Regional Assessment of Human Fecal Contamination in Southern California Coastal Drainages
Abstract
:1. Introduction
2. Materials and Methods
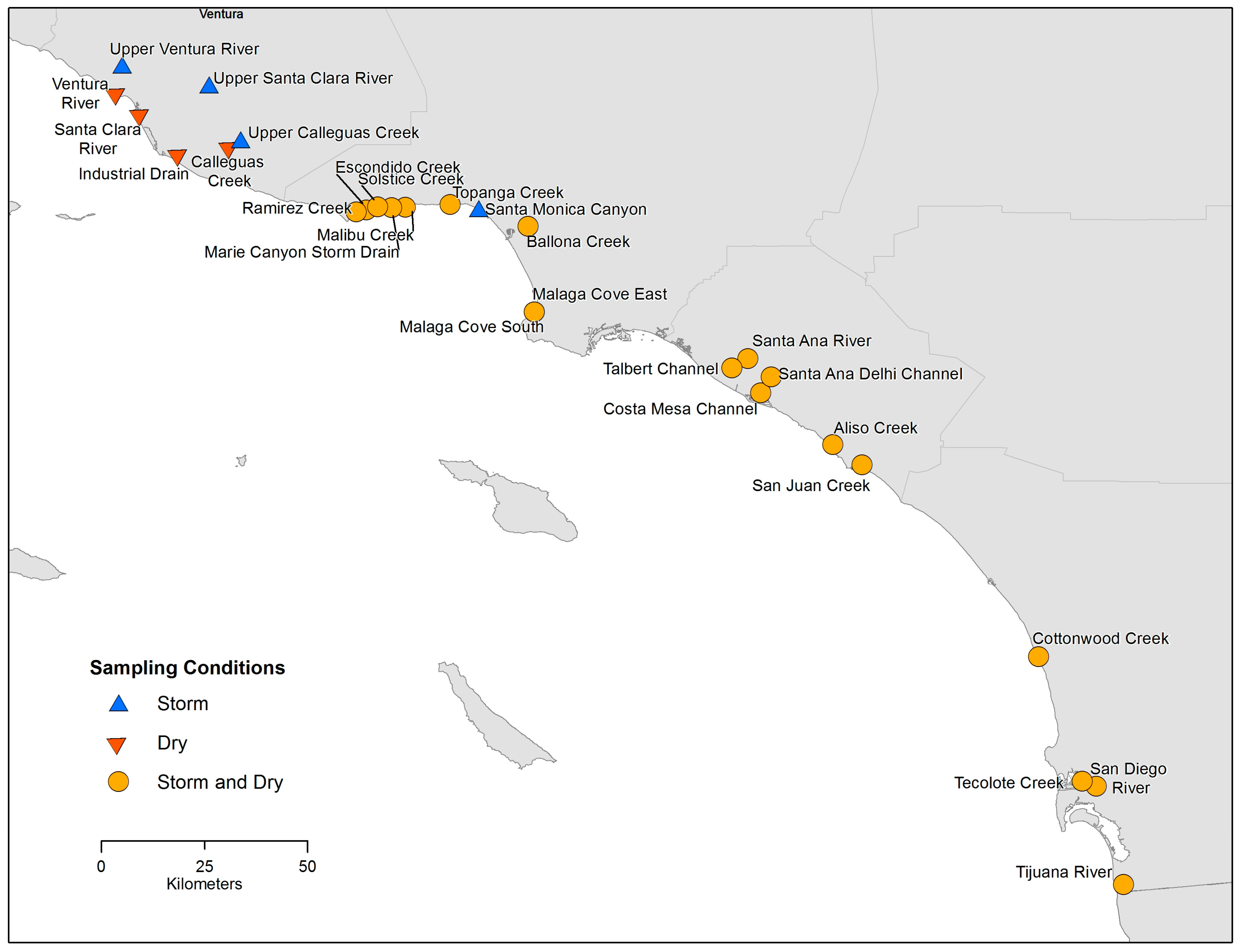
2.1. Sites and Sampling
2.2. Sample Processing and Quantitative Polymerase Chain Reaction Analysis
2.3. Data Analysis
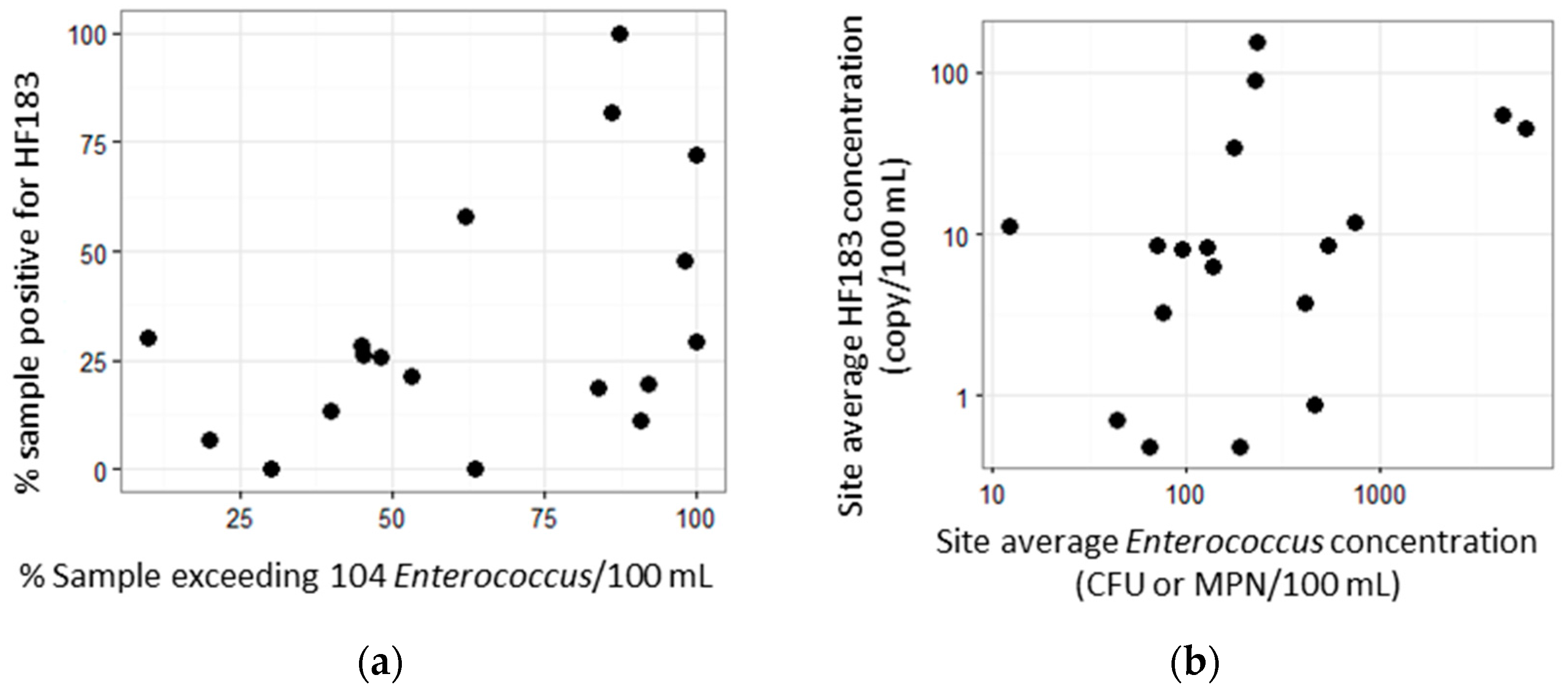
3. Results
3.1. Sampling and Results Summary
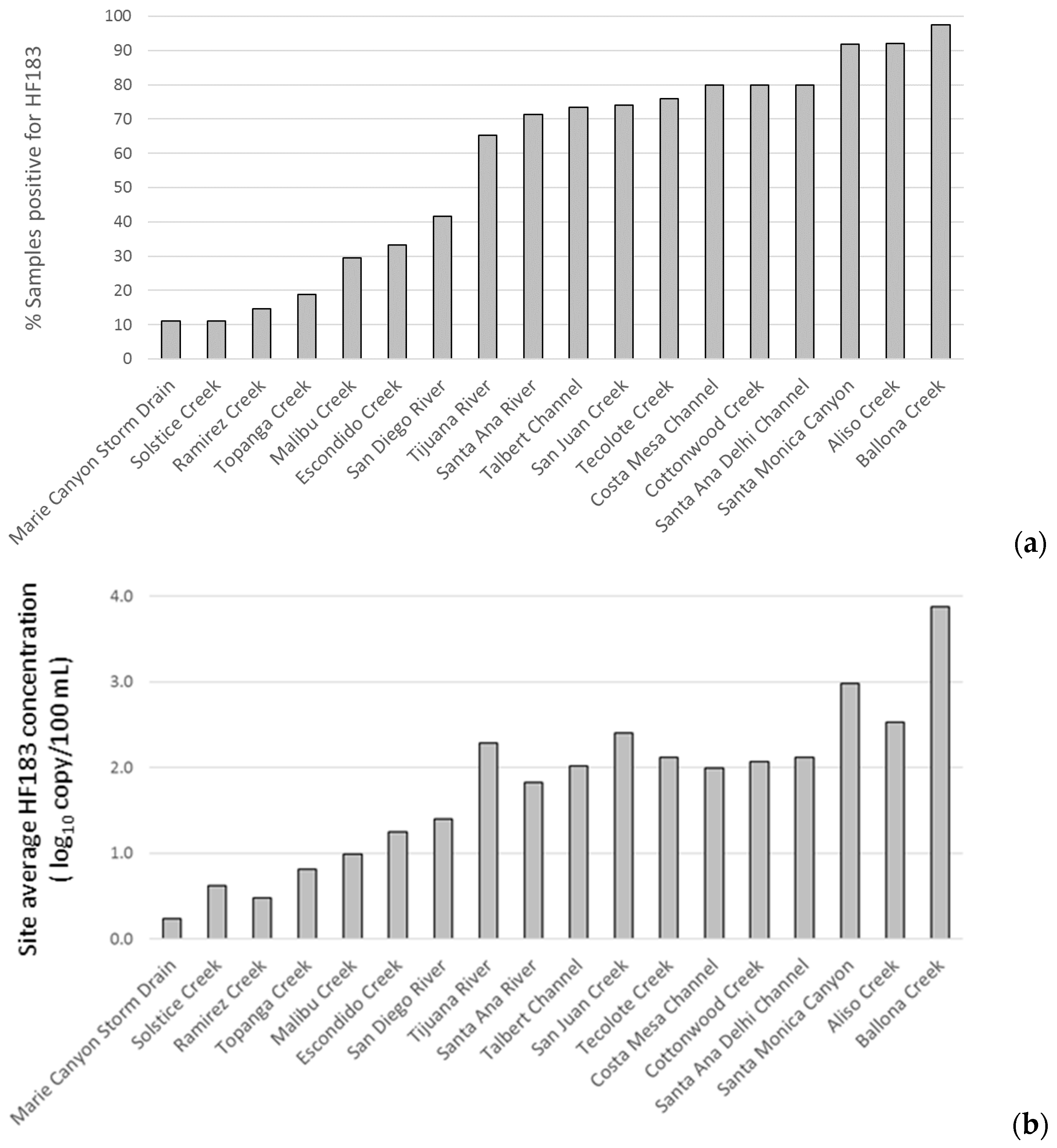
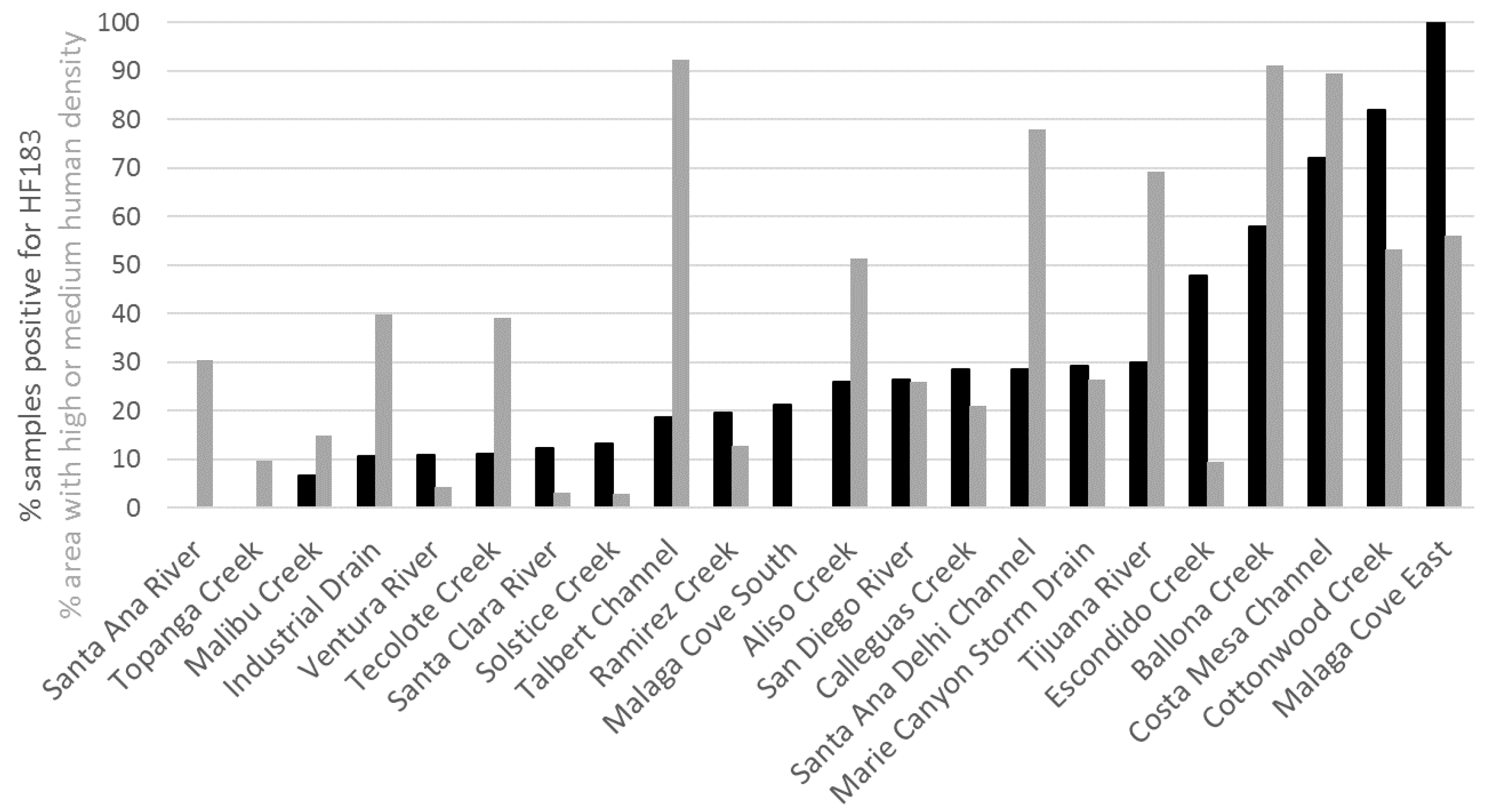
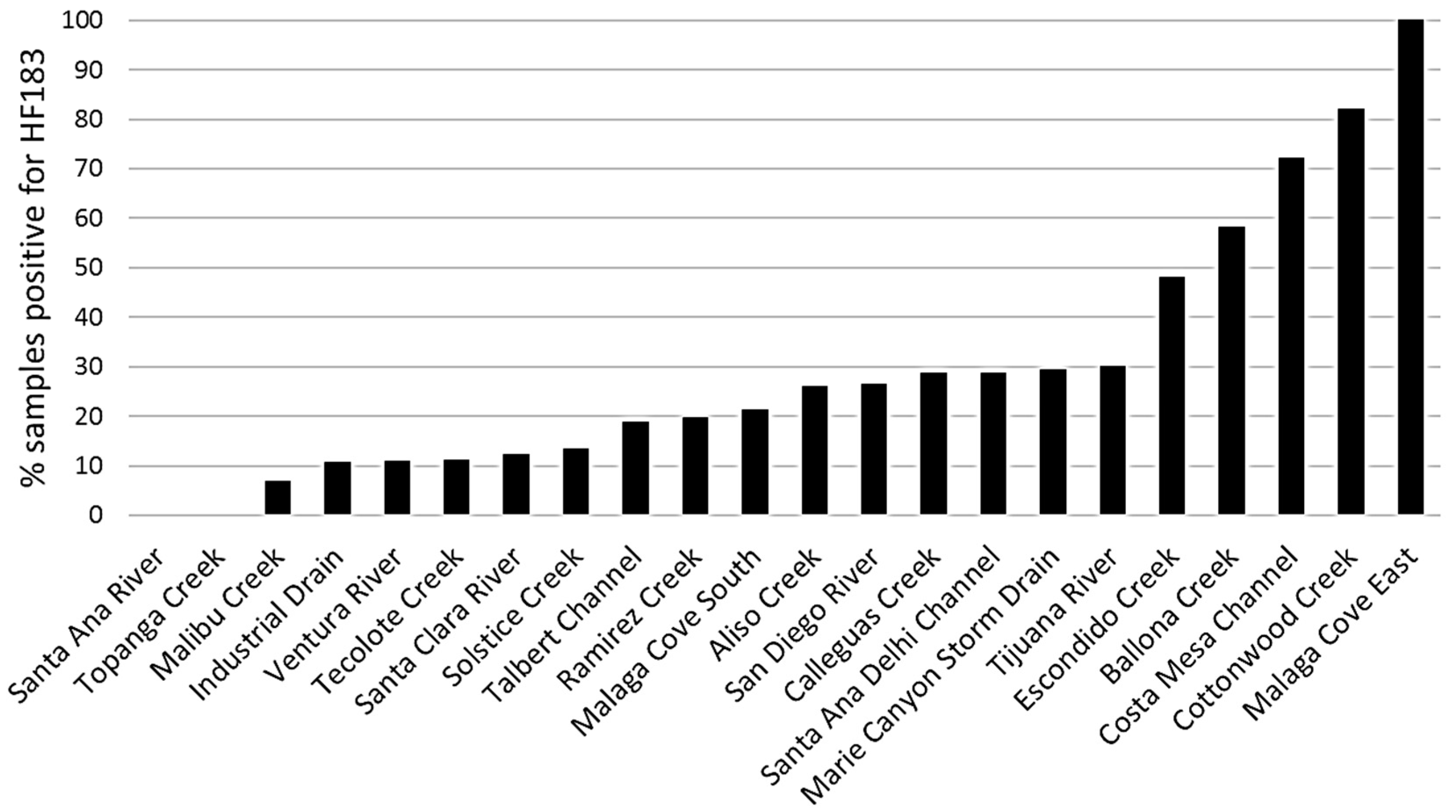
