Bisphenol A and Metabolic Diseases: Challenges for Occupational Medicine
Abstract
1. Introduction
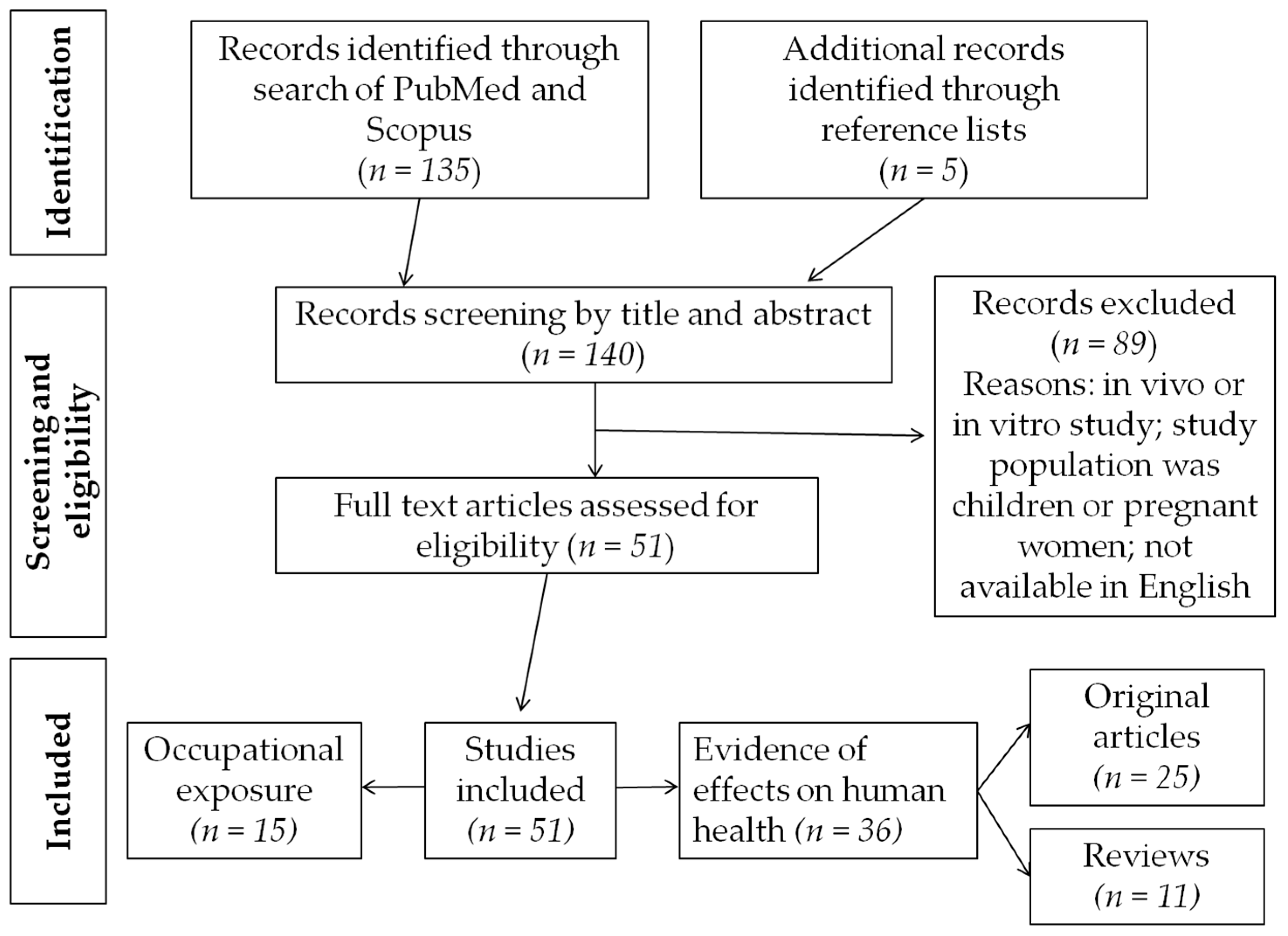
2. Materials and Methods
3. Evidence of the Effects of Metabolic Type of Exposure to Bisphenol A on Health
3.1. Population Studies
3.2. The Workplace: Epidemiological and Exposure Data
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Chevalier, N.; Fenichel, P. Endocrine disruptors: A missing link in the pandemy of type 2 diabetes and obesity? Presse Med. 2016, 45, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Fenichel, P. Endocrine disruptors: New players in the pathophysiology of type 2 diabetes? Diabetes Metab. 2015, 41, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Environmental estrogens and obesity. Mol. Cell. Endocrinol. 2009, 304, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Valentino, R.; D’Esposito, V.; Ariemma, F.; Cimmino, I.; Beguinot, F.; Formisano, P.; Bisphenol, A. Environmental exposure and the detrimental effects on human metabolic health: Is it necessary to revise the risk assessment in vulnerable population? J. Endocrinol. Invest. 2016, 39, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to Bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ariemma, F.; D’Esposito, V.; Liguoro, D.; Oriente, F.; Cabaro, S.; Liotti, A.; Ciammino, I.; Longo, M.; Beguinot, F.; Formisano, P.; et al. Low dose bisphenol A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PLoS ONE 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Biemann, R.; Fischer, B.; Navarrete Santos, A. Adipogenic effects of a combination of the endocrine disrupting compounds bisphenol A, diethylhexylphthalate, and tributyltin. Obes. Facts 2014, 7, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular mechanisms of action of BPA. Dose Response 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Somm, E.; Schwitzgebel, V.M.; Toulotte, A.; Cederroth, C.R.; Combescure, C.; Nef, S.; Aubert, M.L.; Hüppi, P.S. Perinatal Exposure to bisphenol A alters early adipogenesis in the rat. Environ. Health Perspect. 2009, 117, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Tremblay-Franco, M.; Cabaton, N.J.; Canlet, C.; Gautier, R.; Schaeberle, C.M.; Jourdan, F.; Sonnenschein, C.; Vinson, F.; Soto, A.M.; Zalko, D. Dynamic metabolic disruption in rats perinatally exposed to low doses of bisphenol A. PLoS ONE 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Di Iorio, M.; Chalifour, L.E. Metabolic response to chronic bisphenol A exposure in C57bl/6n mice. Toxicol. Rep. 2014, 1, 522–532. [Google Scholar] [CrossRef]
- Nadal, A.; Alonso Magdalena, P.; Soriano, S.; Queseda, I.; Ropero, A.B. The pancreatic β-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol. Cell. Endocrinol. 2009, 304, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Alonsp-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006, 114, 106–112. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Ropero, A.B.; Carrera, M.P.; Cederroth, C.R.; Baquié, M.; Gauthier, B.R.; Nef, S.; Stefani, E.; Nadal, A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE 2008, 3, e2069. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, L.; Zhang, H.; Wei, W.; Jia, L. Perinatal BPA exposure induces hyperglycemia oxidative stress and decreased adiponectin production in later life of male rat offspring. Int. J. Environ. Res. Public Health 2014, 11, 3728–3742. [Google Scholar] [CrossRef] [PubMed]
