Efficacy of a Low Dose of Hydrogen Peroxide (Peroxy Ag+) for Continuous Treatment of Dental Unit Water Lines: Challenge Test with Legionella pneumophila Serogroup 1 in a Simulated Dental Unit Waterline
Abstract
:1. Introduction
2. Methods
2.1. Antimicrobial Efficacy of Peroxy Ag+: In Vitro Tests
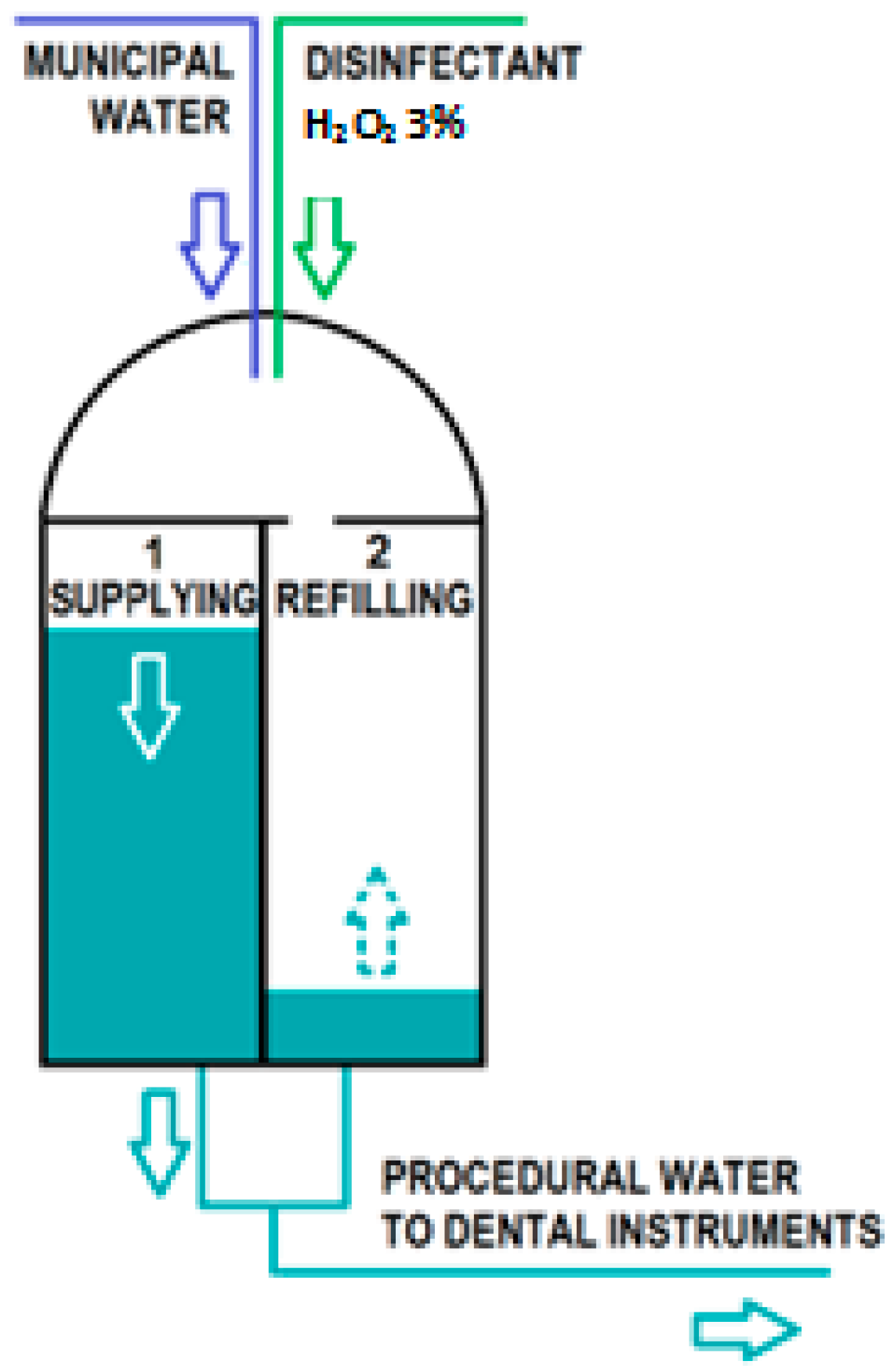
2.2. Antimicrobial Efficacy of Peroxy Ag+: Challenge Tests
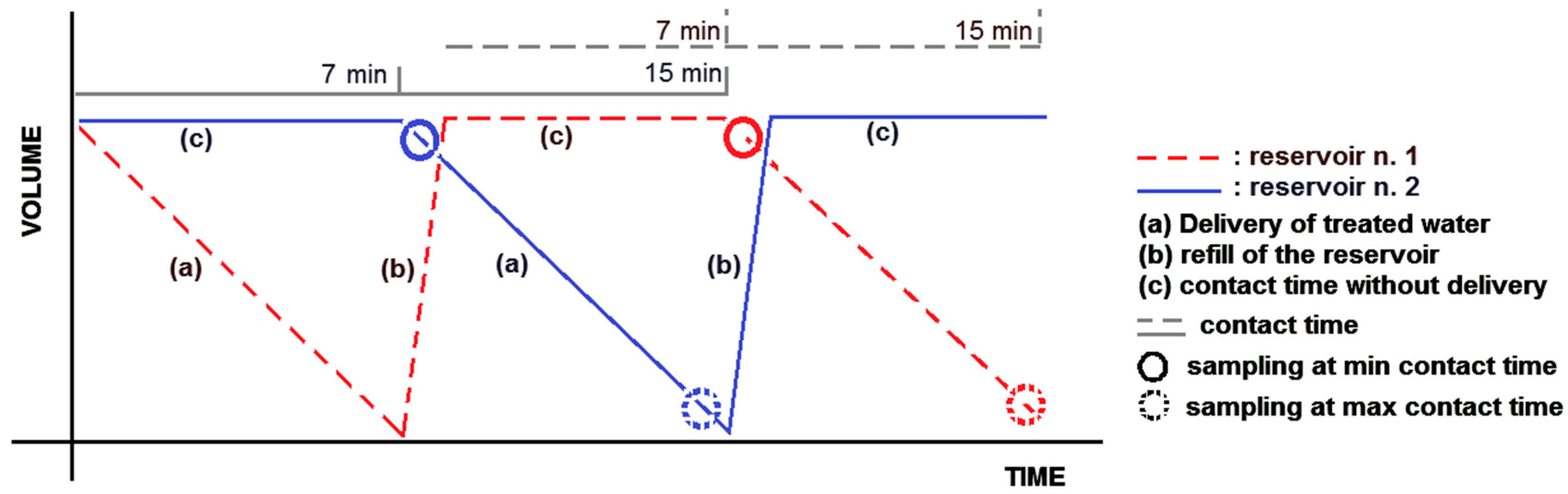
2.2.1. Collection of Water Samples
2.2.2. Laboratory Procedure
3. Results
3.1. In Vitro Test
3.2. Challenge Test
3.2.1. Continuous Mode
3.2.2. At Rest Stagnation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abel, L.C.; Miller, R.L.; Micik, R.E.; Ryge, G. Studies on dental aerobiology. IV. Bacterial contamination of water delivered by dental units. J. Dent. Res. 1971, 50, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbon, E.J.; Bartzokas, C.A.; Martin, M.V.; Gibson, M.F.; Graham, R. The source, frequency and extent of bacterial contamination of dental unit water systems. Br. Dent. J. 1984, 157, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.J.; Broderius, C. Evaluation of a dental unit designed to prevent retraction of oral fluids. Quintessence Int. Berl. Ger. 1985 1990, 21, 47–51. [Google Scholar]
- Bagga, B.S.; Murphy, R.A.; Anderson, A.W.; Punwani, I. Contamination of dental unit cooling water with oral microorganisms and its prevention. J. Am. Dent. Assoc. 1939 1984, 109, 712–716. [Google Scholar] [CrossRef]
- Fayle, S.A.; Pollard, M.A. Decontamination of dental unit water systems: A review of current recommendations. Br. Dent. J. 1996, 181, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Testarelli, L.; Vaia, F.; De Luca, M.; Dolci, G. Efficacy of anti-retraction devices in preventing bacterial contamination of dental unit water lines. J. Dent. 2003, 31, 105–110. [Google Scholar] [CrossRef]
- Montebugnoli, L.; Dolci, G.; Spratt, D.A.; Puttaiah, R. Failure of anti-retraction valves and the procedure for between patient flushing: A rationale for chemical control of dental unit waterline contamination. Am. J. Dent. 2005, 18, 270–274. [Google Scholar] [PubMed]
- Blake, J. The incidence and control of infection in dental spray reservoirs. Brit. Dent. J. 1963, 115, 412–416. [Google Scholar]
- Martin, M.V. The air/water syringe: A potential source of microbial contamination. Br. Dent. J. 1998, 184, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Ma’ayeh, S.Y.; Al-Hiyasat, A.S.; Hindiyeh, M.Y.; Khader, Y.S. Legionella pneumophila contamination of a dental unit water line system in a dental teaching centre. Int. J. Dent. Hyg. 2008, 6, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, R.L.; Peters, E.; Lizotte, J.; Lilge, C. Influence of biofilms on microbial contamination in dental unit water. J. Dent. 1991, 19, 290–295. [Google Scholar] [CrossRef]