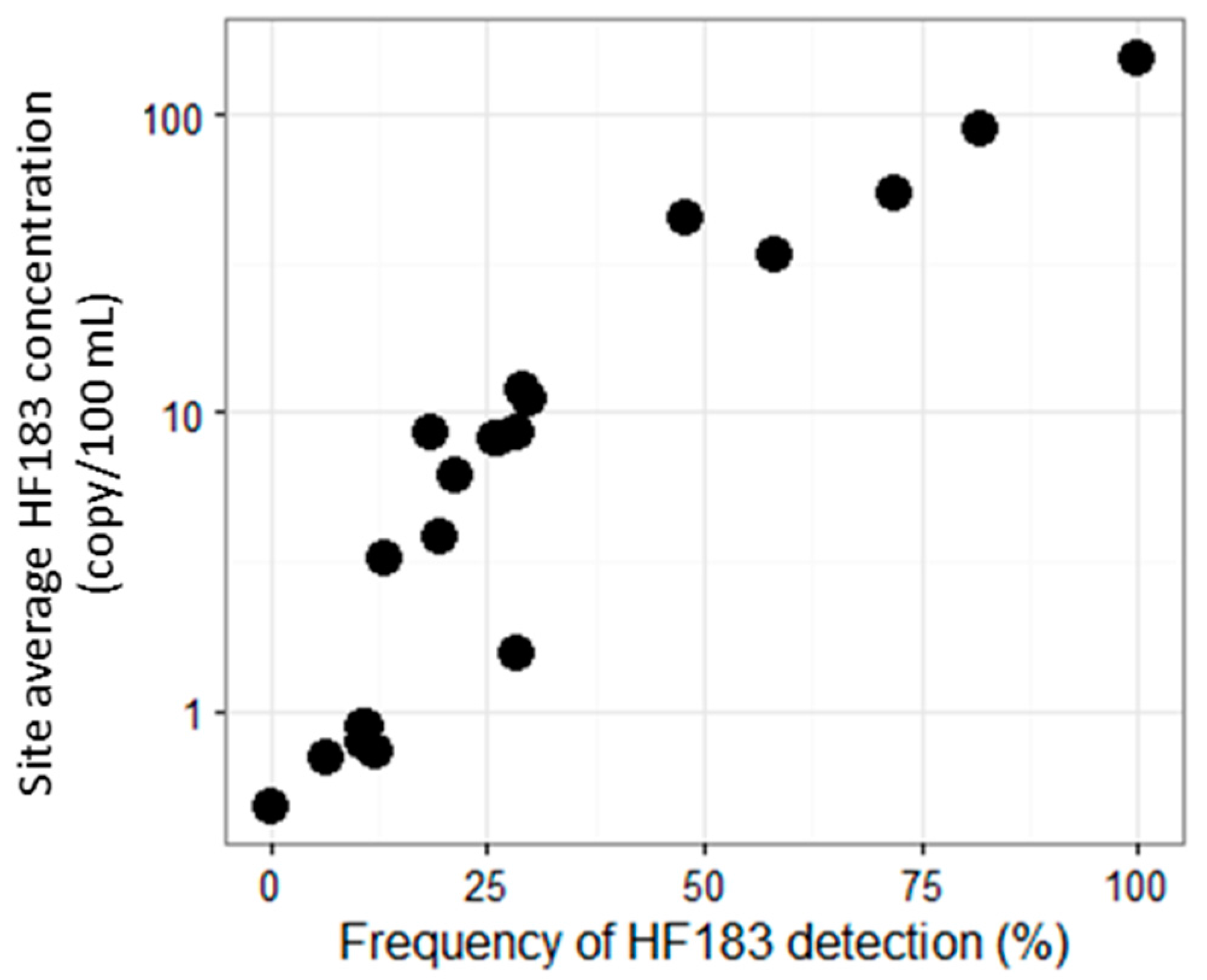
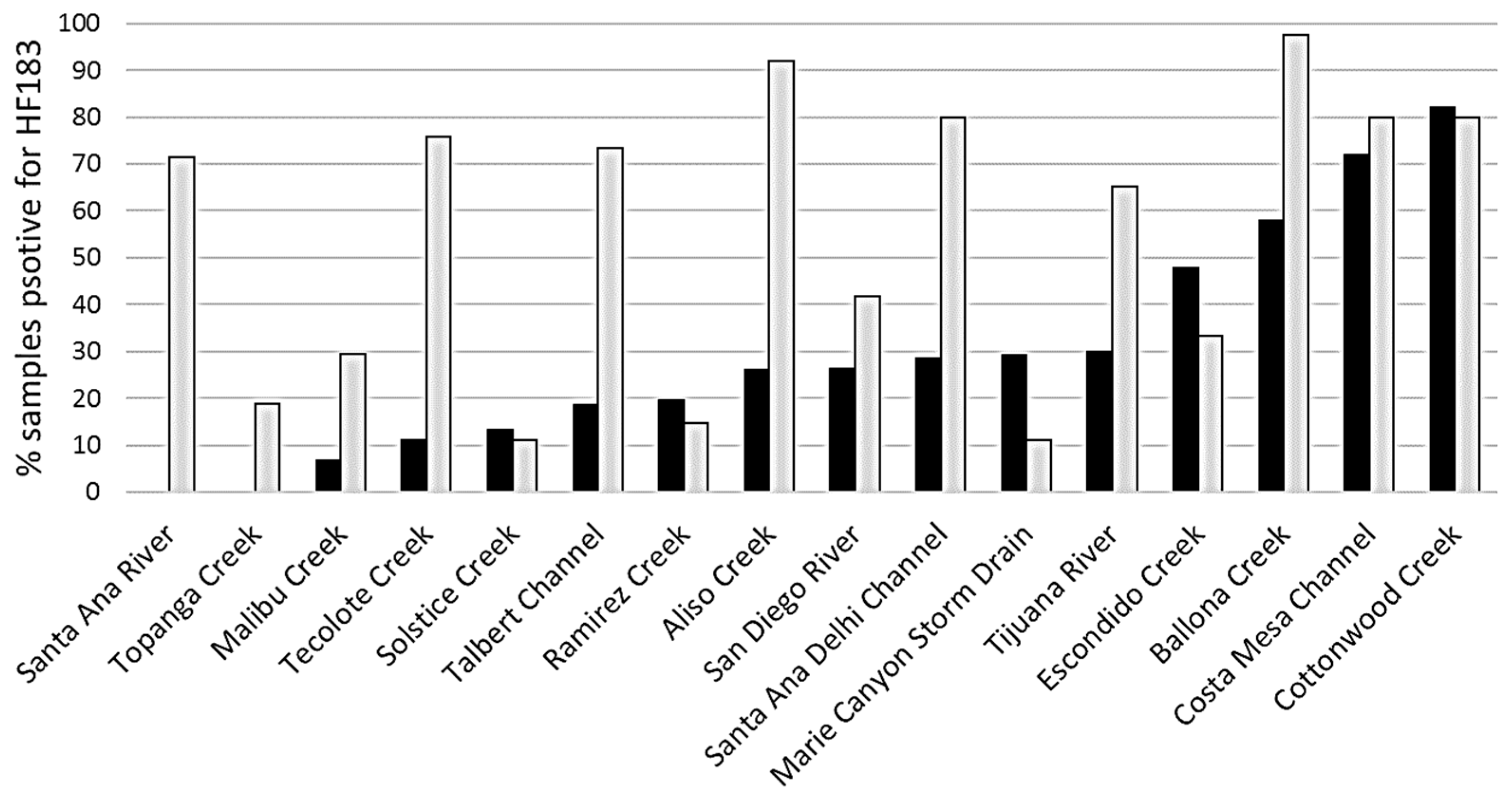
3.2. Site Prioritization
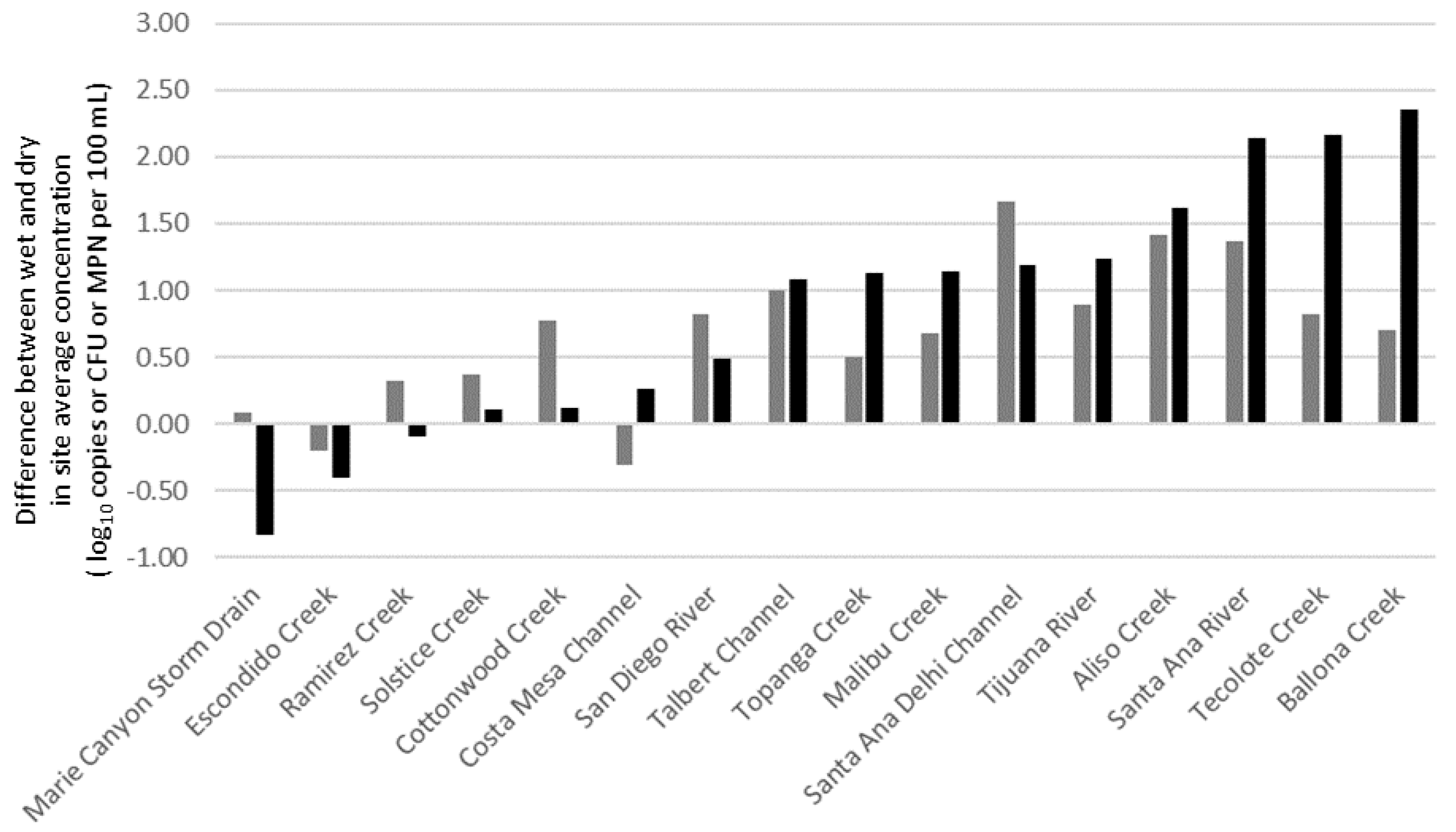
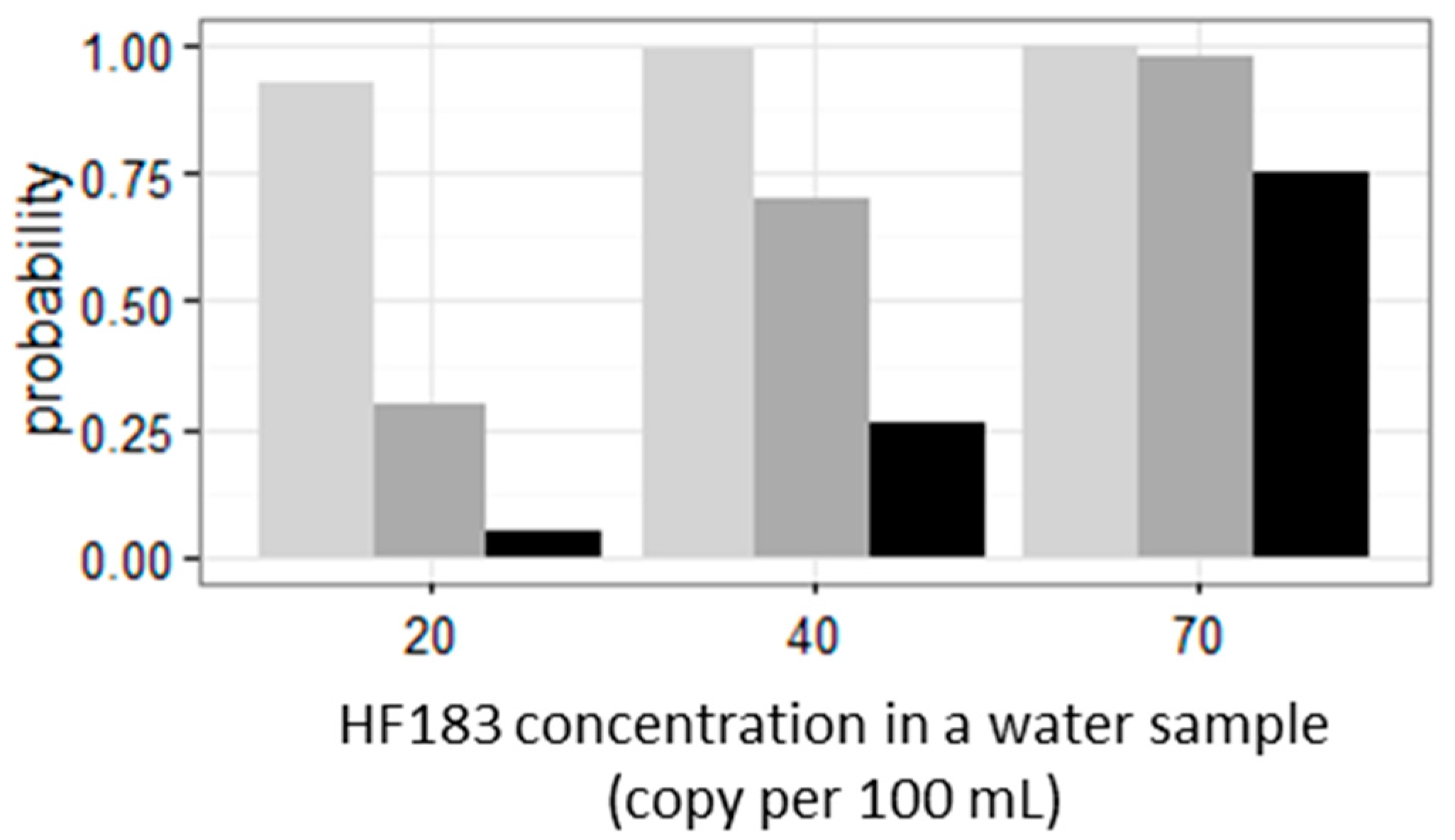
4. Discussion
5. Conclusions
- The human fecal-associated HF183 marker was ubiquitous in drainages that discharge into the U.S. southern California coast, but the frequency and magnitude of detection differed greatly across sites.