- Menale, C.; Mita, D.G.; Diano, N.; Diano, S. Adverse effects of bisphenol A exposure on glucose metabolism regulation. Open Biotechol. J. 2016, 10, 122–130. [Google Scholar] [CrossRef]
- Trasande, L.; Cronk, C.; Durkin, M.; Weiss, M.; Schoeller, D.A.; Gall, E.A.; Hewitt, J.B.; Carrel, A.L.; Landrigan, P.J.; Gillman, M.W. Environment and obesity in the National Children’s study. Environ. Health Perspect. 2009, 117, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Zhou, Y.; Tang, C.X.; Wu, J.G.; Chen, Y.; Jiang, Q.W. Association between bisphenol A exposure and body mass index in Chinese school children: A cross-sectional study. Environ. Health 2012, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Miao, M.; Zhou, Z.; Wu, C.; Shi, H.; Liu, X.; Wang, S.; Yuan, W. Urine bisphenol A level in relation to obesity and overweight in school-age children. PLoS ONE 2013, 8, e65399. [Google Scholar] [CrossRef] [PubMed]
- Pornkunwilai, S.; Nosoongnoen, W.; Jantarat, C.; Wachrasindhu, S.; Supornsilchai, V. Urinary bisphenol A detection is significantly associated with young and obese Thai children. Asian Biomed. 2015, 9, 363–372. [Google Scholar] [CrossRef]
- Menale, C.; Grandone, A.; Nicolucci, C.; Cirillo, G.; Crispi, S.; Si Sessa, A.; Marzuillo, P.; Rossi, S.; Mita, D.G.; Perrone, L.; et al. Bisphenol A is associated with insulin resistance and modulates adiponectin and resistin gene expression in obese children. Pediatr. Obes. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hoepner, L.A.; Whyatt, R.M.; Widen, E.M.; Hassoun, A.; Oberfield, S.E.; Mueller, N.T.; Diaz, D.; Calafat, A.M.; Perera, F.P.; Rundlr, A.G. Bisphenol A and adiposity in an inner-city birth cohort. Environ. Health Perspect. 2016, 124, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Magdalen, P.A.; Quesada, I.; Nadal, A. Prenatal exposure to BPA and Offspring outcomes: The diabesogenic behavior of BPA. Dose Response 2015, 1–8. [Google Scholar] [CrossRef]
- Braun, J.M.; Lanphear, B.P.; Calafat, A.M.; Deria, S.; Khoury, J.; Howe, C.J.; Venners, S.A. Early-life bisphenol A exposure and child body mass index: A prospective cohort study. Environ. Health Perspect. 2014, 122, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadi, M.; Rouneliotaki, T.; Myridakis, A.; Chalkiadaki, G.; Fthenou, E.; Dermitzaki, E.; Karachaliou, M.; Sarri, K.; Vassilaki, M.; Stephanou, E.G.; et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ. Res. 2016, 146, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Candura, F.; Candura, S.M. Elementi di Tecnologia Industriale a uso dei Cultori di Medicina del Lavoro, 1st ed.; Casa Editrice La Tribuna: Piacenza, Italy, 2002. [Google Scholar]
- Sabanayagam, C.; Teppala, S.; Shankar, A. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol. 2013, 50, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S. Relationship between urinary bisphenol A levels and diabetes mellitus. J. Clin. Endocrinol. Metab. 2011, 96, 3822–3826. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Sung, Y.A.; Hong, Y.S.; Ha, E.; Jeong, K.; Chung, H.; Lee, H. Urinary bisphenol A is associated with insulin resistance and obesity in reproductive-aged women. Clin. Epidemiol. 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ning, G.; Bi, Y.; Wang, T.; Xu, M.; Xu, Y.; Huang, Y.; Li, M.; Li, X.; Wang, W.; Chen, Y. Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: A cross-sectional analysis. Ann. Intern. Med. 2011, 155, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, H. Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: A population based cross sectional study. Int. J. Hyg. Environ. Health 2013, 216, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Rice, N.E.; Lewis, C.; Henley, W.E.; Galloway, T.S. Association of urinary bisphenol A concentration with heart disease: Evidence from NHANES 2003/6. PLoS ONE 2010, 5, e8673. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; Tarantino, G.; D’Esposito, V.; Passaretti, F.; Cabaro, S.; Liotti, A.; Liguoro, D.; Perruolo, G.; Ariemma, F.; Finelli, C.; et al. Bisphenol A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: A cross sectional study on adult male population. J. Transl. Med. 2015, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.K.; O’Neil, M.S.; Sowers, M.R.; Park, S.K. Urinary bisphenol A and type-2 diabetes in U.S. adults: Data from NHANES 2003–2008. PLoS ONE 2011, 6, e26868. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Gates, P.; Osborn, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Schofield, P.; Mosedale, D.; et al. Urinary bisphenol A concentration and angiography-defined coronary artery stenosis. PLoS ONE 2012, 7, e43378. [Google Scholar] [CrossRef]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.T.; Wareham, N.J.; et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hauser, R.; Hu, F.B.; Franke, A.A.; Liu, S.; Sun, Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: A prospective investigation in US women. Int. J. Obes. 2014, 38, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol A and peripheral arterial disease: Results from the NHANES. Environ. Health Perspect. 2012, 120, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, J.H.; Lim, Y.H.; Park, H.Y.; Hong, Y.C. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension 2012, 60, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Carwile, J.L.; Michels, K.B. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. 2011, 111, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Rönn, M.; Lind, L.; Örberg, J.; Kullberg, J.; Söderberg, S.; Larsson, A.; Johansson, L.; Ahlström, H.; Lind, P.M. Bisphenol A is related to circulating levels of adiponectin, leptin and ghrelin, but not to fat mass or fat distribution in humans. Chemosphere 2014, 112, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, M.; Chen, B.; Xu, M.; Xu, Y.; Huang, Y.; Lu, J.; Chen, Y.; Wang, W.; Li, X.; et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2012, 97, E223–E227. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.S.; Makris, K.C. Association between urinary levels of bisphenol A and its monochlorinated derivative and obesity. J. Environ. Sci. Health Part A 2015, 50, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Bi, Y.F.; Ma, L.Y.; Zhao, I.; Wang, T.G.; Zhang, I.Z.; Tao, B.; Sun, L.H.; Zhao, Y.J.; Wang, W.Q.; et al. The effects of bisphenol A (BPA) exposure on fat mass and serum leptin concentrations have no impact on bone mineral densities in non-obese premenopausal women. Clin. Biochem. 2012, 45, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Urinary bisphenol A levels and measures of obesity: Results from the national health and nutrition examination survey 2003–2008. ISRN Endocrinol. 2012, 2012, 965243. [Google Scholar] [CrossRef] [PubMed]
- Metwally, F.M.; Mohamed, M.M.; Sharaf, N.E.; Ghazy, M.A.; El Mishad, A.M.; Elfiky, A. The Impact of bisphenol A (BPA) as environmental obesogen on lipids and lipids metabolism. Int. J. Pharm. Clin. Res. 2016, 8, 1323–1330. [Google Scholar]
