BMI and BMD: The Potential Interplay between Obesity and Bone Fragility
Abstract
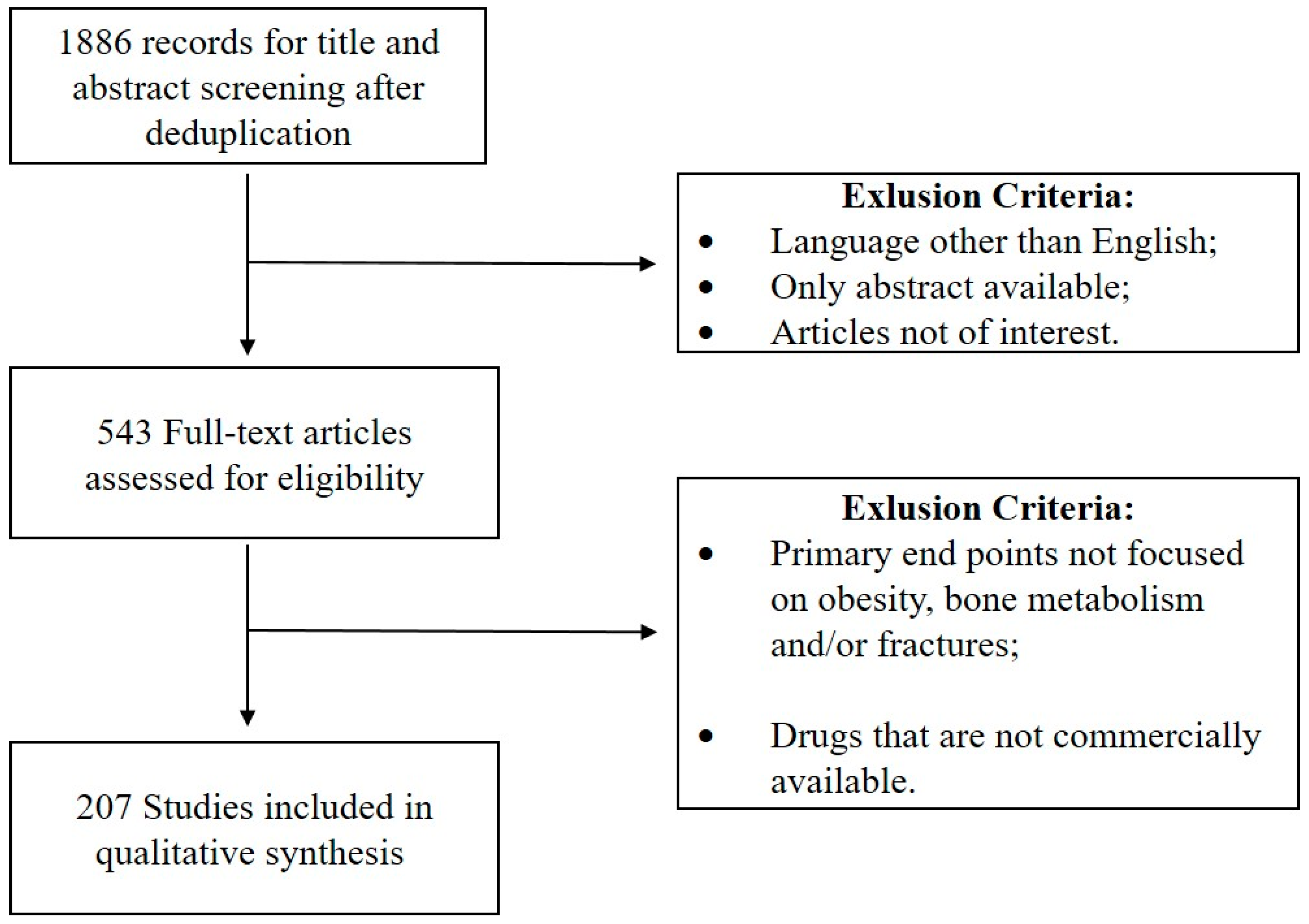
:1. Introduction
2. Materials and Methods
3. Interplay between BMI and BMD: Epidemiology of Fracture Risk in Obese Subjects
4. Physiopathology of the Bone-Body Cross Talk
5. Environmental Factors
6. Anti-Obesity Drugs and Bone Metabolism
7. Weight Reduction and Bone Health. Is It Actually Worthwhile?
8. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| BMD | bone mineral density |
| BMC | bone mineral content |
| SHBG | sex hormone binding globulin |
| CRFs | clinical risk factors |
| TBMC | body compartments on total BMC |
| RBMC | regional BMC |
| DFF | distal forearm fracture |
| HRT | ormone replacement therapy |
| CSI | Compression Strength Index |
| BSI | Bending Strength Index |
| ISI | Impact Strength Index |
References
- Greco, E.A.; Lenzi, A.; Migliaccio, S. The obesity of bone. Ther. Adv. Endocrinol. Metab. 2015, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Kado, D.M.; Huang, M.-H.; Karlamangla, A.S.; Barrett-Connor, E.; Greendale, G.A. Hyperkyphotic posture predicts mortality in older community-dwelling men and women: A prospective study. J. Am. Geriatr. Soc. 2004, 52, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Rössner, S. Obesity: The disease of the twenty-first century. Int. J. Obes. Relat. Metab. Disord. 2002, 26. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Overweight and obesity in women: Health risks and consequences. J. Womens Health (Larchmt) 2003, 12, 163–172. [Google Scholar] [CrossRef] [PubMed]
- WHO Study Group. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, i–xii, 1–253. [Google Scholar]
- Greco, E.A.; Fornari, R.; Rossi, F.; Santiemma, V.; Prossomariti, G.; Annoscia, C.; Aversa, A.; Brama, M.; Marini, M.; Donini, L.M.; et al. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int. J. Clin. Pract. 2010, 64, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-C.; Shin, D.-H.; Lee, S.-Y.; Im, J.-A.; Duk-Chul, L. Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med. J. 2010, 51, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; Flahive, J.; Hosmer, D.W.; Watts, N.B.; Siris, E.S.; Silverman, S.; Saaq, K.G.; Roux, C.; Rossini, M.; Pfeilschifter, J.; et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: The Global Longitudinal Study of Osteoporosis in Women (GLOW). J. Bone Miner. Res. 2014, 29, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Fact sheet N°311. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 12 January 2016).
- Cawley, J.; Meyerhoefer, C. The medical care costs of obesity: An instrumental variables approach. J. Health Econ. 2012, 31, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff. (Millwood) 2009, 28. [Google Scholar] [CrossRef] [PubMed]
- Cawley, J.; Rizzo, J.A.; Haas, K. Occupation-specific absenteeism costs associated with obesity and morbid obesity. J. Occup. Environ. Med. 2007, 49, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Gates, D.M.; Succop, P.; Brehm, B.J.; Gillespie, G.L.; Sommers, B.D. Obesity and presenteeism: The impact of body mass index on workplace productivity. J. Occup. Environ. Med. 2008, 50, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Müller-Riemenschneider, F.; Reinhold, T.; Berghöfer, A.; Willich, S.N. Health-economic burden of obesity in Europe. Eur. J. Epidemiol. 2008, 23, 499–509. [Google Scholar] [CrossRef] [PubMed]
- WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994, 843, 1–129. [Google Scholar]
- Cooper, C.; Campion, G.; Melton, L.J. Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992, 2, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Shuler, F.D.; Conjeski, J.; Kendall, D.; Salava, J. Understanding the burden of osteoporosis and use of the World Health Organization FRAX. Orthopedics 2012, 35, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Elffors, I.; Allander, E.; Kanis, J.A.; Gullberg, B.; Johnell, O.; Dequeker, J.; Dilsen, G.; Gennari, C.; Lopes Vaz, A.A.; Lyritis, G.; et al. The variable incidence of hip fracture in southern Europe: The MEDOS Study. Osteoporos. Int. 1994, 4, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O.; de Laet, C.; Jonsson, B.; Oden, A.; Ogelsby, A.K. International variations in hip fracture probabilities: Implications for risk assessment. J. Bone Miner. Res. 2002, 17, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.A. Racial and ethnic differences in osteoporosis. J. Am. Acad. Orthop. Surg. 2007, 15, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Bone Health and Osteoporosis: A Report of the Surgeon General. Available online: http://www.ncbi.nlm.nih.gov/pubmed/?term=20945569 (accessed on 15 March 2016).