- Site prioritization for remediation was relatively consistent between prioritization based on frequency of HF183 detection and prioritization based on site average concentration of HF183 detection.
- Compared to dry weather conditions, the extent of human fecal pollution expanded considerably during wet weather events, and site prioritization outcomes also differed under wet weather. These results suggest site ranking is needed for both wet and dry conditions and that different management remediation strategies may be needed to address pollution sources during dry and wet weather time periods.
- Site prioritization based on enterococci, which do not distinguish between pollution sources, differed from site prioritization based on the HF183 marker, which does distinguish higher risk human fecal source from other lower risk sources, indicating the added value of the human fecal-associated marker.
- This study provided a valuable assessment of the status quo of human fecal marker prevalence in a highly urbanized environment and demonstrated the utility of the HF183 marker as a routine monitoring tool.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Oligonucleotide | Name | Sequence (5′ → 3′) |
|---|---|---|
| forward primer | HF183 | ATCATGAGTTCACATGTCCG |
| reverse primer | BacR287 | CTTCCTCTCAGAACCCCTATCC |
| HF183 probe | BacP234MGB | FAM-CTAATGGAACGCATCCC-MGB |
| IAC probe | BacP234IAC | VIC-AACACGCCGTTGCTACA-MGB |
| HF183 standard | Standard | ATCATGAGTTCACATGTCCGCATGATTAAAGGTATTTTCCGGTAGACGATGGGGATGCGTTCCATTAGCTCGAGATAGTAGGCGGGGTAACGGCCCACCTAGTCAACGATGGATAGGGGTTCTGAGAGGAAGGTCCCCCACATTGGAACTGAGACACGGTCCAAACTCCTACG |
| IAC standard | HF183/BacR287 IAC | ATCATGAGTTCACATGTCCGCATGATTAAAGGTATTTTCCGGTAGACGATGTGTAGCAACGGCGTGTTATAGTAGGCGGGGTAACGGCCCACCTAGTCAACGATGGATAGGGGTTCTGAGAGGAAG |
References
- Field, K.G.; Samadpour, M. Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 2007, 41, 3517–3538. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Recreational Water Quality Criteria; 820-F-12-058; Office of Water: Washington, DC, USA, 2012.