- Barbeau, J.; Gauthier, C.; Payment, P. Biofilms, infectious agents, and dental unit waterlines: A review. Can. J. Microbiol. 1998, 44, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.T.; Bradshaw, D.J.; Bennett, A.M.; Fulford, M.R.; Martin, M.V.; Marsh, P.D. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl. Environ. Microbiol. 2000, 66, 3363–3367. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Boyle, M.A.; Russell, R.J.; Coleman, D.C. Management of dental unit waterline biofilms in the 21st century. Future Microbiol. 2011, 6, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.E. The dental unit waterline controversy: Defusing the myths, defining the solutions. J. Am. Dent. Assoc. 2000, 131, 1427–1441. [Google Scholar] [CrossRef]
- Martin, M.V. The significance of the bacterial contamination of dental unit water systems. Br. Dent. J. 1987, 163, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Reinthaler, F.; Mascher, F. Demonstration of Legionella pneumophila in dental units. Zentralblatt Bakteriol. Mikrobiol. Hyg. B 1986, 183, 86–88. [Google Scholar]
- Fotos, P.G.; Westfall, H.N.; Snyder, I.S.; Miller, R.W.; Mutchler, B.M. Prevalence of Legionella-specific IgG and IgM antibody in a dental clinic population. J. Dent. Res. 1985, 64, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Kelstrup, J.; Funder-Nielsen, T.D.; Theilade, J. Microbial aggregate contamination of water lines in dental equipment and its control. Acta Pathol. Microbiol. Scand. B 1977, 85, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Leoni, E.; Dallolio, L.; Stagni, F.; Sanna, T.; D’Alessandro, G.; Piana, G. Impact of a risk management plan on Legionella contamination of dental unit water. Int. J. Environ. Res. Public Health 2015, 12, 2344–2358. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M.; Williams, J.F.; Huntington, M.K. Legionella contamination of dental-unit waters. Appl. Environ. Microbiol. 1995, 61, 1208–1213. [Google Scholar] [PubMed]
- Ricci, M.L.; Fontana, S.; Pinci, F.; Fiumana, E.; Pedna, M.F.; Farolfi, P.; Sabattini, M.A.B.; Scaturro, M. Pneumonia associated with a dental unit waterline. Lancet 2012, 379, 684. [Google Scholar] [CrossRef]