- Reid, I.R. Fat and bone. Arch. Biochem. Biophys. 2010, 503, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-J.; Jiang, H.; Papasian, C.J.; Maulik, D.; Drees, B.; Hamilton, J.; Deng, H.-W. Correlation of obesity and osteoporosis: Effect of fat mass on the determination of osteoporosis. J. Bone Miner. Res. 2008, 23, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Obesity and bone. Curr. Osteoporos. Rep. 2013, 11, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Nielson, C.M.; Srikanth, P.; Orwoll, E.S. Obesity and fracture in men and women: An epidemiologic perspective. J. Bone Miner. Res. 2012, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.A.; Bloch, D.A.; Fries, J.F. Weight-bearing exercise, overexercise, and lumbar bone density over age 50 years. Arch. Intern. Med. 1989, 149, 2325–2329. [Google Scholar] [CrossRef] [PubMed]
- Albala, C.; Yáñez, M.; Devoto, E.; Sostin, C.; Zeballos, L.; Santos, J.L. Obesity as a protective factor for postmenopausal osteoporosis. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 1027–1032. [Google Scholar] [PubMed]
- Goulding, A.; Taylor, R.W. Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif. Tissue Int. 1998, 63, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Bauer, R.L. The association of obesity and glucose and insulin concentrations with bone density in premenopausal and postmenopausal women. Metabolism 1993, 42, 735–738. [Google Scholar] [CrossRef]
- Holmberg, A.H.; Johnell, O.; Nilsson, P.M.; Nilsson, J.; Berglund, G.; Akesson, K. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos. Int. 2006, 17, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Fraser, M.; Lovell, F.; Reece, A.; McLellan, A.R. Characteristics of males over 50 years who present with a fracture: Epidemiology and underlying risk factors. J. Bone Jt. Surg. Br. 2008, 90, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.; Johnell, O.; Gullberg, B.; Allander, E.; Elffors, L.; Ranstam, J.; Dequeker, J.; Dilsen, G.; Gennari, C.; Vaz, A.L.; et al. Risk factors for hip fracture in men from southern Europe: The MEDOS study. Mediterranean Osteoporosis Study. Osteoporos. Int. 1999, 9, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Khang, Y.-H.; Lim, K.-H.; Kim, B.-J.; Koh, J.-M.; Kim, G.S.; Kim, H.; Cho, N.H. Clinical risk factors for osteoporotic fracture: A population-based prospective cohort study in Korea. J. Bone Miner. Res. 2010, 25, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Gnudi, S.; Sitta, E.; Lisi, L. Relationship of body mass index with main limb fragility fractures in postmenopausal women. J. Bone Miner. Metab. 2009, 27, 479–484. [Google Scholar] [CrossRef] [PubMed]
- De Laet, C.; Kanis, J.A.; Odén, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Brambilla, P.; Pietrobelli, A.; Beccaria, L.; Bianchessi, A.; Mora, S.; Chiumello, G. Influence of body composition on bone mineral content in children and adolescents. Am. J. Clin. Nutr. 1996, 64, 603–607. [Google Scholar] [PubMed]
- Fischer, S.; Milinarsky, A.; Giadrosich, V.; Dib, G.; Arriagada, M.; Arinoviche, R. X-ray absorptiometry of bone in obese and eutrophic children from Valparaiso, Chile. J. Rheumatol. 2000, 27, 1294–1296. [Google Scholar] [PubMed]
- Correa Rodríguez, M.; Rueda Medina, B.; González Jiménez, E.; Navarro Pérez, C.F.; Schmidt-RioValle, J. The levels of bone mineralization are influenced by body composition in children and adolescents. Nutr. Hosp. 2014, 30, 763–768. [Google Scholar] [PubMed]
- Jeddi, M.; Dabbaghmanesh, M.H.; Ranjbar Omrani, G.; Ayatollahi, S.M.T.; Bagheri, Z.; Bakhshayeshkaram, M. Relative importance of lean and fat mass on bone mineral density in Iranian children and adolescents. Int. J. Endocrinol. Metab. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Goulding, A.; Taylor, R.W.; Jones, I.E.; McAuley, K.A.; Manning, P.J.; Williams, S.M. Overweight and obese children have low bone mass and area for their weight. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Goulding, A.; Taylor, R.W.; Jones, I.E.; Manning, P.J.; Williams, S.M. Spinal overload: A concern for obese children and adolescents? Osteoporos. Int. 2002, 13, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Wetzsteon, R.J.; Petit, M.A.; Macdonald, H.M.; Hughes, J.M.; Beck, T.J.; McKay, H.A. Bone structure and volumetric BMD in overweight children: A longitudinal study. J. Bone Miner. Res. 2008, 23, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Koebnick, C.; Smith, N.; Adams, A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin. Orthop. Relat. Res. 2013, 471, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Paulis, W.D.; Silva, S.; Koes, B.W.; van Middelkoop, M. Overweight and obesity are associated with musculoskeletal complaints as early as childhood: A systematic review. Obes. Rev. 2014, 15, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.D.; Theim, K.R.; Mirch, M.C.; Ghorbani, S.; Tanofsky-Kraff, M.; Adler-Wailes, D.C.; Brady, S.; Reynolds, J.C.; Calis, K.A.; Yanovski, J.A. Orthopedic complications of overweight in children and adolescents. Pediatrics 2006, 117, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.L.; Goulding, A.; Chalmers, D.J. Biomechanical analysis of arm fracture in obese boys. J. Paediatr. Child Health 2003, 39, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Dempster, D.W.; Recker, R.R.; Lappe, J.M.; Zhou, H.; Zwahlen, A.; Müller, R.; Zhao, B.; Guo, X.; Lang, T.; et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: A transiliac bone biopsy study. J. Clin. Endocrinol. Metab. 2013, 98, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Torriani, M.; Ghomi, R.H.; Thomas, B.J.; Brick, D.J.; Gerweck, A.V.; Harrington, L.M.; Breggia, A.; Rosen, C.J.; Miller, K.K. Determinants of bone mineral density in obese premenopausal women. Bone 2011, 48, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Cauley, J.A.; Greendale, G.A.; Nielsen, C.; Karvonen-Gutierrez, C.; Ruppert, K.; Karlamangla, A.S. Pleiotropic effects of obesity on fracture risk: The Study of Women’s Health Across the Nation. J. Bone Miner. Res. 2014, 29, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Premaor, M.O.; Compston, J.E.; Fina Avilés, F.; Pagès-Castellà, A.; Nogués, X.; Díez-Pérez, A.; Prieto-Alhambra, D. The association between fracture site and obesity in men: A population-based cohort study. J. Bone Miner. Res. 2013, 28, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Nielson, C.M.; Marshall, L.M.; Lee, D.C.; Keaveny, T.M.; Orwoll, E.S. The association between BMI and QCT-derived proximal hip structure and strength in older men: A Cross-Sectional Study. J. Bone Miner. Res. 2015, 30, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, A.J.; Holvik, K.; Omsland, T.K.; Tell, G.S.; Dahl, C.; Schei, B.; Falch, J.A.; Eisman, J.A.; Meyer, H.E. Abdominal obesity increases the risk of hip fracture. A population-based study of 43,000 women and men aged 60–79 years followed for 8 years. Cohort of Norway. J. Intern. Med. 2015, 277, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Paganini-Hill, A.; Chao, A.; Ross, R.K.; Henderson, B.E. Exercise and other factors in the prevention of hip fracture: The Leisure World study. Epidemiology 1991, 2, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Nevitt, M.C.; Browner, W.S.; Stone, K.; Fox, K.M.; Ensrud, K.E.; Cauley, J.; Black, D.; Vogt, T.M. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N. Engl. J. Med. 1995, 332, 767–773. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.; Welch, G.A.; Davis, D.R.; Drane, J.W.; Macera, C.A. Body mass and risk of hip fracture among a national cohort of postmenopausal white women: A reanalysis. Obes. Res. 1993, 1, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Joakimsen, R.M.; Fønnebø, V.; Magnus, J.H.; Tollan, A.; Søgaard, A.J. The Tromsø Study: Body height, body mass index and fractures. Osteoporos. Int. 1998, 8, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Van der Voort, D.J.; Geusens, P.P.; Dinant, G.J. Risk factors for osteoporosis related to their outcome: Fractures. Osteoporos. Int. 2001, 12, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.J.; Honkanen, K.; Kröger, H.; Alhava, E.; Tuppurainen, M.; Saarikoski, S. Risk factors for perimenopausal distal forearm fracture. Osteoporos. Int. 2000, 11, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B. Insights from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Nat. Rev. Endocrinol. 