- Mahalingaiah, S.; Meeker, J.D.; Pearson, K.R.; Calafat, A.M.; Ye, X.; Petrozza, J.; Hauser, R. Temporal Variability and Predictors of Urinary Bisphenol A Concentrations in Men and Women. Environ. Health Perspect. 2008, 116, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tsutsumi, O.; Ikezuki, Y.; Taketani, Y. Positive relationship between androgen and the endocrine disruptor bisphenol A in normal women and women with ovarian dysfunction. Endocr. J. 2004, 51, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Valentino, R.; Di Somma, C.; D’Esposito, V.; Passaretti, F.; Pizza, G.; Brancato, V.; Orio, F.; Formisano, P.; Colao, A.; et al. Bisphenol A in polycystic ovary syndrome and its association with liver-spleen axis. Clin. Endocrinol. 2013, 78, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Thayer, K.A.; Heindel, J.J.; Bucher, J.R.; Gallo, M.A. Role of environmental chemicals in diabetes and obesity: A national toxicology program workshop review. Environ. Health Perspect. 2012, 120, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Stojanoska, M.M.; Milosevic, N.; Milic, N.; Abenavoli, L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine 2017, 55, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Ranciere, F.; Lyons, J.G.; Loh, V.H.Y.; Botton, J.; Galloway, T.; Wang, T.; Shaw, J.E.; Magliano, D.J. Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health 2015, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Moon, K.; Thayer, K.A.; Navas-Acien, A. Environmental chemicals and type 2 diabetes: An updated systematic review of the epidemiologic evidence. Curr. Diabetes Rep. 2013, 13, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Oppeneer, S.; Robien, K. Bisphenol A exposure and associations with obesity among adults: A critical review. Public Health Nutr. 2014, 18, 1847–1863. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.; Leone, A.; Battezzati, A. Human Bisphenol A exposure and the “Diabesity Phenotype”. Dose Response 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ndaw, S.; Remy, A.; Jargot, D.; Robert, A. Occupational exposure of cashiers to Bisphenol A via thermal paper: Urinary biomonitoring study. Int. Arch. Occup. Environ. Health 2016, 89, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Maduka, I.C.; Ezeonu, F.C.; Neboh, E.E.; Shu, E.N.; Ikekpeazu, E.J. Urinary bisphenol A output in plastic industry workers: A possible indicator of occupational exposure. Trop. J. Med. Res. 2014, 17, 117–120. [Google Scholar] [CrossRef]
- Wang, F.; Hua, J.; Chen, M.; Xia, Y.; Zhang, Q.I.; Zhao, R.; Zhou, W.; Zhang, Z.; Wang, B. High urinary bisphenol A concentrations in workers and possible laboratory abnormalities. Occup. Environ. Med. 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, Z.; Qing, D.; He, Y.; Wu, T.; Miao, M.; Wang, J.; Weng, X.; Ferber, J.R.; Herrinton, L.J.; et al. Occupational exposure to bisphenol A (BPA) and the risk of self-reported male sexual dysfunction. Hum. Reprod. 2010, 25, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Yuan, W.; Yang, F.; Liang, H.; Zhou, Z.; Li, R.; Gao, E.; Li, D.K. Associations between bisphenol A exposure and reproductive hormones among female workers. Int. J. Environ. Res. Public Health 2015, 12, 13240–13250. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miao, M.; Zhou, Z.; Gao, E.; Chen, J.; Wang, J.; Sun, F.; Yuan, W.; Li, D.K. Exposure to bisphenol A and reproductive hormones among male adults. Environ. Toxicol. Pharmacol. 