- Montebugnoli, L.; Dolci, G. A new chemical formulation for control of dental unit water line contamination: An “in vitro” and clinical “study”. BMC Oral Health 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Kite, P.; Eastwood, K.; Sugden, S.; Percival, S.L. Use of in vivo-generated biofilms from hemodialysis catheters to test the efficacy of a novel antimicrobial catheter lock for biofilm eradication in vitro. J. Clin. Microbiol. 2004, 42, 3073–3076. [Google Scholar] [CrossRef] [PubMed]
- Karpay, R.I.; Plamondon, T.J.; Mills, S.E.; Dove, S.B. Combining periodic and continuous sodium hypochlorite treatment to control biofilms in dental unit water systems. J. Am. Dent. Assoc. 1999, 130, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Schel, A.J.; Marsh, P.D.; Bradshaw, D.J.; Finney, M.; Fulford, M.R.; Frandsen, E.; Østergaard, E.; ten Cate, J.M.; Moorer, W.R.; Mavridou, A.; et al. Comparison of the efficacies of disinfectants to control microbial contamination in dental unit water systems in general dental practices across the European Union. Appl. Environ. Microbiol. 2006, 72, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Kettering, J.D.; Muñoz-Viveros, C.A.; Stephens, J.A.; Naylor, W.P.; Zhang, W. Reducing bacterial counts in dental unit waterlines: Distilled water vs. antimicrobial agents. J. Calif. Dent. Assoc. 2002, 30, 735–741. [Google Scholar] [PubMed]
- Mungara, J.; Dilna, N.C.; Joseph, E.; Reddy, N. Evaluation of microbial profile in dental unit waterlines and assessment of antimicrobial efficacy of two treating agents. J. Clin. Pediatr. Dent. 2013, 37, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Fiehn, N.E. The effect of Sterilex Ultra for disinfection of dental unit waterlines. Int. Dent. J. 2003, 53, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-M.; Svoboda, K.K.H.; Giletto, A.; Seibert, J.; Puttaiah, R. Effects of hydrogen peroxide on dental unit biofilms and treatment water contamination. Eur. J. Dent. 2011, 5, 47–59. [Google Scholar] [PubMed]
- Dallolio, L.; Scuderi, A.; Rini, M.S.; Valente, S.; Farruggia, P.; Sabattini, M.A.B.; Pasquinelli, G.; Acacci, A.; Roncarati, G.; Leoni, E. Effect of different disinfection protocols on microbial and biofilm contamination of dental unit waterlines in community dental practices. Int. J. Environ. Res. Public Health 2014, 11, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Wirthlin, M.R.; Roth, M. Dental unit waterline contamination: A review of research and findings from a clinic setting. Compend. Contin. Educ. Dent. 2015, 36, 216–219. [Google Scholar]