2014, 10, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Premaor, M.O.; Fina Avilés, F.; Hermosilla, E.; Martinez-Laguna, D.; Carbonell-Abella, C.; Nogués, X.; Compston, J.E.; Díez-Pérez, A. The association between fracture and obesity is site-dependent: A population-based study in postmenopausal women. J. Bone Miner. Res. 2012, 27, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Kanis, J.A.; Odén, A.; McCloskey, E.; Chapurlat, R.D.; Christiansen, C.; Cummings, S.R.; Diez-Perez, A.; Eisman, J.A.; Fujiwara, S.; et al. A meta-analysis of the association of fracture risk and body mass index in women. J. Bone Miner. Res. 2014, 29, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Suzawa, M.; Kato, S. Nuclear receptors as targets for drug development: Crosstalk between peroxisome proliferator-activated receptor gamma and cytokines in bone marrow-derived mesenchymal stem cells. J. Pharmacol. Sci. 2005, 97, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Tontonoz, P. Fat’s loss is bone’s gain. J. Clin. Investig. 2004, 113, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Puig, A.J.; Considine R, V.; Jimenez-Liñan, M.; Werman, A.; Pories, W.J.; Caro, J.F.; Flier, J.S. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J. Clin. Investig. 1997, 99, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T.; Pirtskhalava, T.; Han, J.; Karagiannides, I. Adipogenesis and aging: Does aging make fat go MAD? Exp. Gerontol. 2002, 37, 757–767. [Google Scholar] [CrossRef]
- Sepe, A.; Tchkonia, T.; Thomou, T.; Zamboni, M.; Kirkland, J.L. Aging and regional differences in fat cell progenitors—A mini-review. Gerontology 2011, 57, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Thaete, F.L.; Kelley, D.E. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 885–892. [Google Scholar] [PubMed]
- Yim, J.-E.; Heshka, S.; Albu, J.; Heymsfield, S.; Kuznia, P.; Harris, T.; Gallagher, D. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int. J. Obes. (Lond.) 2007, 31, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Wronska, A.; Kmiec, Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol. (Oxf.) 2012, 205, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Nicklas, B.J. Age-related changes in fat deposition in mid-thigh muscle in women: Relationships with metabolic cardiovascular disease risk factors. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Snijder, M.B.; Visser, M.; Dekker, J.M.; Goodpaster, B.H.; Harris, T.B.; Kritchevsky, S.B.; De Rekeneire, N.; Kanaya, A.M.; Newman, A.B.; Tylavsky, F.A.; et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005, 48, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.-E.; Heshka, S.; Albu, J.B.; Heymsfield, S.; Gallagher, D. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J. Appl. Physiol. 2008, 104, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Cartier, A.; Côté, M.; Lemieux, I.; Pérusse, L.; Tremblay, A.; Bouchard, C.; Després, J.-P. Age-related differences in inflammatory markers in men: Contribution of visceral adiposity. Metabolism 2009, 58, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Addison, O.; LaStayo, P.C.; Dibble, L.E.; Marcus, R.L. Inflammation, aging, and adiposity: Implications for physical therapists. J. Geriatr. Phys. Ther. 2012, 35, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.J.; Joseph, L.J.; Brandauer, J.; Katzel, L.I.; Hagberg, J.M.; Ryan, A.S. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J. Clin. Endocrinol. Metab. 2007, 92, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Dubé, M.-C.; Lemieux, S.; Piché, M.-E.; Corneau, L.; Bergeron, J.; Riou, M.-E.; Weisnagel, S.J. The contribution of visceral adiposity and mid-thigh fat-rich muscle to the metabolic profile in postmenopausal women. Obesity (Silver Spring) 2011, 19, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Durheim, M.T.; Slentz, C.A.; Bateman, L.A.; Mabe, S.K.; Kraus, W.E. Relationships between exercise-induced reductions in thigh intermuscular adipose tissue, changes in lipoprotein particle size, and visceral adiposity. Am. J. Physiol. Endocrinol. Metab. 2008, 295. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [PubMed]
- Yoshida, Y.; Marcus, R.L.; Lastayo, P.C. Intramuscular adipose tissue and central activation in older adults. Muscle Nerve 2012, 46, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Roubenoff, R.; Wood, M.; Frontera, W.R.; Evans, W.J.; Fiatarone Singh, M.A. Anthropometric assessment of 10-y changes in body composition in the elderly. Am. J. Clin. Nutr. 2004, 80, 475–482. [Google Scholar] [PubMed]
- Raguso, C.A.; Kyle, U.; Kossovsky, M.P.; Roynette, C.; Paoloni-Giacobino, A.; Hans, D.; Genton, L.; Pichard, C. A 3-year longitudinal study on body composition changes in the elderly: Role of physical exercise. Clin. Nutr. 2006, 25, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.; Melton, L.J.; Atkinson, E.J.; Achenbach, S.J.; Holets, M.F.; Peterson, J.M.; Khosla, S.; Drake, M.T. Relationship of adiposity to bone volumetric density and microstructure in men and women across the adult lifespan. Bone 2013, 55, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Katz, M.S.; Stern, M.P.; Dunn, J.F. Relationship of sex hormone binding globulin to overall adiposity and body fat distribution in a biethnic population. Int. J. Obes. 1989, 13, 1–9. [Google Scholar] [PubMed]
- MacDonald, P.C.; Edman, C.D.; Hemsell, D.L.; Porter, J.C.; Siiteri, P.K. Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am. J. Obstet. Gynecol. 1978, 130, 448–455. [Google Scholar] [CrossRef]
- Cleland, W.H.; Mendelson, C.R.; Simpson, E.R. Effects of aging and obesity on aromatase activity of human adipose cells. J. Clin. Endocrinol. Metab. 1985, 60, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Vattikuti, S.; Yarramaneni, J.; Giri, T.K.; Nekkalapu, S.; Qualls, C.; Armamento-Villareal, R.C. Increased 2-hydroxylation of estrogen is associated with lower body fat and increased lean body mass in postmenopausal women. Maturitas 2012, 72, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, A.; Breer, S.; Wallaschofski, H.; Nauck, M.; Baumeister, S.E.; Barvencik, F.; Amling, M.; Schinke, T.; Haring, R.; Keller, J. Osteocalcin is associated with testosterone in the general population and selected patients with bone disorders. Andrology 2013, 1, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Sumara, G.; Sumara, O.; Ferron, M.; Chang, H.; Smith, C.E.; Hermo, L.; Suarez, S.; Roth, B.L. Endocrine regulation of male fertility by the skeleton. Cell 2011, 144, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Shahnazari, M.; Orwoll, E.S.; Lane, N.E. Osteoporosis in men: Findings from the Osteoporotic Fractures in Men Study (MrOS). Ther. Adv. Musculoskelet. Dis. 2016, 8, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E. Pathogenesis of bone fragility in women and men. Lancet 2002, 359, 1841–1850. [Google Scholar] [CrossRef]
- Sundh, D.; Mellström, D.; Nilsson, M.; Karlsson, M.; Ohlsson, C.; Lorentzon, M. Increased cortical porosity in older men with fracture. J. Bone Miner. Res. 2015, 30, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.J.; Cook, A.; Thomson, R.G. Incidence of fractures in a geographically defined population. J. Epidemiol. Community Health 1990, 44, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. The mutual dependence between bone and gonads. J. Endocrinol. 2012, 213, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Melton, L.J.; Atkinson, E.J.; O’Fallon, W.M. Relationship of serum sex steroid levels to longitudinal changes in bone density in young vs. elderly men. J. Clin. Endocrinol. Metab. 2001, 86, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Bobjer, J.; Bogefors, K.; Isaksson, S.; Leijonhufvud, I.; Åkesson, K.; Giwercman, Y.L.; Giwercman, A. High prevalence of hypogonadism and associated impaired metabolic and bone mineral status in subfertile men. Clin. Endocrinol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Haring, R.; Völzke, H.; Felix, S.B.; Schipf, S.; Dörr, M.; Rosskopf, D.; Nauck, M.; Schöfl, C.; Wallaschofski, H. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes 2009, 58, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.; Nettleship, J.E.; Talib, R.A.; Almehmadi, Y.; Doros, G. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male 2016, 19, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E. Current topics in testosterone replacement of hypogonadal men. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Prats-Puig, A.; Mas-Parareda, M.; Riera-Pérez, E.; González-Forcadell, D.; Mier, C.; Mallol-Guisset, M.; Díaz, M.; Bassols, J.; de Zegher, F.; Ibáñez, L.; et al. Carboxylation of osteocalcin affects its association with metabolic parameters in healthy children. Diabetes Care 2010, 33, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Bernstein, C.N.; Leslie, W.D. Inflammatory bowel disease and the risk of osteoporosis and fracture. Maturitas 2013, 76, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.; Bonnet, F.; Dubois, S.; Massart, C.; Grosheny, C.; Bachelot, A.; Aubé, C.; Balkau, B.; Ducluzeau, P.-H. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin. Endocrinol. 2013, 78, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Lenchik, L.; Register, T.C.; Hsu, F.C.; Lohman, K.; Nicklas, B.J.; Freedman, B.I.; Langefeld, C.D.; Carr, J.J.; Bowden, D.W. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 2003, 33, 646–651. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Berner, H.S.; Lyngstadaas, S.P.; Spahr, A.; Monjo, M.; Thommesen, L.; Drevon, C.A.; Syversen, U.; Reseland, J.E. Adiponectin and its receptors are expressed in bone-forming cells. Bone 2004, 35, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.L.; Xu, J.Y.; Lu, G.; Xu, L.Y.; Cooper, G.J.S.; Xu, A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J. Biol. Chem. 2005, 280, 18341–18347. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.A.; Wang, Y.; Callon, K.E.; Watson, M.; Lin, J.; Lam, J.B.B.; Costa, J.L.; Orpe, A.; Broom, N.; Naot, D.; et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology 2009, 150, 3603–3610. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.B.; Valdes, A.M.; Burling, K.; Perks, U.C.; Spector, T.D. Serum adiponectin and bone mineral density in women. J. Clin. Endocrinol. Metab. 2007, 92, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Jürimäe, J.; Jürimäe, T. Plasma adiponectin concentration in healthy pre- and postmenopausal women: Relationship with body composition, bone mineral, and metabolic variables. Am. J. Physiol. Endocrinol. Metab. 2007, 293. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-D.; Xie, H.; Zhao, Q.; Wu, X.-P.; Sun, Z.-Q.; Liao, E.-Y. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin. Chim. Acta 2008, 387, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Pedone, C.; Pozzilli, P.; Lauretani, F.; Ferrucci, L.; Incalzi, R.A. Adiponectin and bone mass density: The InCHIANTI study. Bone 2010, 47, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Yoneda, M.; Yamane, K.; Nakanishi, S.; Nakashima, R.; Okubo, M.; Kohno, N. Serum leptin and adiponectin are positively associated with bone mineral density at the distal radius in patients with type 2 diabetes mellitus. Metabolism 2007, 56, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, M.D.; Dafni, U.G.; Routsias, J.G.; Skopouli, F.N. Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. J. Bone Miner. Res. 2004, 19, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Henry, M.J.; Kotowicz, M.A.; Collier, G.R.; Ball, M.J.; Ugoni, A.M.; Nicholson, G.C. Serum leptin levels are associated with bone mass in nonobese women. J. Clin. Endocrinol. Metab. 2001, 86, 1884–1887. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Takeda, S.; Ebihara, K.; Magre, J.; Patano, N.; Kim, C.A.; Ogawa, Y.; Liu, X.; Ware, S.M.; Craigen, W.J.; et al. Serum leptin level is a regulator of bone mass. Proc. Natl. Acad. Sci. USA 2004, 101, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Amling, M.; Takeda, S.; Priemel, M.; Schilling, A.F.; Beil, F.T.; Shen, J.; Vinson, C.; Rueger, J.M.; Karsenty, G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 2000, 100, 197–207. [Google Scholar] [CrossRef]
- Blain, H.; Vuillemin, A.; Guillemin, F.; Durant, R.; Hanesse, B.; de Talance, N.; Doucet, B.; Jeandel, C. Serum leptin level is a predictor of bone mineral density in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Shaarawy, M.; Abassi, A.F.; Hassan, H.; Salem, M.E. Relationship between serum leptin concentrations and bone mineral density as well as biochemical markers of bone turnover in women with postmenopausal osteoporosis. Fertil. Steril. 2003, 79, 919–924. [Google Scholar] [CrossRef]
- Holecki, M.; Wiecek, A. Relationship between body fat mass and bone metabolism. Pol. Arch. Med. Wewn. 2010, 120, 361–367. [Google Scholar] [PubMed]
- Couce, M.E.; Green, D.; Brunetto, A.; Achim, C.; Lloyd, R.V.; Burguera, B. Limited brain access for leptin in obesity. Pituitary 2001, 4, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Lamghari, M.; Tavares, L.; Camboa, N.; Barbosa, M.A. Leptin effect on RANKL and OPG expression in MC3T3-E1 osteoblasts. J. Cell. Biochem. 2006, 98, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Shintani, M.; Ogawa, Y.; Ebihara, K.; Aizawa-Abe, M.; Miyanaga, F.; Takaya, K.; Hayashi, T.; Inoue, G.; Hosoda, K.; Kojima, M.; et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 2001, 50, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Small, C.J.; Abbott, C.R.; Dhillo, W.S.; Seal, L.J.; Cohen, M.A.; Batterham, R.L.; Taheri, S.; Stanley, S.A.; Ghatei, M.A.; et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001, 50, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, A.; Inui, A.; Kaga, T.; Yuzuriha, H.; Nagata, T.; Ueno, N.; Makino, S.; Fujimiya, M.; Niijima, A.; Fujino, M.A.; et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001, 120, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, N.; Hanada, R.; Teranishi, H.; Fukue, Y.; Tachibana, T.; Ishikawa, H.; Takeda, S.; Takeuchi, Y.; Fukumoto, S.; Kangawa, K.; et al. Ghrelin directly regulates bone formation. J. Bone Miner. Res. 2005, 20, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M. Marrow adipose cells and hemopoiesis: An interpretative review. Exp. Hematol. 1984, 12, 139–146. [Google Scholar] [PubMed]
- Rosen, C.J.; Bouxsein, M.L. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006, 2, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Klibanski, A. Bone, fat, and body composition: Evolving concepts in the pathogenesis of osteoporosis. Am. J. Med. 2009, 122, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Elefteriou, F.; Karsenty, G. Common endocrine control of body weight, reproduction, and bone mass. Annu. Rev. Nutr. 2003, 23, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Schellinger, D.; Lin, C.S.; Hatipoglu, H.G.; Fertikh, D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. Am. J. Neuroradiol. 2001, 22, 1620–1627. [Google Scholar] [PubMed]
- Bredella, M.A.; Gill, C.M.; Gerweck, A.V.; Landa, M.G.; Kumar, V.; Daley, S.M.; Torriani, M.; Miller, K.K. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology 2013, 269, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Parhami, F. Possible role of oxidized lipids in osteoporosis: Could hyperlipidemia be a risk factor? Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 373–378. [Google Scholar] [CrossRef]
- Rajamannan, N.M. Low-density lipoprotein and aortic stenosis. Heart 2008, 94, 1111–1112. [Google Scholar] [CrossRef] [PubMed]
- Zernicke, R.F.; Salem, G.J.; Barnard, R.J.; Schramm, E. Long-term, high-fat-sucrose diet al.ters rat femoral neck and vertebral morphology, bone mineral content, and mechanical properties. Bone 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Demigné, C.; Bloch-Faure, M.; Picard, N.; Sabboh, H.; Besson, C.; Rémésy, C.; Geoffroy, V.; Gaston, A.-T.; Nicoletti, A.; Hagège, A.; et al. Mice chronically fed a westernized experimental diet as a model of obesity, metabolic syndrome and osteoporosis. Eur. J. Nutr. 2006, 45, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.G.; Lee, B.Y.; Lim, D.; Kim, H.S. Relationship between nutrition factors and osteopenia: Effects of experimental diets on immature bone quality. J. Biomech. 2009, 42, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.M.; Kiefer, F.W.; Varga, P.; Pail, P.; Rauner, M.; Stupphann, D.; Resch, H.; Moser, D.; Zysset, P.K.; Stulnig, T.M.; et al. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism 2011, 60, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Sun, L.; Gao, H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann. N. Y. Acad. Sci. 2010, 1192, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Ionova-Martin, S.S.; Wade, J.M.; Tang, S.; Shahnazari, M.; Ager, J.W.; Lane, N.E.; Yao, W.; Alliston, T.; Vaisse, C.; Ritchie, R.O. Changes in cortical bone response to high-fat diet from adolescence to adulthood in mice. Osteoporos. Int. 2011, 22, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, C.; Reimer, R.A.; Boyd, S.K.; Zernicke, R.F. High-fat, sucrose diet impairs geometrical and mechanical properties of cortical bone in mice. Br. J. Nutr. 2010, 103, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Zernicke, R.F.; Salem, G.J.; Barnard, R.J.; Woodward, J.S.; Meduski, J.W.; Meduski, J.D. Adaptations of immature trabecular bone to exercise and augmented dietary protein. Med. Sci. Sports Exerc. 1995, 27, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.X.; Glasier, A.; Norman, J.; Kelly, R.W.; Baird, D.T.; McNeilly, A.S. The effects of the antiprogestin mifepristone, in vivo, and progesterone in vitro on prolactin production by the human decidua in early pregnancy. Hum. Reprod. 1990, 5, 627–631. [Google Scholar] [PubMed]