2015, 39, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Wu, K.; Wang, Y.; Zhu, H.; Deng, Z.; Peng, L.; Zhu, G. Association of serum bisphenol A concentration and male reproductive function among exposed workers. Arch. Environ. Contam. Toxicol. 2015, 68, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Miao, M.; Ran, M.; Ding, L.; Bai, L.; Wu, T.; Yuan, W.; Gao, E.; Wang, J.; Li, G.; et al. Serum bisphenol A concentration and sex hormone levels in men. Fertil. Steril. 2013, 100, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Zhou, Z.; Miao, M.; He, Y.; Qing, D.; Wu, T.; Wang, J.; Weng, X.; Ferber, J.; Herrinton, L.J.; et al. Relationship between urine bisphenol A level and declining male sexual function. J. Androl. 2010, 31, 500–506. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Miao, M.; Herrinton, L.J.; Wu, C.; Yuan, W.; Zhou, Z.; Li, D.K. Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ. Res. 2009, 109, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, T.; Kawamura, N.; Hara, K.; Tsugane, S. Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup. Environ. Med. 2002, 59, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, M.; Saeedi, A.; Poorbaghi, S.L.; Sepehrimanesh, M.; Fattahi, M. Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environ. Sci. Pollut. Res. 2016, 23, 23546–23550. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Yang, Y.J.; Hong, Y.P.; Myung, S.C.; Kim, S.C. Distribution of serum bisphenol A diglycidyl ether and its metabolite in Korean adult men and its association with reproductive hormone levels. Mol. Cell. Toxicol. 2015, 11, 71–78. [Google Scholar] [CrossRef]
- Li, D.K.; Zhou, Z.; Miao, M.; He, Y.; Wang, J.; Ferber, J.; Herrington, L.J.; Gao, E.; Yuan, W. Urine bisphenol A (BPA) level in relation to semen quality. Fertil. Steril. 2011, 95, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.B.; Shi, J.L.; He, G.H. Investigation into serum BPA and sex hormone level of workers in epoxy resin manufacture. J. Environ. Occup. Med. 2005, 22, 295–298. [Google Scholar]
- Newbold, R.R. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones 2010, 9, 206–2017. [Google Scholar] [CrossRef] [PubMed]
- Legler, J.; Fletcher, T.; Govarts, E.; Porta, M.; Blumberg, B.; Heindel, J.J.; Trasande, L. Obesity, diabetes and associated costs of exposure to endocrine disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Fenichel, P. Bisphenol A: Targeting metabolic tissues. Rev. Endocr. Metab. Disord. 2015, 16, 299–309. [Google Scholar] [CrossRef] [PubMed]
| Sources of Contamination from BPA | Concentration of BPA (Range) |
|---|---|
| Aquatic environment | 8.000–21.000 ng/L |
| Air | 0.002–0.208 ng/L |
| Dust | 800–10.000 ng/g |
| Thermal paper | 54.000–79.000 ng/cm2 |
| Meats | 17–602 ng/g |
| Fish | 5–109 ng/g |
| Vegetables and fruits | 9–76 ng/g |
| Beverages | 1–18 ng/g |
| Dairy products | 21–43 ng/g |
| Infant formula | 0.1–13 ng/g |
| Cans | 2–82 ng/g |
| Plastics | 0.2–26 ng/g |
| Dental materials | 13.000–30.000 ng |
| Focus | Study Type | Number of Subjects | Population Type | Average BPA Concentration | Results | Ref. |
|---|---|---|---|---|---|---|
| Diabetes | Cross-sectional | 3516 | Prediabetes subjects (glucose: 100–125 mg/dL) older than 20 | 1.93–2.22 a | Independent of traditional diabetes, risk factors higher, urinary BPA levels were found to be associated with prediabetes situation. | [28] |
| Diabetes/cardiovascular disease/obesity | Cross-sectional | 1455 | adults aged 18–74 | 4.5–4.7 a | Positive correlation between urinary BPA levels and increased diagnosis of cardiovascular dosease, type 2 diabetes, but not BMI. | [29] |