- Schaeffer, A.J.; Jones, J.M.; Amundsen, S.K. Bacterial effect of hydrogen peroxide on urinary tract pathogens. Appl. Environ. Microbiol. 1980, 40, 337–340. [Google Scholar] [PubMed]
- Omidbakhsh, N.; Sattar, S.A. Broad-spectrum microbicidal activity, toxicologic assessment, and materials compatibility of a new generation of accelerated hydrogen peroxide-based environmental surface disinfectant. Am. J. Infect. Control 2006, 34, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Linger, J.B.; Molinari, J.A.; Forbes, W.C.; Farthing, C.F.; Winget, W.J. Evaluation of a hydrogen peroxide disinfectant for dental unit waterlines. J. Am. Dent. Assoc. 1939 2001, 132, 1287–1291. [Google Scholar] [CrossRef]
- Szymańska, J. Antifungal efficacy of hydrogen peroxide in dental unit waterline disinfection. Ann. Agric. Environ. Med. 2006, 13, 313–317. [Google Scholar] [PubMed]
- European Committee for Standardization (CEN). Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Bactericidal Activity against Legionella of Chemical Disinfectants for Aqueous System—Test Method and Requirements (Phase 2, Step 1); BSI Standards Publication: London, UK, 2010. [Google Scholar]
- Bolyard, E.A.; Tablan, O.C.; Williams, W.W.; Pearson, M.L.; Shapiro, C.N.; Deitchmann, S.D. Guideline for infection control in healthcare personnel, Hospital Infection Control Practices Advisory Committee. Infect. Control Hosp. Epidemiol. 1998, 19, 407–463. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Mittal, S.; Kaur, P. Dental unit waterline management: Historical perspectives and current trends. J. Investig. Clin. Dent. 2012, 3, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, P.; Byrne, B.; Chan, Y.; Swanson, M.S.; Steinman, H.M. Legionella pneumophila Catalase-Peroxidases Are Required for Proper Trafficking and Growth in Primary Macrophages. Infect. Immun. 2003, 71, 4526–4535. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, J.; Tanguay, R.; Faucher, E.; Avezard, C.; Trudel, L.; Côté, L.; Prévost, A.P. Multiparametric analysis of waterline contamination in dental units. Appl. Environ. Microbiol. 1996, 62, 3954–3959. [Google Scholar] [PubMed]
- Williams, J.F.; Johnston, A.M.; Johnson, B.; Huntington, M.K.; Mackenzie, C.D. Microbial contamination of dental unit waterlines: Prevalence, intensity and microbiological characteristics. J. Am. Dent. Assoc. 1993, 124, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Mercier, A.; Gravouil, K.; Lesobre, J.; Delafont, V.; Bousseau, A.; Verdon, J.; Imbert, C. Pyrosequencing analysis of bacterial diversity in dental unit waterlines. Water Res. 2015, 81, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ditommaso, S.; Giacomuzzi, M.; Ricciardi, E.; Zotti, C.M. Cultural and Molecular Evidence of Legionella spp. Colonization in Dental Unit Waterlines: Which Is the Best Method for Risk Assessment? Int. J. Environ. Res. Public Health 2016, 13, 211. [Google Scholar] [CrossRef] [PubMed]