- Tsanzi, E.; Light, H.R.; Tou, J.C. The effect of feeding different sugar-sweetened beverages to growing female Sprague-Dawley rats on bone mass and strength. Bone 2008, 42, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Yarrow, J.F.; Toklu, H.Z.; Balaez, A.; Phillips, E.G.; Otzel, D.M.; Chen, C.; Wronski, T.J.; Aguirre, J.I.; Sakarya, Y.; Tümer, N.; et al. Fructose consumption does not worsen bone deficits resulting from high-fat feeding in young male rats. Bone 2016, 85, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Fried, A.; Manske, S.L.; Eller, L.K.; Lorincz, C.; Reimer, R.A.; Zernicke, R.F. Skim milk powder enhances trabecular bone architecture compared with casein or whey in diet-induced obese rats. Nutrition 2012, 28, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2011, 93, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Plasma phosphatidylcholine concentrations of polyunsaturated fatty acids are differentially associated with hip bone mineral density and hip fracture in older adults: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2012, 27, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Martin-Bautista, E.; Muñoz-Torres, M.; Fonolla, J.; Quesada, M.; Poyatos, A.; Lopez-Huertas, E. Improvement of bone formation biomarkers after 1-year consumption with milk fortified with eicosapentaenoic acid, docosahexaenoic acid, oleic acid, and selected vitamins. Nutr. Res. 2010, 30, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.; Kunz, I.; Bendik, I.; Prudence, K.; Weber, P.; Recker, R.; Heaney, R.P. Effect of a combination of genistein, polyunsaturated fatty acids and vitamins D3 and K1 on bone mineral density in postmenopausal women: A randomized, placebo-controlled, double-blind pilot study. Eur. J. Nutr. 2013, 52, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Sahni, S.; Kerstetter, J.E.; Kenny, A.M.; Hannan, M.T. Polyunsaturated fatty acids and their relation with bone and muscle health in adults. Curr. Osteoporos. Rep. 2013, 11, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Alexy, U.; Remer, T.; Manz, F.; Neu, C.M.; Schoenau, E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am. J. Clin. Nutr. 2005, 82, 1107–1114. [Google Scholar] [PubMed]
- Remer, T.; Manz, F.; Alexy, U.; Schoenau, E.; Wudy, S.A.; Shi, L. Long-term high urinary potential renal acid load and low nitrogen excretion predict reduced diaphyseal bone mass and bone size in children. J. Clin. Endocrinol. Metab. 2011, 96, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Bounds, W.; Skinner, J.; Carruth, B.R.; Ziegler, P. The relationship of dietary and lifestyle factors to bone mineral indexes in children. J. Am. Diet. Assoc. 2005, 105, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, H.; Bailey, D.A.; Baxter-Jones, A.D.G.; Whiting, S.J. The effects of dietary protein on bone mineral mass in young adults may be modulated by adolescent calcium intake. J. Nutr. 2007, 137, 2674–2679. [Google Scholar] [PubMed]
- Hoppe, C.; Mølgaard, C.; Michaelsen, K.F. Bone size and bone mass in 10-year-old Danish children: Effect of current diet. Osteoporos. Int. 2000, 11, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Iuliano-Burns, S.; Stone, J.; Hopper, J.L.; Seeman, E. Diet and exercise during growth have site-specific skeletal effects: A co-twin control study. Osteoporos. Int. 2005, 16, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Chevalley, T.; Bonjour, J.-P.; Ferrari, S.; Rizzoli, R. High-protein intake enhances the positive impact of physical activity on BMC in prepubertal boys. J. Bone Miner. Res. 2008, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Marotte, C.; Bryk, G.; Gonzales Chaves, M.M.S.; Lifshitz, F.; de Portela, M.L.P.M.; Zeni, S.N. Low dietary calcium and obesity: A comparative study in genetically obese and normal rats during early growth. Eur. J. Nutr. 2014, 53, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Therapy of osteoporosis: Calcium, vitamin D, and exercise. Am. J. Med. Sci. 1996, 312, 278–286. [Google Scholar] [CrossRef]
- Salamone, L.M.; Cauley, J.A.; Black, D.M.; Simkin-Silverman, L.; Lang, W.; Gregg, E.; Palermo, L.; Epstein, R.S.; Kuller, L.H.; Wing, R. Effect of a lifestyle intervention on bone mineral density in premenopausal women: A randomized trial. Am. J. Clin. Nutr. 1999, 70, 97–103. [Google Scholar] [PubMed]
- Langlois, J.A.; Mussolino, M.E.; Visser, M.; Looker, A.C.; Harris, T.; Madans, J. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: The NHANES I epidemiologic follow-up study. Osteoporos. Int. 2001, 12, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Fullman, R.L.; Barrett-Connor, E.; Cauley, J.A.; Stefanick, M.L.; Fink, H.A.; Lewis, C.E.; Orwoll, E. Voluntary weight reduction in older men increases hip bone loss: The osteoporotic fractures in men study. J. Clin. Endocrinol. Metab. 2005, 90, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, K.; Cumming, R.G.; Naganathan, V.; Travison, T.G.; Sambrook, P.N.; Blyth, F.M.; Handelsman, D.J.; Le Couteur, D.G.; Waite, L.M.; Creasey, H.M.; et al. The role of fat and lean mass in bone loss in older men: Findings from the CHAMP study. Bone 2011, 49, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Cifuentes, M.; Pleshko, N.L.; Ambia-Sobhan, H.; Shapses, S.A. Energy restriction is associated with lower bone mineral density of the tibia and femur in lean but not obese female rats. J. Nutr. 2010, 140, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.M.; Cifuentes, M.; Dunn, M.G.; Shapses, S.A. Energy restriction reduces bone density and biomechanical properties in aged female rats. J. Nutr. 2001, 131, 2382–2387. [Google Scholar] [PubMed]
- Devlin, M.J.; Stetter, C.M.; Lin, H.-M.; Beck, T.J.; Legro, R.S.; Petit, M.A.; Lieberman, D.E.; Lloyd, T. Peripubertal estrogen levels and physical activity affect femur geometry in young adult women. Osteoporos. Int. 2010, 21, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Labouesse, M.A.; Gertz, E.R.; Piccolo, B.D.; Souza, E.C.; Schuster, G.U.; Witbracht, M.G.; Woodhouse, L.R.; Adams, S.H.; Keim, N.L.; Van Loan, M.D. Associations among endocrine, inflammatory, and bone markers, body composition and weight loss induced bone loss. Bone 2014, 64, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Zibellini, J.; Seimon, R.V.; Lee, C.M.; Gibson, A.A.; Hsu, M.S.; Shapses, S.A.; Nguyen, T.V.; Sainsbury, A. Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J. Bone Miner. Res. 2015, 30, 2168–2178. [Google Scholar] [CrossRef] [PubMed]
- Pop, L.C.; Sukumar, D.; Tomaino, K.; Schlussel, Y.; Schneider, S.H.; Gordon, C.L.; Wang, X.; Shapses, S.A. Moderate weight loss in obese and overweight men preserves bone quality. Am. J. Clin. Nutr. 2015, 101, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Skerry, T.M. The response of bone to mechanical loading and disuse: Fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch. Biochem. Biophys. 2008, 473, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Armamento-Villareal, R.; Parimi, N.; Chode, S.; Sinacore, D.R.; Hilton, T.N.; Napoli, N.; Qualls, C.; Villareal, D.T. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J. Bone Miner. Res. 2011, 26, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; Dunstan, D.W.; Owen, N.; Jolley, D.; Shaw, J.E.; Zimmet, P.Z. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos. Int. 2005, 16, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Legge, M.; Stapleton, J.P.; Evans, M.C.; Grey, A.B. Regular exercise dissociates fat mass and bone density in premenopausal women. J. Clin. Endocrinol. Metab. 1995, 80, 1764–1768. [Google Scholar] [PubMed]
- Meyer, H.E.; Willett, W.C.; Flint, A.J.; Feskanich, D. Abdominal obesity and hip fracture: Results from the Nurses’ Health Study and the Health Professionals Follow-Up Study. Osteoporos. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Endocrine Disruption. Available online: https://www.epa.gov/endocrine-disruption (accessed on 13 January 2016).