- Stoeckel, D.M.; Harwood, V.J. Performance, design, and analysis in microbial source tracking studies. Appl. Environ. Microbiol. 2007, 73, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.F.; Layton, B.A.; Boehm, A.B.; Holden, P.A.; Jay, J.A.; Hagedorn, C.D.; McGee, C.D.; Weisberg, S.B. The California Microbial Source Identification Manual: A Tiered Approach to Identifying Fecal Pollution Sources to Beaches. Available online: http://www.waterboards.ca.gov/water_issues/programs/beaches/cbi_projects/docs/sipp_manual.pdf (accessed on 1 August 2017).
- United States Environmental Protection Agency. Microbial Source Tracking Guide Document; Office of Research and Development: Cincinnatti, OH, USA, 2005; p. 150.
- Soller, J.A.; Schoen, M.E.; Bartrand, T.; Ravenscroft, J.E.; Ashbolt, N.J. Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res. 2010, 44, 4674–4691. [Google Scholar] [CrossRef] [PubMed]
- Boehm, A.B.; Van De Werfhorst, L.C.; Griffith, J.F.; Holden, P.A.; Jay, J.A.; Shanks, O.C.; Wang, D.; Weisberg, S.B. Performance of forty-one microbial source tracking methods: A twenty-seven lab evaluation study. Water Res. 2013, 47, 6812–6828. [Google Scholar] [CrossRef] [PubMed]
- Layton, B.A.; Cao, Y.; Ebentier, D.L.; Hanley, K.; Ballesté, E.; Brandão, J.; Byappanahalli, M.; Converse, R.; Farnleitner, A.H.; Gentry-Shields, J.; et al. Performance of human fecal anaerobe-associated PCR-based assays in a multi-laboratory method evaluation study. Water Res. 2013, 47, 6897–6908. [Google Scholar] [CrossRef] [PubMed]
- Green, H.C.; Haugland, R.A.; Varma, M.; Millen, H.T.; Borchardt, M.A.; Field, K.G.; Walters, W.A.; Knight, R.; Sivaganesan, M.; Kelty, C.A.; et al. Improved HF183 quantitative real-time PCR assay for characterization of human fecal pollution in ambient surface water samples. Appl. Environ. Microbiol. 2014, 80, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Hughes, B.; Harwood, V. Current status of marker genes of Bacteroides and related taxa for identifying sewage pollution in environmental waters. Water 2016, 8, 231. [Google Scholar] [CrossRef]
- Ervin, J.S.; Van De Werfhorst, L.C.; Murray, J.L.; Holden, P.A. Microbial source tracking in a coastal California watershed reveals canines as controllable sources of fecal contamination. Environ. Sci. Technol. 2014, 48, 9043–9052. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Staley, C.; Sadowsky, M.J.; Gyawali, P.; Sidhu, J.P.; Palmer, A.; Beale, D.J.; Toze, S. Toolbox approaches using molecular markers and 16s rRNA gene amplicon data sets for identification of fecal pollution in surface water. Appl. Environ. Microbiol. 2015, 81, 7067–7077. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hagedorn, C.; Shanks, O.C.; Wang, D.; Ervin, J.; Griffith, J.F.; Layton, B.A.; McGee, C.D.; Riedel, T.E.; Weisberg, S.B. Towards establishing a human fecal contamination index in microbial source tracking. Int. J. Environ. Eng. Syst. 2013, 4, 46–58. [Google Scholar]
- Griffith, J.F.; Schiff, K.C.; Lyon, G.S.; Fuhrman, J.A. Microbiological water quality at non-human influenced reference beaches in southern California during wet weather. Mar. Pollut. Bull. 2010, 60, 500–508. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Method 1611: Enterococci in Water by TaqMan® Quantitative Polymerase Chain Reaction (qPCR) Assay; EPA-821-R-12-008; Office of Water: Washington, DC, USA, 2012.
- Ebentier, D.L.; Hanley, K.T.; Cao, Y.; Badgley, B.; Boehm, A.; Ervin, J.; Goodwin, K.D.; Gourmelon, M.; Griffith, J.; Holden, P.; et al. Evaluation of the repeatability and reproducibility of a suite of PCR-based microbial source tracking methods. Water Res. 2013, 47, 6839–6848. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; Boehm, A.B.; Dubinsky, E.A.; Fong, T.T.; Goodwin, K.D.; Griffith, J.F.; Noble, R.T.; Shanks, O.C.; Vijayavel, K.; Weisberg, S.B. Recommendations following a multi-laboratory comparison of microbial source tracking methods. Water Res. 2013, 47, 6829–6838. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.P.; Ahmed, W.; Gernjak, W.; Aryal, R.; McCarthy, D.; Palmer, A.; Kolotelo, P.; Toze, S. Sewage pollution in urban stormwater runoff as evident from the widespread presence of multiple microbial and chemical source tracking markers. Sci. Total Environ. 2013, 463–464, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Sercu, B.; Van De Werfhorst, L.C.; Murray, J.; Holden, P.A. Storm drains are sources of human fecal pollution during dry weather in three urban Southern California watersheds. Environ. Sci. Technol. 2009, 43, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Sauer, E.P.; Vandewalle, J.L.; Bootsma, M.J.; McLellan, S.L. Detection of the human specific Bacteroides genetic marker provides evidence of widespread sewage contamination of stormwater in the urban environment. Water Res. 2011, 45, 4081–4091. [Google Scholar] [CrossRef] [PubMed]
- Templar, H.A.; Dila, D.K.; Bootsma, M.J.; Corsi, S.R.; McLellan, S.L. Quantification of human-associated fecal indicators reveal sewage from urban watersheds as a source of pollution to Lake Michigan. Water Res. 2016, 100, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Sercu, B.; Van De Werfhorst, L.C.; Murray, J.L.S.; Holden, P.A. Terrestrial sources homogenize bacterial water quality during rainfall in two urbanized watersheds in Santa Barbara, CA. Microb. Ecol. 2011, 62, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Sercu, B.; Van De Werfhorst, L.C.; Murray, J.L.; Holden, P.A. Sewage exfiltration as a source of storm drain contamination during dry weather in urban watersheds. Environ. Sci. Technol. 2011, 45, 7151–7157. [Google Scholar] [CrossRef] [PubMed]