| Time of Exposure | Inoculum (N0) | Outcome (Na) | lgR | Kill Rate % |
|---|---|---|---|---|
| 10 min | 4.89 × 107 (7.69) | 3.47 × 107 (7.54) | 0.15 | 29.20 |
| 60 min | 4.89 × 107 (7.69) | 1.95 × 105 (5.29) | 2.40 | 99.60 |
| 75 min | 4.89 × 107 (7.69) | 3.16 × 103 (3.50) | 4.19 | 99.993 |
| 15 h | 4.89 × 107 (7.69) | 2.1 × 102 (2.30) | 5.39 | 99.9995 |
| Time of Exposure | Inoculum (N0) | Outcome (Na) | lgR | Kill Rate % |
|---|---|---|---|---|
| 8 min 30 s * | 5.70 × 103 (3.75) | 6.33 × 102 (2.80) | 0.95 | 88.77 |
| 16 min 30 s * | 5.70 × 103 (3.75) | 1.02 × 103 (3.00) | 0.75 | 82.21 |
| 75 min ** | 5.00 × 104 (4.69) | 0 | ≥4.69 | 99.997 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditommaso, S.; Giacomuzzi, M.; Ricciardi, E.; Zotti, C.M. Efficacy of a Low Dose of Hydrogen Peroxide (Peroxy Ag+) for Continuous Treatment of Dental Unit Water Lines: Challenge Test with Legionella pneumophila Serogroup 1 in a Simulated Dental Unit Waterline. Int. J. Environ. Res. Public Health 2016, 13, 745. https://doi.org/10.3390/ijerph13070745
Ditommaso S, Giacomuzzi M, Ricciardi E, Zotti CM. Efficacy of a Low Dose of Hydrogen Peroxide (Peroxy Ag+) for Continuous Treatment of Dental Unit Water Lines: Challenge Test with Legionella pneumophila Serogroup 1 in a Simulated Dental Unit Waterline. International Journal of Environmental Research and Public Health. 2016; 13(7):745. https://doi.org/10.3390/ijerph13070745
Chicago/Turabian StyleDitommaso, Savina, Monica Giacomuzzi, Elisa Ricciardi, and Carla M. Zotti. 2016. "Efficacy of a Low Dose of Hydrogen Peroxide (Peroxy Ag+) for Continuous Treatment of Dental Unit Water Lines: Challenge Test with Legionella pneumophila Serogroup 1 in a Simulated Dental Unit Waterline" International Journal of Environmental Research and Public Health 13, no. 7: 745. https://doi.org/10.3390/ijerph13070745
APA StyleDitommaso, S., Giacomuzzi, M., Ricciardi, E., & Zotti, C. M. (2016). Efficacy of a Low Dose of Hydrogen Peroxide (Peroxy Ag+) for Continuous Treatment of Dental Unit Water Lines: Challenge Test with Legionella pneumophila Serogroup 1 in a Simulated Dental Unit Waterline. International Journal of Environmental Research and Public Health, 13(7), 745. https://doi.org/10.3390/ijerph13070745