- Birnbaum, L.S. When environmental chemicals act like uncontrolled medicine. Trends Endocrinol. Metab. 2013, 24, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Thayer, K.A.; Heindel, J.J.; Bucher, J.R.; Gallo, M.A. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ. Health Perspect. 2012, 120, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Kopras, E.; Potluri, V.; Bermudez, M.-L.; Williams, K.; Belcher, S.; Kasper, S. Actions of endocrine-disrupting chemicals on stem/progenitor cells during development and disease. Endocr. Relat. Cancer 2014, 21. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Ishihara, Y.; Miyagawa-Tomita, S.; Hagiwara, H. Inhibition of ossification in vivo and differentiation of osteoblasts in vitro by tributyltin. Biochem. Pharmacol. 2004, 68, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Salmela, E.; Sahlberg, C.; Alaluusua, S.; Lukinmaa, P.-L. Tributyltin impairs dentin mineralization and enamel formation in cultured mouse embryonic molar teeth. Toxicol. Sci. 2008, 106, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Salmela, E.; Alaluusua, S.; Sahlberg, C.; Lukinmaa, P.-L. Tributyltin alters osteocalcin, matrix metalloproteinase 20 and dentin sialophosphoprotein gene expression in mineralizing mouse embryonic tooth in vitro. Cells Tissues Organs 2012, 195, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Blumberg, B. (2006) Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006, 147. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Watanabe, H.; Zamanian, Z.; Maeda, L.; Arima, K.; Cubacha, R.; Gardiner, D.M.; Kanno, J.; Iguchi, T.; Blumberg, B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006, 20, 2141–2155. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-J.; Han, S.-H. Blood cadmium is associated with osteoporosis in obese males but not in non-obese males: The Korea National Health and Nutrition Examination Survey 2008–2011. Int. J. Environ. Res. Public Health 2015, 12, 12144–12157. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V. Pleiotropic effects of incretins. Indian J. Endocrinol. Metab. 2012, 16. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Luce, V.; Ventura, A.; Colaianni, G.; Colucci, S.; Cavallo, L.; Grano, M.; Brunetti, G. Skeleton and glucose metabolism: A bone-pancreas loop. Int. J. Endocrinol. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Itokawa, T.; Sridhar, S.; Ding, K.-H.; Xie, D.; Kang, B.; Bollag, W.B.; Bollag, R.J.; Hamrick, M.; Insogna, K.; et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am. J. Physiol. Endocrinol. Metab. 2007, 292. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Pantoja, E.L.; Ranganath, L.R.; Gallagher, J.A.; Wilson, P.J.M.; Fraser, W.D. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Nuche-Berenguer, B.; Portal-Núñez, S.; Moreno, P.; González, N.; Acitores, A.; López-Herradón, A.; Esbrit, P.; Valverde, I.; Villanueva-Peñacarrillo, M.L. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J. Cell. Physiol. 2010, 225, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y. Incretin and bone. Clin. Calcium 2009, 19, 1312–1317. [Google Scholar] [PubMed]
- Nuche-Berenguer, B.; Moreno, P.; Esbrit, P.; Dapía, S.; Caeiro, J.R.; Cancelas, J.; Haro-Mora, J.J.; Villanueva-Peñacarrillo, M.L. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif. Tissue Int. 2009, 84, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Nuche-Berenguer, B.; Moreno, P.; Portal-Nuñez, S.; Dapía, S.; Esbrit, P.; Villanueva-Peñacarrillo, M.L. Exendin-4 exerts osteogenic actions in insulin-resistant and type 2 diabetic states. Regul. Pept. 2010, 159, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Nuche-Berenguer, B.; Lozano, D.; Gutiérrez-Rojas, I.; Moreno, P.; Mariñoso, M.L.; Esbrit, P.; Villanueva-Peñacarrillo, M.L. GLP-1 and exendin-4 can reverse hyperlipidic-related osteopenia. J. Endocrinol. 2011, 209, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Jeyabalan, J.; Jørgensen, C.S.; Hopkinson, M.; Al-Jazzar, A.; Roux, J.P.; Chavassieux, P.; Orriss, I.R.; Cleasby, M.E.; Chenu, C. Chronic administration of Glucagon-like peptide-1 receptor agonists improves trabecular bone mass and architecture in ovariectomised mice. Bone 2015, 81, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Sun, H.; Yu, J.; Wang, X.; Liu, D.; Zhao, L.; Sun, L.; Zhao, H.; Tao, B.; Liu, J. Glucagon-like peptide-1 receptor agonist Liraglutide has anabolic bone effects in ovariectomized rats without diabetes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, D.B.; Alexandersen, P.; Hartmann, B.; Adrian, C.L.; Byrjalsen, I.; Bone, H.G.; Holst, J.J. Christiansen C Four-month treatment with GLP-2 significantly increases hip BMD: A randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone 2009, 45, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Bunck, M.C.; Eliasson, B.; Cornér, A.; Heine, R.J.; Shaginian, R.M.; Taskinen, M.-R.; Yki-Järvinen, H.; Smith, U.; Diamant, M. Exenatide treatment did not affect bone mineral density despite body weight reduction in patients with type 2 diabetes. Diabetes Obes. Metab. 2011, 13, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Marre, M.; Holst, J.J.; Garber, A.; Baeres, F.M.M.; Thomsen, H.; Pratley, R.E. Comparison of the long-term effects of liraglutide and glimepiride monotherapy on bone mineral density in patients with type 2 diabetes. Endocr. Pract. 2015. [Google Scholar] [CrossRef] [PubMed]
- Iepsen, E.W.; Lundgren, J.R.; Hartmann, B.; Pedersen, O.; Hansen, T.; Jørgensen, N.R.; Jensen, J.-E.B.; Holst, J.J.; Madsbad, S.; Torekov, S.S. GLP-1 Receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J. Clin. Endocrinol. Metab. 2015, 100, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Mabilleau, G.; Mieczkowska, A.; Chappard, D. Use of glucagon-like peptide-1 receptor agonists and bone fractures: A meta-analysis of randomized clinical trials (-1:meta). J. Diabetes 2014, 6, 260–236. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Sheng, H.; Zhang, M.; Bu, L.; Yang, P.; Li, L.; Li, F.; Sheng, C.; Han, Y.; Qu, S.; Wang, J. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists’ treatment: A meta-analysis of randomized controlled trials. Endocrine 2014. [Google Scholar] [CrossRef] [PubMed]
- Pace, D.G.; Blotner, S.; Guerciolini, R. Short-term orlistat treatment does not affect mineral balance and bone turnover in obese men. J. Nutr. 2001, 131, 1694–1699. [Google Scholar] [PubMed]
- Gotfredsen, A.; Westergren Hendel, H.; Andersen, T. Influence of orlistat on bone turnover and body composition. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; Laskey, M.A.; Croucher, P.I.; Coxon, A.; Kreitzman, S. Effect of diet-induced weight loss on total body bone mass. Clin. Sci. 1992, 82, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, M.M.; Sainsbury, A.; Eisman, J.A.; Baldock, P.A.; Center, J.R. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes. Rev. 2013, 14, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.E.; Wadden, T.A.; Herzog, R.J. Changes in bone mineral content in obese dieting women. Metabolism 1997, 46, 857–861. [Google Scholar] [CrossRef]
- Jensen, L.B.; Kollerup, G.; Quaade, F.; Sørensen, O.H. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J. Bone Miner. Res. 2001, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, O.L.; Hassager, C.; Christiansen, C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am. J. Med. 1993, 95, 131–140. [Google Scholar] [CrossRef]
- Riedt, C.S.; Cifuentes, M.; Stahl, T.; Chowdhury, H.A.; Schlussel, Y.; Shapses, S.A. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J. Bone Miner. Res. 2005, 20, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Ricci, T.A.; Chowdhury, H.A.; Heymsfield, S.B.; Stahl, T.; Pierson, R.N.; Shapses, S.A. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J. Bone Miner. Res. 1998, 13, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Ramsdale, S.J.; Bassey, E.J. Changes in bone mineral density associated with dietary-induced loss of body mass in young women. Clin. Sci. 1994, 87, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Van Loan, M.D.; Johnson, H.L.; Barbieri, T.F. Effect of weight loss on bone mineral content and bone mineral density in obese women. Am. J. Clin. Nutr. 1998, 67, 734–738. [Google Scholar] [PubMed]
- Shapses, S.A.; Von Thun, N.L.; Heymsfield, S.B.; Ricci, T.A.; Ospina, M.; Pierson, R.N.; Stahl, T. Bone turnover and density in obese premenopausal women during moderate weight loss and calcium supplementation. J. Bone Miner. Res. 2001, 16, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Riedt, C.S.; Schlussel, Y.; von Thun, N.; Ambia-Sobhan, H.; Stahl, T.; Field, M.P.; Sherrell, R.M.; Shapses, S.A. Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. Am. J. Clin. Nutr. 2007, 85, 972–980. [Google Scholar] [PubMed]