| Type 2 diabetes | Cross-sectional | 3967 | Adults older than 20 | 3.9–4.0 a | Increased type 2 diabetes was significantly associated with higer urinary levels of BPA. | [30] |
| Diabetes/obesity | Crosssectional | 296 | Reproductive aged women between 30–49 | 1.38 c | Urinary BPA levels were positively correlated with BMI, waist circumference, and insulin resistance. | [31] |
| Type 2 diabetes | Cross-sectional | 3423 | Adults older than 40 | 0.8 a | A weak association was found between urinary BPA levels and increased diabetes. | [32] |
| Type 2 diabetes | Cross-sectional | 1210 | Adults older than 40 | 2.1 a | A weak association was found between urinary BPA levels and increased diabetes. | [33] |
| Diabetes/cardiovascular disease/liver function | Cross-sectional | 2948 | Adults aged 18–74 | 1.8–2.5 a | Positive correlation between urinary BPA levels and increased diagnosis of cardiovascular disease, type 2 diabetes and liver enzymes, but with fewer associations in most recent data. | [34] |
| Inflammatory markers/obesity/diabetes | Cross-sectional | 76 | Male aged 47–59 | 1.04 d | Data support the BPA role in visceral obesity-related low grade chronic inflammation. | [35] |
| Type 2 diabetes | Cross-sectional | 4389 | Adults older than 20 | 2.0 a | Higher urinary BPA levels were significantly and positively associated with incidence of type 2 diabetes and hemoglobin A1c. | [36] |
| Cardiovascular disease | Cross-sectional | 591 | Subjects with and without CAD | 1.3–1.5 b,a | Compared to controls, people with CAD had shown significantly higher urinary BPA levels. | [37] |
| Cardiovascular disease | Case/control | 1619 | Adults aged 40–74 with or without CAD | 1.2–1.4 a | Higher incident of CAD during 10.8 years of follow-up was positively associated with higher urinary BPA levels. | [38] |
| Obesity | Prospective cohort | 977 | Adults older than 40 | 0.8–5.0 a,b | Weak associationbetween BPA levels and greater weight. | [39] |
| Cardiovascular disease | Cross-sectional | 745 | Adults older than 40 | 2.3 a | Positive association between prevalence of peripheral arterial disease and BPA levels in urine. | [40] |
| Cardiovascular disease | Cross-sectional | 521 | Adults older than 60 | 1.2 c | Positive association of reduced heart rate variability and increased hypertension with urinary levels of BPA. | [41] |
| Obesity | Cross-sectional | 2747 | Adults aged 18–74 | 2.1 c | Higher urinary BPA was significantly associated with higher BMI and waist circumference. | [42] |
| Obesity/hormones | Prospective cohort | 890 | Adults older than 70 | 2.1–3.9 d | No significant relationship between BPA levels and indices of at mass or fat distribution were found. | [43] |
| Obesity/type 2 diabetes | Cross-sectional | 3390 | Adults older than 40 | 0.8 a | Higher urinary BPA was significantly associated with higher BMI, abdominal obesity, and insulin resistance. | [44] |
| Obesity | Cross-sectional | 223 | Adults older than 18 | 2.85 c | Weak positive association between urinary BPA levels and BMI. | [45] |
| Obesity/sex hormone concentrations | Cross-sectional | 282 | Healthy premenopausal, non–obese women aged 20–55 | 2.3 a | Positive association between body weight, BMI, fat mass, and serum leptin concentrations with urinary BPA levels. | [46] |
| Obesity | Cross-sectional | 3967 | Adults older than 20 | 3.9–4.0 a | Higher urinary BPA was significantly associated with higher BMI and waist circumference. | [47] |
