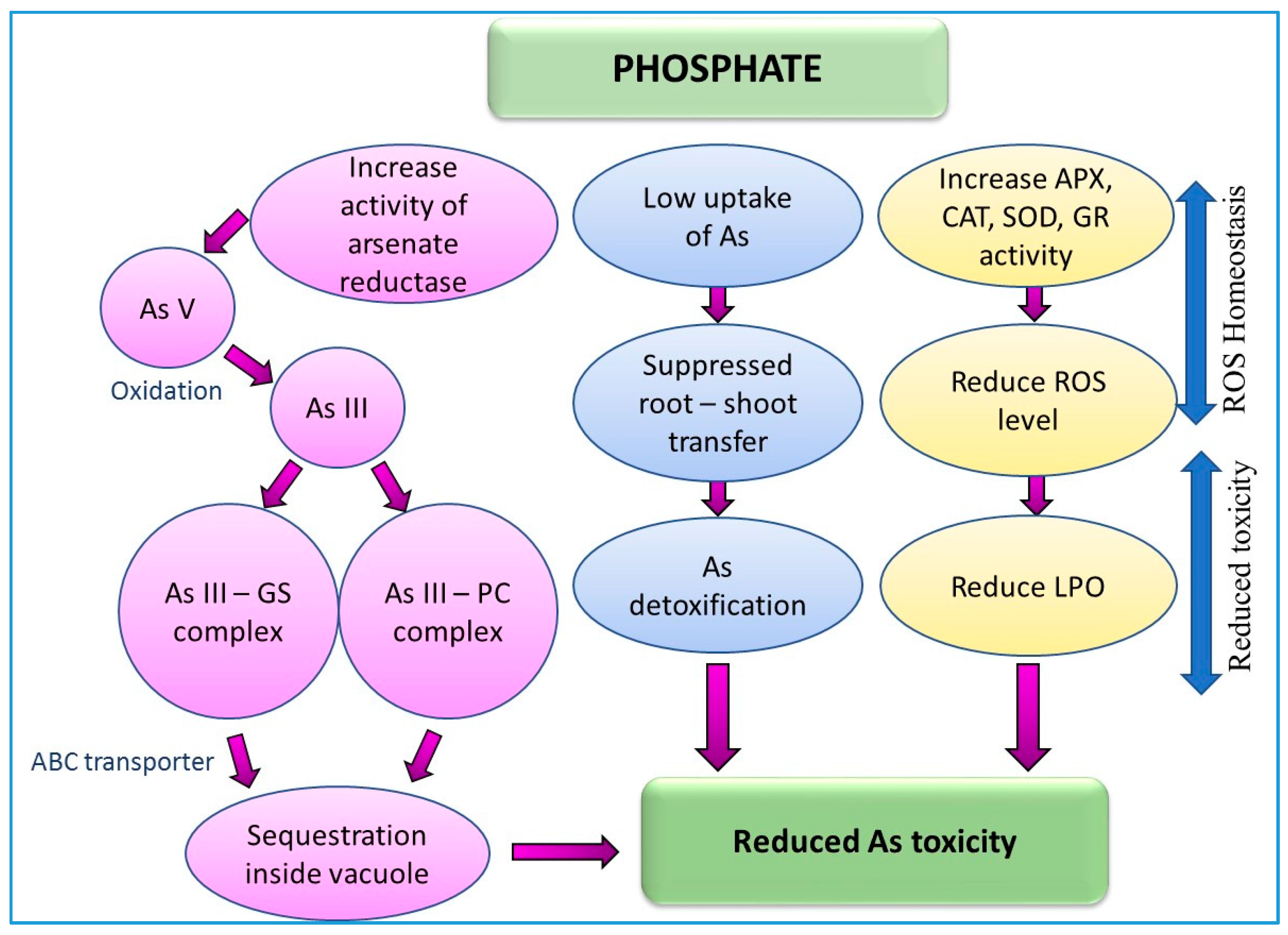
| Plant Species | Growth Medium | As(V/III) Concentration | Effects | References |
|---|---|---|---|---|
| Cicer arietinum L. | Soil | As(V) (0, 20 mg kg−1) | Reduction in essential and non-essential amino acids and Fe concentrations. Over expression of dehydration responsive genes (MIPS, PGIP, and DRE). Reduction in antioxidant enzyme activities (GR, CAT, SOD, APX, and GPX). | [45] |
| Zea mays L. | Soil | As(III) (0, 150 μM) | Reduction in gas exchange attributes (photosynthetic rate, transpiration rate, stomatal conductance) and chlorophyll concentrations. | [33] |
| Zea mays L. | Soil | As(V) (0, 40, 80, 120 mg kg−1), | Increase in shoot As and P concentrations, reduction in pigment concentrations (chlorophyll A, chlorophyll B, and total chlorophyll) and gas exchange attributes. | [8] |
| Brassica napus and Brassica juncea | Soil | As(V) (0, 25, 50 and 75 mg As kg−1) | Reduction in growth attributes (leaf area, plant height, number of leaves, shoot and root dry biomass), gas exchange attributes (photosynthetic rate, transpiration rate, stomatal conductance), photosynthetic pigments and water use efficiency. | [7] |
| Vigna mungo L. | Soil | As(V) (0, 100 and 200 µM) | Chlorophyll a, Chlorophyll b, total chlorophyll, and carotenoids decreased with increasing As concentration. Lipid peroxidation was increased. The activities of antioxidative enzymes (SOD, POD, and APX) except CAT were increased. | [34] |
| Glycine max | Soil | As(V) and As(III) (0, 25 µM) | Changes in the expression of a key messenger (Phosphatidic acid) via phospholipase D and phospholipase C. Moreover, a rapid and significant stomatal closure. | [43] |
| Glycine max | Soil | As(V) and As(III) (0, 25, 50, 100 and 200 µM) | Reduction in chlorophyll content and increase in lipid peroxidation. Reduction in root cortex area, broken cells in the outer cortical layer and cell death of root tips. Dark deposits in cortex cells and within phloem cell walls and xylem vessel elements. | [21] |
| Oryza sativa L. | Hydroponic | As(V) (0, 50 µM) | Increased leakage of electrolytes and increased root arsenate reductase activity along with relatively lower root to shoot As translocation in As tolerant rice genotype BRRI 33 than in sensitive genotype BRRI 51. Decrease in Pi content and increase in PCs content in roots. | [32] |
| Boehmeria nivea L. | Hydroponic | As(III) (0, 5, 10, 15, and 20 mg L−1) | Reduction in chlorophyll concentrations, relative water concentrations, SOD and CAT activities. Increase in H2O2, malondialdehyde (MDA) content, and electrolyte leakage. | [163] |
| Oryza sativa L. | Hydroponic | As(III) (0, 25 mM) | Carbohydrate metabolism and photosynthesis were greatly affected, however, As did not caused any significant oxidative damage to plants. | [164] |
| Oryza sativa L. var. Triguna | Hydroponic | As(III) (0, 50 µM) | Reduction in shoot and root growth, biomass production, and protein concentrations. | [165] |
| Aquatic plants species (Vallisneria gigantea, Azolla filiculoides and Lemna minor | Hydroponic | As(V) 2 ppm | Changes in fluorescence spectra and damage to photosystem II. | [166] |