- Pritchard, J.E.; Nowson, C.A.; Wark, J.D. Bone loss accompanying diet-induced or exercise-induced weight loss: A randomised controlled study. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 513–520. [Google Scholar] [PubMed]
- Bakhireva, L.N.; Barrett-Connor, E.; Kritz-Silverstein, D.; Morton, D.J. Modifiable predictors of bone loss in older men: A prospective study. Am. J. Prev. Med. 2004, 26, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, G.M.; Sievänen, H.T.; Kukkonen-Harjula, T.K.; Pasanen, M.E. Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int. 2001, 12, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.C.; Fisher, G.; Roy, J.L.; Gower, B.A.; Hunter, G.R. The effects of weight loss on relative bone mineral density in premenopausal women. Obesity 2013, 21, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Villalon, K.L.; Gozansky, W.S.; van Pelt, R.E.; Wolfe, P.; Jankowski, C.M.; Schwartz, R.S.; Kohrt, W.M. A losing battle: weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity 2011, 19, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Gower, B.A.; Casazza, K. Divergent effects of obesity on bone health. J. Clin. Densitom. 2013, 16, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Armamento-Villareal, R.; Sadler, C.; Napoli, N.; Shah, K.; Chode, S.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J. Bone Miner. Res. 2012, 27, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Shapses, S.A.; Riedt, C.S. Bone, body weight, and weight reduction: What are the concerns? J. Nutr. 2006, 136, 1453–1456. [Google Scholar] [PubMed]
- Sundh, D.; Rudäng, R.; Zoulakis, M.; Nilsson, A.G.; Darelid, A.; Lorentzon, M. A high amount of local adipose tissue is associated with high cortical porosity and low bone material strength in older women. J. Bone Miner. Res. 2016, 31, 749–757. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Country | Type of Study | Subjects | RR/OR (95% CI) | Results |
|---|---|---|---|---|---|
| Michel BA, 1988 [28] | U.S. | Cross-sectional study | 78 healthy subjects, ≥50 years | - | Moderate weight bearing exercise may increase lumbar bone density. Comment of the author: maybe, extremely vigorous exercise could be detrimental to bone density in individuals after age 50 |
| Haffner SM, 1993 [31] | U.S. | Cross-sectional study | 317 premenopausal and postmenopausal women | - | Lumbar spine and femoral neck density were positively correlated with BMI. The same between femoral neck density with fasting insulin level in younger women after adjustment for age (r = 0.214, p < 0.01). After adjustment for BMI, femoral neck density was not significantly correlated with fasting insulin level (p = 0.08). Adjustment for glucose and insulin levels does not explain the linkage between bone density and obesity |
| DiPietro L, 1993 [57] | U.S. | Cross-sectional study | 2285 postmenopausal women, aged 50–77 years | Baseline body mass index in the highest quartile (>37 kg/m2) experienced a 70% lower rate of hip fracture compared with women in the lowest quartile (28.7 kg/m2) (RR = 0.32; 95% CI 0.12–0.82) | Although reported education level, physical activity level, smoking history and estrogen replacement were significantly (p < 0.0001) associated with BMI, these covariates were not related to hip fracture in the multivariable analysis |
| Albala C, 1996 [29] | Chile | Case-control study | 113 obese and 50 non-obese postmenopausal women | In Obese women, a decreased risk of osteopenia in femoral neck (Age adjusted OR = 0.36, 0.17–0.75); lumbar spine (Age adjusted OR = 0.43, 0.20–0.91) | Obese women showed a higher BMD; obesity exerts protection due to a decreased SHBG thus increasing free sex steroids. Hyperinsulinemia may produce a decrease in the production of IGFBG-1, leading to an increase of IGF-1, that could stimulate the proliferation of osteoblasts |
| Manzoni P, 1996 [38] | Italy | Cross-sectional study | 65 obese and 50 normal-weight children and adolescents (age range: 5–18 years relative body weight: 160% ± 23% and 101% ± 12%, respectively) | - | No differences in TBMC and RBMC among obese and normal-weight children groups, after correction for the confounding variables age and sex |
| Goulding A, 1998 [30] | New Zealand | Cross-sectional study | 54 postmenopausal women | - | No evidence for an association between plasma levels of leptin and biochemical markers of either osteoclastic or osteoblastic activity |
| Kanis J, 1999 [34] | UK | Case-control study | 730 men with hip fracture, 1132 age-stratified controls, 50 years or more. | The effect of BMI on risk was linear, with a change for each unit of BMI of 6.8% (95% CI 4%–9%) | A low BMI was associated with a significantly increased risk of hip fracture in a dose-dependent manner |
| Fischer S, 2000 [39] | Chile | Cross-sectional, case control study | 16 obese children (8 male, 8 female) aged 5 to 13 years. 16 healthy eutrophic children matched for sex, chronological age, height, and pubertal stage were enrolled as controls | - | Obese children have more total body BMC than eutrophic children. No significant difference was showed in regional hip BMD and lumbar spine BMD in the group of obese and normal children |
| Goulding A, 2000 [42] | Cross-sectional study | 200 girls and 136 boys, aged 3–19 years | - | Girls and boys (in overweight and obese) showed a mismatch between body weight and bone development during growth: their bone mass and bone area are low for their body weight | |
| van der Voort DJ, 2001 [59] | The Netherlands | Cross-sectional study | 4725 postmenopausal women, 50–80 years of age | BMI > 30 kg/m2 and fractures elsewhere: OR 1.4 (1.0–1.9). | Women with normal BMD showed statistically significant lower fracture risk than osteoporotic women. Women with a possibly decreased BMI were most often osteoporotic and had sustained more fractures during the past 5 years’ than expected. Women who had (probably) always been obese were less often osteoporotic and had a much lower fracture risk |
| Goulding A, 2002 [43] | New Zealand | Cross-sectional study | 202 boys and 160 girls, aged 3–19 years | Overweight and obese groups were 0.92 (95% CI 0.87–0.97) and 0.88 (95% CI 0.80–0.96) for girls and 0.96 (95% CI 0.91–1.02, NS) and 0.87 (95% CI 0.78–0.96) for boys, respectively | During growth children (in overweight and obese) do not increase their spinal BMC due to a compensation for their excessive weight |
| Davidson PL, 2003 [48] | New Zealand | Cross-sectional study | 50 boys (25 obese pair-matched with 25 non-obese subjects), aged 4–17 years | - | Environmental modifications are unlikely to lower the risk of arm fracture in obese children to the same levels showed by non-obese children |
| Taylor ED, 2006 [47] | U.S. | Cross-sectional study | 227 overweight and 128 nonoverweight children and adolescents | The prevalence of documented skeletal fractures in overweight than in nonoverweight children and adolescents (odds ratio (OR): 4.54; 95% confidence interval (CI): 1.6–13.2 p = 0.0053) | Fractures, impaired mobility, musculoskeletal difficulties, and lower extremity malalignment were more prevalent in overweight than nonoverweight children and adolescents |
| Sharma S, 2008 [33] | UK | Cross-sectional study | 2035 men aged over 50 years | - | A low BMI, showed significantly, more hip fractures than those with fractures elsewhere |
| Gnudi S, 2009 [36] | Italy | Cross-sectional study | 2235 postmenopausal women including those with fragility fractures of the hip (187), ankle (108), wrist (226) and humerus (85) | BMI had a protective effect against hip fracture: OR 0.949 (0.900–0.999); higher risk of humerus fracture: OR 1.077 (1.017–1.141) | Decreasing BMI increases the risk for hip fracture, whereas increasing BMI increases the risk for humerus fractures |
| Bredella MA, 2011 [50] | U.S. | Cross-sectional study | 68 healthy obese premenopausal women | - | VAT exerts detrimental effects, whereas muscle mass exerts positive effects on BMD in premenopausal obese women. IGF-1 could be a mediator of the bad effects of VAT on bone health through effects on bone formation |