| Obesity | Cross-sectional | 85 | Female aged 16–58 | 1.5–1.7 a | Positively significant correlation between BMI and BPA, cholesterol, LDL-c and leptin; while a negative correlation between BMI and adiponectin and HDL-c. | [48] |
| Obesity | Cross-sectional | 82 | Men and women with subfertility | 1.3 a | None association between BPA levels and BMI. | [49] |
| Obesity/sex hormones/PCOS | Case/control | 73 | Women with and without PCOS, obese and not | 0.7–1.2 d | Positive association between increased serum BPA, BMI, and sex hormone concentrations. | [50] |
| Diabetes/sex hormones/PCOS | Cross-sectional | 171 | Women with and without PCOS, obese and not | 0.7–1.1 d | Positive association between increased serum BPA and sex hormone concentrations. BPA was positively correlated with insulin resistance. | [51] |
| Type 2 diabetes/PCOS/inflammation | Cross-sectional | 60 | Lean and obese women with and without PCOS, aged 23–33 | 0.1–0.7 d | Women with higher levels of serum BPA had more severe insulin resistance, increased markers of chronic inflammation. Women with PCOS had higher serum BPA levels than controls. | [52] |
| Focus | Study Type | Number of Subjects | Population Type | Average BPA Concentration (Subject or Controls-Cases) | Results | Ref. |
|---|---|---|---|---|---|---|
| Urinary biomonitoring | Case/control | 90 cases/44 controls | Cashiers exposed by thermal paper (dermal exposure) and not. | 2.89 c–6.76 c | A significant increase in urinary total BPA concentration was observed for cashiers handling daily thermal paper receipts. | [59] |
| Urinary biomonitoring | Case/control | 108 cases/88 controls | Workers of a plastic industry and not | 25.10 a–43.88 a | There was significant increase in the mean urinary BPA output by industry workers, especially male;, those who had spent ≥6 years in the industry showed a significant increase in BPA output compared to those who spent <6 years. | [60] |
| Urinary biomonitoring and laboratory abnormalities | Cross-sectional | 28 | Workers in two semiautomatic epoxy resin factories | 31.96 b,c | Higher BPA concentrations were associated with clinically abnormal concentrations of FT3,FT4,TT3,TT4,TSH, glutamic-oxaloacetic transaminase and, γ-glutamyl transferase. | [61] |
| Male sexual dysfunction | Case/control | 230 cases/404 controls | Workers of BPA manufacturer and epoxy resin manufacturers and not. | 1.2 c–57.9 c | Exposed workers had a statistically increased risk of erectile difficulty (OR = 4.5, 95% CI 2.1–9.8) and ejaculation difficulty (OR = 7.1, 95% CI 2.9–17.6). | [62] |
| Urinary biomonitoring and reproductive hormones | Case/control | 106 cases/250 controls | Female workers from manufacturers of epoxy resin | 0.9 b,c–22.2 b,c | A significant positive association was found between urine BPA level and serum prolactin and progesterone concentration. | [63] |
| Urinary biomonitoring and reproductive hormones | Cross-sectional | 592 | Male workers in industry | 685.9 c,d | Males, whose urine BPA level was in the second, third, and highest quartiles had respectively 1.58-, 1.33- and 3.09-fold increased prevalence of having high prolactin levels, and the highest quartile was associated with 1.63- and 1.50-fold increased prevalence of having elevated estradiol and sex hormone-binding globulin levels. | [64] |
| Serum biomonitoring and reproductive function | Case/control | 281 cases/278 controls | Workers occupationally exposed to BPA | 0.0 e–18.75 e | Increased serum BPA level was associated with decreased mean serum androstenedione level (0.18 ng/mL, 95% CI 0.22–0.13) and increased mean serum SHBG level (2.79 nmol/L 95%, CI 2.11–3.46). | [65] |