| Brassica juncea L. | Soil | As(V) (0.0, 0.1, 0.2, and 0.3 mM) | Affected plant growth and biochemical stress indicators such as protein content, lipid peroxidation, and antioxidative enzymes (SOD, CAT, POD, APX, GR). | [167] |
| Pisum sativum L. | Hydroponic containing NaHS (0, 100 µM) | As(V) (0, 50 µM) | As uptake caused reduction in chlorophyll fluorescence, nitrogen content concentrations of H2S and nitric oxide (NO). The activities of cysteine desulfhydrase and nitrate reductase were also decreased. Increasing levels of ROS caused damage to lipids, proteins, and membranes. | [168] |
| Oryza sativa L. | Hydroponic | As(V) (0, 100 µM) | Increases in hydrogen peroxide and lipid peroxidation. | [24] |
| Anadenanthera peregrine, Myracrodruon urundeuva | Soil | As(V) (0, 10, 50, and 100 mg L−1) | Increase in hydrogen peroxide and lipid peroxidation. | [128] |
| Trigonella foe num_graecum L. | Soil | As(V) (0, 10, 20, and 30 mg As kg−1) | Reductions in radicle length, dry weight, and chlorophyll content. | [37] |
| Hydrilla verticillata | Hydroponic | As(V) (0, 100, and 500 µΜ) | Decline in chlorophyll content and rate of photosynthesis. | [169] |
| Oryza sativa L. | Hydroponic | As(III) (0, 50, 150, and 300 μM) | Reductions in seed germination; root and shoot length; chlorophyll and protein content, and genomic stability. | [170] |
| Oryza sativa L. | Soil culture (field study) | Groundwater As concentrations (17, 27, and 53 μg L−1) and soil As concentrations (10.4, 12.6, and 15.5 μg g−1) | Both essential and non-essential amino acids were decreased as the grain As concentration was increased in high As accumulating rice genotypes. Non-essential amino acids were increased in low As accumulating rice genotypes. | [171] |
| Vigna mungo | Soil | As(V) (0, 2.8 mM) | Delayed nodule formation and reduction in nitrogenase activity. | [172] |
| Cicer arietinum L. | Soil | As(V) (0, 5 mg kg−1) | Reduction in chlorophyll, relative leaf water, sucrose, proteins, starch, and sugars concentrations. Reduction in Ca, P, Fe, and amino acids like; Lys, Met, Pro, Thr, Trp, and Val. | [159] |
| Lemna minor L. | Hydroponic | As(V) and As(III) (0, 1, 4, 16, and 64 µM) | Reduction in chlorophyll, and increase in electrolyte leakage and lipid peroxidation. | [162] |
| Helianthus annuus | Soil | As(V) (0, 30, and 60 mg kg−1) | Reductions in plant growth and ionic concentrations (K, Ca, Mg, Si, Fe, Zn, Cu, Rb, and Sr). | [161] |
| Festuca arundinacea | Hydroponic | As(V) (0, 25 mΜ) | Excessive ROS accumulation, membrane perturbation and lipid peroxidation. | [173] |
| Cicer arietinum L. | Soil | As(V) (0, 30, and 60 mg kg−1) | Increase in H2O2 content and lipid peroxidation. Reduction in SOD and non-enzymatic antioxidants activities. Increase in CAT and APX activities. | [174] |
| Trifolium pretense | Soil | As(V) (0, 5, 10, and 50 mg kg−1) | Increase in SOD, POD, and glutathione activities. Reduction in chlorophyll and carotenoid concentrations. | [175] |
| Plant Species | Growth Medium | As(V/III) Concentration | Mechanisms/Effects | References |
|---|---|---|---|---|