- Soller, J.A.; Schoen, M.E.; Varghese, A.; Ichida, A.M.; Boehm, A.B.; Eftim, S.; Ashbolt, N.J.; Ravenscroft, J.E. Human health risk implications of multiple sources of faecal indicator bacteria in a recreational waterbody. Water Res. 2014, 66, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Ervin, J.S.; Russell, T.L.; Layton, B.A.; Yamahara, K.M.; Wang, D.; Sassoubre, L.M.; Cao, Y.; Kelty, C.A.; Sivaganesan, M.; Boehm, A.B.; et al. Characterization of fecal concentrations in human and other animal sources by physical, culture, and quantitative real-time PCR methods. Water Res. 2013, 47, 6873–6882. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, K.M.; Walters, S.P.; Boehm, A.B. Growth of Enterococci in unaltered, unseeded beach sands subjected to tidal wetting. Appl. Environ. Microbiol. 2009, 75, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Byappanahalli, M.N.; Roll, B.M.; Fujioka, R.S. Evidence for occurrence, persistence, and growth potential of Escherichia coli and enterococci in Hawaii’s soil environments. Microbes Environ. 2012, 27, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.M.; Weisberg, S.B.; Hagedorn, C.; De Leon, K.; Mofidi, V.; Wolfe, J.; Zimmerman, M.; Jay, J.A. Enterococcus growth on eelgrass (Zostera marina): Implications for water quality. FEMS Microbiol. Ecol. 2016, 92, fiw047. [Google Scholar] [CrossRef] [PubMed]
- Imamura, G.J.; Thompson, R.S.; Boehm, A.B.; Jay, J.A. Wrack promotes the persistence of fecal indicator bacteria in marine sands and seawater. FEMS Microbiol. Ecol. 2011, 77, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Harwood, V.J.; Levine, A.D.; Scott, T.M.; Chivukula, V.; Lukasik, J.; Farrah, S.R.; Rose, J.B. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl. Environ. Microbiol. 2005, 71, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Shanks, O.C.; Kelty, C.A.; Oshiro, R.; Haugland, R.A.; Madi, T.; Brooks, L.; Field, K.G.; Sivaganesan, M. Data acceptance criteria for standardized human-associated fecal source identification quantitative real-time PCR methods. Appl. Environ. Microbiol. 2016, 82, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Griffith, J.F.; Weisberg, S.B. The next generation PCR-based quantification method for ambient waters: Digital PCR. In Methods in Molecular Biology Series: Marine Genomics; Bourlat, S.J., Ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Cao, Y.; Raith, M.R.; Griffith, J.F. Droplet digital PCR for simultaneous quantification of general and human-associated fecal indicators for water quality assessment. Water Res. 2015, 70, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Raith, M.R.; Griffith, J.F. A duplex digital PCR assay for simultaneous quantification of the Enterococcus spp. and the human fecal-associated HF183 marker in waters. J. Vis. Exp. 2016, e53611. [Google Scholar] [CrossRef] [PubMed]
- Helsel, D.R. Statistics for Censored Environmental Data Using Minitab and R; John Wiley and Sons: New York, NY, USA, 2012. [Google Scholar]
- Boehm, A.B.; Soller, J.A.; Shanks, O.C. Human-associated fecal quantitative polymerase chain reaction measurements and simulated risk of gastrointestinal illness in recreational waters contaminated with raw sewage. Environ. Sci. Technol. Lett. 2015, 2, 270–275. [Google Scholar] [CrossRef]
- Cao, Y.; Andersen, G.L.; Boehm, A.A.; Holden, P.A.; Jay, J.A.; Griffith, J.F. Determination of DNA-Based Fecal Marker Aging Characteristics for Use in Quantitative Microbial Source Tracking; Technical Report 978; Southern California Coastal Water Research Project Authority: Costa Mesa, CA, USA, 2017. [Google Scholar]
- Hughes, B.; Beale, D.; Dennis, P.G.; Cook, S.; Ahmed, W. Cross-comparison of human wastewater-associated molecular markers in relation to fecal indicator bacteria and enteric viruses in recreational beach waters. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Boyer, T.C.; Hanson, T.; Singer, R.S. Estimation of low quantity genes: A hierarchical model for analyzing censored quantitative real-time PCR data. PLoS ONE 2013, 8, e64900. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Raith, M.R.; Smith, P.D.; Griffith, J.F.; Weisberg, S.B.; Schriewer, A.; Sheldon, A.; Crompton, C.; Amenu, G.G.; Gregory, J.; et al. Regional Assessment of Human Fecal Contamination in Southern California Coastal Drainages. Int. J. Environ. Res. Public Health 2017, 14, 874. https://doi.org/10.3390/ijerph14080874
Cao Y, Raith MR, Smith PD, Griffith JF, Weisberg SB, Schriewer A, Sheldon A, Crompton C, Amenu GG, Gregory J, et al. Regional Assessment of Human Fecal Contamination in Southern California Coastal Drainages. International Journal of Environmental Research and Public Health. 2017; 14(8):874. https://doi.org/10.3390/ijerph14080874
Chicago/Turabian StyleCao, Yiping, Meredith R. Raith, Paul D. Smith, John F. Griffith, Stephen B. Weisberg, Alexander Schriewer, Andrew Sheldon, Chris Crompton, Geremew G. Amenu, Jason Gregory, and et al. 2017. "Regional Assessment of Human Fecal Contamination in Southern California Coastal Drainages" International Journal of Environmental Research and Public Health 14, no. 8: 874. https://doi.org/10.3390/ijerph14080874
APA StyleCao, Y., Raith, M. R., Smith, P. D., Griffith, J. F., Weisberg, S. B., Schriewer, A., Sheldon, A., Crompton, C., Amenu, G. G., Gregory, J., Guzman, J., Goodwin, K. D., Othman, L., Manasjan, M., Choi, S., Rapoport, S., Steele, S., Nguyen, T., & Yu, X. (2017). Regional Assessment of Human Fecal Contamination in Southern California Coastal Drainages. International Journal of Environmental Research and Public Health, 14(8), 874. https://doi.org/10.3390/ijerph14080874