| Serum biomonitoring and sex hormone levels | Cross-sectional | 290 | Male workers, with and without BPA exposure | 0.276 d,e–3.198 d,e | Increasing serum BPA concentration was statistically associated with decreased androstenedione levels, free testosterone levels, free androgen index, and increased sex hormone binding globulin levels. | [66] |
| Urinary biomonitoring and reproductive function | Cross-sectional | 427 | Male workers in BPA and epoxy resin industry, exposed and not to BPA | 1.2 c,d–53.7 c,d | Increasing urine BPA level was associated with more difficulty having an erection and lower ejaculation strength. | [67] |
| Urinary and serum Biomonitoring | Cross-sectional | 952 | Workers of industrial factories and family members | 24.93 c and 2.84 e | Half of the study subjects had detectable BPA in their urine samples, BPA levels were influenced by gender and smoking status. | [68] |
| Urinary biomonitoring and reproductive function | Case/control | 42 exposed male workers and 42 controls | Workers whose job was to spray epoxy resin | 0.52 d,f–1.06 d,f | Results suggest that bisphenol A may disrupt secretion of gonadotrophic hormones in men | [69] |
| Serum biomonitoring and polycystic ovary syndrome (PCOS) | Case/control | 62 PCOS women and 62 controls | PCOS women, working as market seller and healthly women | 0.16 e–0.48 e | In BPA-exposed PCOS women, BPA level was higher than healthy women, together with higher levels of triglyceride, cholesteriol, TSH and LH:FSH ratio. | [70] |
| Serum biomonitoring and reproductive function | Case/control | 110 workers and 113 controls | Petrolchemical factory workers and non-petrochemical workers | 0.628 *–0.457 * | The serum BADGE concentrations were sufficiently high to produce hormonal alterations in adult men but didn’t show a statistically significant difference between cases and controls. | [71] |
| Urinary biomonitoring and semen quality | Case/control | 130 cases 88 controls | Workers in factories with and without BPA exposure | 1.4 c,d–38.7 c,d | The inverse correlation between increased urine BPA levels and descreased sperm concentration and total count was statistically significant. | [72] |
| Serum biomonitoring and sex hormones levels | Cross-sectional | 33 | Workers in factories of epoxy resin production | 64.4 e | No association between serum BPA levels and sex hormone levels was noted. | [73] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporossi, L.; Papaleo, B. Bisphenol A and Metabolic Diseases: Challenges for Occupational Medicine. Int. J. Environ. Res. Public Health 2017, 14, 959. https://doi.org/10.3390/ijerph14090959
Caporossi L, Papaleo B. Bisphenol A and Metabolic Diseases: Challenges for Occupational Medicine. International Journal of Environmental Research and Public Health. 2017; 14(9):959. https://doi.org/10.3390/ijerph14090959
Chicago/Turabian StyleCaporossi, Lidia, and Bruno Papaleo. 2017. "Bisphenol A and Metabolic Diseases: Challenges for Occupational Medicine" International Journal of Environmental Research and Public Health 14, no. 9: 959. https://doi.org/10.3390/ijerph14090959
APA StyleCaporossi, L., & Papaleo, B. (2017). Bisphenol A and Metabolic Diseases: Challenges for Occupational Medicine. International Journal of Environmental Research and Public Health, 14(9), 959. https://doi.org/10.3390/ijerph14090959