| Glycine max L. | Soil with two P levels (21 and 8 mg P kg−1) | As(V) (0, 10, 50 and 100 mg As kg−1) with different levels of fluoride (F) | As caused more oxidative damage in low P soil than in high P soil. High soil P mitigated oxidative stress by higher increase in antioxidant activities (SOD, CAT, POX, and glutathione) and an increase in chlorophyll concentrations. | [54] |
| Brassica juncea cultivars; Varuna and Pusa Jagannath (PJn) | Hydroponic | As(III) (0, 50, 150, 300 µM) | Sulfur concentrations, thiol-related proteins, and phytochemicals played their protective role against oxidative stress. The higher levels of total and aliphatic glucosinolate (GSL) were responsible for higher As tolerance in in Varuna than PJn. | [25] |
| Brassica species | Soil with P levels (0, 50 and 100 (mg kg−1) | As(V) (0, 25, 50, 75 mg kg−1) | Pi application under As stress improved plant growth, photosynthetic pigments, gas exchange attributes (photosynthetic rate, transpiration rate, stomatal conductance), and water use efficiency. | [7] |
| Helianthus annuus L. | Hydroponic with SA concentrations (0, 10, 50, and 100 μM) | As(V) (0, 10 µΜ) | SA application mitigated the adverse effects of As on plant growth by reducing the oxidative stress and increasing the activities of (CAT), (APX), and (GPX), whereas the activities of (SOD) and (POD) were decreased. | [52] |
| Oryza sativa L. | Hydroponic with SNP (0.0 and 30 μM) as NO donor | As(III) (0.0, 25 µΜ) | SNP supply caused a reduction in As accumulation, ROS production and cell death. NO reduced As toxicity by modulating metal transporters (NIP, NRAMP, ABC, and iron transporters), stress-related genes, and secondary metabolism genes, signaling, amino acid and hormones such as jasmonic acid concentrations. | [39] |
| Oryza sativa L. | Hydroponic with salicylic acid (SA; 40 µM) and nitric oxide (NO as SNP; 30 µM) | As(III) (0, 25 µM) | Exogenous supply of SA lessened As(III)induced oxidative stress by increasing the activities of antioxidant enzymes, particularly SOD, CAT, and APX. SA and NO both restricted the accumulation of As in shoots possibly by downregulating OsLsi2 gene. Nitric oxide mitigated As(III)induced chlorosis by increasing shoot Fe uptake. | [26] |
| Pteris vittata and Vetiveria zizanioides | Hydroponic | As(V) (0, 10, 20, 30, and 50 mg L−1) | Pteris vittata accumulated more As in fronds and showed higher activities of antioxidant enzymes (SOD, APX, CAT, and GPX) in fronds. Vetiveria zizanioides accumulated more As in roots and showed higher activities of antioxidant enzymes in roots. | [273] |
| Glycine max | Soil | As(V) and As(III) (0, 25, 50, 100, and 200 µM) | Increased activities of antioxidant enzymes such as total peroxidases (Px) and superoxide dismutase (SOD) both in shoot and root. | [43] |
| Oryza sativa L. | Hydroponic | As(V) (0, 50 µM) | Increased root arsenate reductase activity and PCs content in roots resulting in relatively lower root to shoot As translocation. Increased activities of antioxidants (CAT, POD, SOD, GR) and an increase in concentrations of amino acids (glutathione, cysteine methionine, and proline) in As tolerant rice genotype. | [32] |
| Oryza sativa L. | Hydroponic with three S levels (0.5, 3.5 and 5.0 mM) | As(V) (50 µM) | Exogenous supply of sulfur (S) increased As accumulation in roots and decreased its transport to shoot by reducing the expression of potent transporters (OsLsi1 and OsLsi2). The activities of antioxidant enzymes were increased and the synthesis of PCs was increased which caused As complexation in the roots. | [51] |
| As-hyperaccumulator Pteris vittata | MS agar medium containing arsenic resistant bacteria | As(V) (37.5 mg kg−1) | Reduced As induced toxicity by efficient As III efflux into external environment and As III translocation to the fronds. | [298] |
| Arabidopsis thaliana | Hydroponic | As(III) (5 mg/L) and As(V) (10 mg/L) | Reduction of As(V) to As(III)by the effect of root excreted organic acids and efflux of As(III)from plant roots after in vivo reduction of As(V) to As(III). | [299] |
| Oryza sativa L. | Hydroponic with 0 and 2 mM Si | As(III) (0 and 25 mM) | Application of Silicon (Si) reduced As uptake by plants and improved photosynthetic attributes by changing the expression of genes involved in As uptake and translocation. | [164] |
| Oryza sativa L. var. Triguna | Hydroponic inoculated with alga; Chlorella vulgaris and Nannochlropsis sp. | As(III) (0, 50 µM) | Algal inoculum reduced As toxicity and improved plant As tolerance by reducing As uptake and modulating the activities of antioxidant enzymes. | [165] |
| Nicotiana tabacum L. | Hydroponic inoculated with Endomycorrhizal fungus Funneliformis mosseae | As(V) (0, 1 and 30 µM) | Endomycorrhizal fungus Funneliformis mosseae increased the concentrations of PCs and antioxidant glutathione (GSH) and reduced the uptake of As and Cd in roots and leaves. | [50] |
| Oryza sativa L. | Hydroponic with sulfur (0, 0.5, 3.5, 5.0 mM) | As(III) 0, 25 µM), and As(V) (0, 50µM) | Exogenous application of S particularly the highest level, restricted As in roots due to its complexation with non-protein thiols and PCs. Oxidative stress was mitigated by limited generation of hydrogen peroxide and higher activities of antioxidant enzyme. | [300] |
| Brassica juncea L. | Hydroponic | As(V) (0.0, 0.1, 0.2, and 0.3 mM) | Synthesis of brassinosteroids (castasterone, teasterone, 24-epibrassinolide, and ty-phasterol) and overexpression of antioxidant enzymes. | [167] |
| Oryza sativa L. | Hydroponic with Se (0, 20 µM) and auxin (0, 3 µM) | As(III) (0, 150 µM) | Co-application of selenium (Se) and auxin to rice seedlings reduced As toxicity by increasing growth, chlorophyll, protein cysteine and proline concentrations and decreasing MDA level in the cell. | [47] |
| Lettuce sativa L. | Hydroponic with 100 μM sodium nitroprusside (SNP) | As(V) (0, (50 µM) | Exogenous supply of NO in the form of SPN reduced root to shoot translocation of As and decreased the oxidative damage by decreasing the concentrations of H2O2 and MDA. | [272] |
| Pisum sativum L. | Hydroponic containing NaHS (0, 100 µM) | As(V) 0, (50 µM) | Addition of hydrogen sulfide to growth medium improved plant As tolerance by increasing the concentrations of H2S and NO and reducing the oxidative damage caused by ROS. Arsenic accumulation was decreased and AsA–GSH cycle was upregulated to offset ROS-mediated damage to cell. | [168] |
| Solanum melongena L. | Hydroponic with 25 µM proline | As(V) (0, 5, and 25 µM) | Exogenous application of proline reduced As accumulation. Deleterious effects of As on photosystem-II (PSII) were ameliorated, and chlorophyll concentrations were improved. Oxidative stress was mitigated by the higher activities of antioxidant enzymes (SOD, POD, CAT, and glutathione-S-transferase; GST). The activity of proline biosynthesis enzyme; Δ1-pyrroline-5-carboxylatesynthetase was upregulated, and proline dehydrogenase was downregulated. | [277] |
| Oryza sativa L. | Hydroponic culture with Si (0, 2 mM) | As(III) (0, 25 μM) | Silicon (Si) application decreased As accumulation in leaves and improved photosynthetic performance (net CO2 assimilation rate, stomatal conductance, and mesophyll conductance) of rice plants in a genotype and time-dependent manner. | [164] |
| Cicer aritenum L. | Soil with bacterial inoculation (Acinetobacter sp) | As(V) (0, 10 mg kg−1) | Bacterial inoculation significantly increased root and shoot biomass, total chlorophyll protein and carotenoid concentrations and decreased As uptake and electrolyte leakage by reducing MDA concentrations. | [271] |
| Oryza sativa L. | Hydroponic | As(III) (0, 10, and 25 µM) and As(V) (0, 10, and 50 µM) | Higher activities of antioxidant enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and guiacol peroxidase (GPX), and higher concentrations of stress responsive amino acids (glycine, cysteine, proline, glutamic acid) in high As accumulating genotype than low As accumulating genotypes. | [289] |
| Triticum aestivum L. | Hydroponic with 0 and 0.25 mM SNP | As(V) (0, 0.25, and 0.5 mM) | Exogenous application of nitric oxide (NO) in the form of sodium nitroprusside, (SNP) increased the RWC, chlorophyll and proline concentrations, AsA and GSH, glyoxalase I and glyoxalase II concentrations, and the activities of antioxidants (CAT, GPX, GR, dehydroascorbate reductase (DHAR). | [301] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. https://doi.org/10.3390/ijerph15010059
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M, Natasha. Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. International Journal of Environmental Research and Public Health. 2018; 15(1):59. https://doi.org/10.3390/ijerph15010059
Chicago/Turabian StyleAbbas, Ghulam, Behzad Murtaza, Irshad Bibi, Muhammad Shahid, Nabeel Khan Niazi, Muhammad Imran Khan, Muhammad Amjad, Munawar Hussain, and Natasha. 2018. "Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects" International Journal of Environmental Research and Public Health 15, no. 1: 59. https://doi.org/10.3390/ijerph15010059
APA StyleAbbas, G., Murtaza, B., Bibi, I., Shahid, M., Niazi, N. K., Khan, M. I., Amjad, M., Hussain, M., & Natasha. (2018). Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. International Journal of Environmental Research and Public Health, 15(1), 59. https://doi.org/10.3390/ijerph15010059