| Prieto-Alhambra D, 2012 [62] | Spain | Cross-sectional study | 832,775 women aged ≥50 years were categorized into underweight/normal (n: 302,414), overweight (n: 266,798), and obese (n: 263,563) | Hip fractures were significantly less common in overweight and obese women than in normal/underweight women (rate ratio (RR) 0.77 (95% confidence interval (CI) 0.68 to 0.88), RR 0.63 (95% CI 0.64–0.79), p < 0.001, respectively). Pelvis fracture rates were lower in the overweight (RR 0.78 (95% CI 0.63–0.96), p = 0.017) and obese (RR 0.58 (95% CI 0.47–0.73), p < 0.001) groups. Conversely, obese women were at significantly higher risk of proximal humerus fracture than the normal/underweight group (RR 1.28 (95% CI 1.04–1.58), p = 0.018) | An age-related increase in incidence was showed for all BMI groups at all fracture sites; obese women with hip, clinical spine and pelvis fracture were significantly younger at the time of fracture than normal/underweight women, whereas those with wrist fracture were significantly older. The association between obesity and fracture in postmenopausal women is site-dependent, obesity being protective against hip and pelvis fractures but associated with an almost 30% increase in risk for proximal humerus fractures when compared with normal/underweight women |
| Kessler J, 2013 [45] | U.S. | Cross-sectional study | Electronic medical records of 913,178 patients, aged 2 to 19 years | Overweight, moderately obese, and extremely obese patients all had an increased OR of fractures of the foot (1.14, 1.23, and 1.42, respectively, (1.04–1.24, 1.12–1.35, and 1.26–1.61), respectively- along with the ankle, knee, and leg (1.27, 1.28, and 1.51, respectively, with 1.16–1.39, 1.15–1.42, and 1.33–1.72, respectively) | Increasing BMI is associated with increased odds of foot, leg, ankle and knee fractures in children |
| Cohen A, 2013 [49] | U.S. | Cross-sectional study | 40 healthy premenopausal women | - | At the tissue level, premenopausal women with more central adiposity showed inferior bone quality and stiffness and markedly lower bone formation |
| Correa Rodriguez M, 2014 [40] | Spain | Cross-sectional study | 157 adolescents (93 women and 64 men) Mean age: 14.22 ± 1.41 year | - | BMD increases in response to increased muscle mass in adolescents with overweight and/or obesity |
| Jeddi M, 2015 [41] | Iran | Cross-sectional study | 472 subjects (235 girls, 237 boys) aged 9–18 years | - | Lean mass was the main predictor of BMD in both genders. Physical activity appears to positively impact on lean mass |
| Shen J, 2015 [53] | U.S. | Cross-sectional Study | 672 men (mean age: 73 years) | Obese men were 4 times more likely to have aload-to-strength ratio >1.0 compared to normal-weight men (OR: 4.66; 95% CI 2.16–10.05; p < 0.0001). | About non-obese men (BMI < 30), increasing BMI was associated with higher integral, cortical and trabecular BMD, integral volume, cross-sectional area, and percent cortical volume (all p < 0.001). About obese men (BMI ≥ 30), increasing BMI was not associated with any of those parameters. Furthermore, compared to non-obese men, obese men had a higher hip strength, but also a higher ratio of impact force to strength (p < 0.0001), in theory increasing their risk of hip fracture despite their increased strength |
| Author, Year | Country | Type of Study | Subjects | RR/OR (95% CI) | Results |
|---|---|---|---|---|---|
| Joakimsen RM, 1998 [58] | Norway | Cohort study | 12,270 (922 persons with fractures) middle-aged | Change in body mass index was not associated with fractures among men, except for a lower incidence of hip fractures (not only low-energy) among those who had gained weight (RR 0.69, 95% CI 0.50–0.95, age adjusted per unit BMI increase). Women who had increased their body mass index had a lower risk of all low-energy fractures (RR 0.95, 95% CI 0.90–1.01, age adjusted per unit BMI increase) and of low-energy fractures in the lower extremities (RR 0.88, 95% CI 0.80–0.97, age adjusted per unitBMI increase) | High body height is a risk factor for fractures, and 1 in 4 low-energy fractures among women today could be ascribed to the increase in average stature since the turn of the century. Low BMI was associated with a higher risk of fractures |
| Honkanen RJ, 2000 [60] | Finland | Cohort study | 11,798 women. Mean baseline age of these women was 52.3 (SD 2.9) years (range 47–56 years) and 68% were postmenopausal | Overweight (BMI > 25 kg/m2) decreased the perimenopausal distal forearm fracture by 36% (p = 0.0002) | Overweight protects against perimenopausal distal forearm fracture |
| Holmberg AH, 2006 [32] | Sweden | Cohort study | 22,444 men and 10,902 women, mean age 44 and 50 years | High BMI and forearm fractures (RR 0.88, 95% CI 0.81–0.96) High BMI and risk of proximal humerus and ankle fractures (RR 1.21–1.33). High BMI and forearm fractures (RR 0.88, 95% CI 0.81–0.96) | High BMI significantly increased the risk of proximal humerus and ankle fractures while, by contrast, lowering the risk of forearm fractures |
| Wetzsteon RJ, 2008 [44] | U.S. | Cohort study | 302 children healthy weight and 143 children overweight, (9–11 years) | - | Bone strength did not adapt to excess body fat. Rather, bone strength was adapted to the greater muscle area in overweight group of children. |
| Lee SH, 2010 [35] | Korea | Cohort study | 9351 subjects (4732 men and 4619 women) aged 40 to 69 years were followed for a mean of 46.3 ± 2.2 months | In women, Obesity and risk of fracture 1.29 (0.76–2.18) | Older age, lower BMI, and previous fracture history were positively associated with fracture risk in men and women |
| Premaor MO, 2013 [52] | Brazil | Cohort study | 139,419 men: underweight/normal (n = 26,298), overweight (n = 70,851), and obese (n = 42,270), ≥65 years | A statistically significant reduction in clinical spine and hip fractures was observed in obese (relative risk (RR), 0.65; 95% confidence interval (CI), 0.53–0.80 and RR, 0.63; 95% CI 0.54–0.74, respectively), and overweight men (RR, 0.77; 95% CI 0.64–0.92 and RR, 0.63; 95% CI 0.55–0.72, respectively) when compared with underweight/normal men. Additionally, obese men had significantly fewer wrist/forearm (RR, 0.77; 95% CI 0.61–0.97) and pelvic (RR, 0.44; 95% CI 0.28–0.70) fractures than underweight/normal men. Conversely, multiple rib fractures were more frequent in overweight (RR, 3.42; 95% CI 1.03–11.37) and obese (RR, 3.96; 95% CI 1.16–13.52) men | Obesity was associated with a reduced risk of clinical spine, pelvis, hip, and wrist/forearm fracture and increased risk of multiple rib fractures when compared to normal or underweight men |
| Ishii S, 2014 [51] | Japan | Cohort study | 1924 women, premenopausal or early perimenopausal | The relative increment in fracture hazard in obese women compared to normal weight women was also statistically significant: 78% (95% CI 13%–181%, p ¼ 0.01). In stark contrast, obesity was significantly associated with decreased fracture hazard when adjusted instead for any of the composite indices of femoral neck strength relative to load: relative decrement in fracture hazard in obese relative to low weight women was 57% (95% CI 24%–76%) after adjusting for CSI, 41% (95% CI 1%–65%) after adjusting for BSI, and 53% (95% CI 16%–74%) after adjusting for ISI | There are 3 major mechanisms by which obesity influences fracture risk: increased impact forces, increased BMD in response to greater skeletal loading, and greater absorption of impact forces by soft tissue padding |
| Søgaard AJ, 2015 [54] | Norway | Cohort study | 19,918 women and 23,061 men, aged 60–79 years | Compared to women with a BMI of <22 kg·m−2, the HR for hip fracture was 0.76 (95% CI 0.65–0.89) in women with a BMI between 22 and 24 kg·m−2, 0.56 (95% CI 0.48–0.65) in women with a BMI between 25 and 29 kg·m−2, and 0.42 (95% CI 0.35–0.51) in women with a BMI ≥ 30 kg·m−2. In men, the corresponding HRs for hip fracture were 0.62 (95% CI 0.50–0.77), 0.49 (95% CI 0.40–0.60) and 0.49 (95% CI 0.37–0.63), respectively | Abdominal obesity was associated with an increased risk of hip fracture when body mass index was taken into account |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palermo, A.; Tuccinardi, D.; Defeudis, G.; Watanabe, M.; D’Onofrio, L.; Lauria Pantano, A.; Napoli, N.; Pozzilli, P.; Manfrini, S. BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. Int. J. Environ. Res. Public Health 2016, 13, 544. https://doi.org/10.3390/ijerph13060544
Palermo A, Tuccinardi D, Defeudis G, Watanabe M, D’Onofrio L, Lauria Pantano A, Napoli N, Pozzilli P, Manfrini S. BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. International Journal of Environmental Research and Public Health. 2016; 13(6):544. https://doi.org/10.3390/ijerph13060544
Chicago/Turabian StylePalermo, Andrea, Dario Tuccinardi, Giuseppe Defeudis, Mikiko Watanabe, Luca D’Onofrio, Angelo Lauria Pantano, Nicola Napoli, Paolo Pozzilli, and Silvia Manfrini. 2016. "BMI and BMD: The Potential Interplay between Obesity and Bone Fragility" International Journal of Environmental Research and Public Health 13, no. 6: 544. https://doi.org/10.3390/ijerph13060544
APA StylePalermo, A., Tuccinardi, D., Defeudis, G., Watanabe, M., D’Onofrio, L., Lauria Pantano, A., Napoli, N., Pozzilli, P., & Manfrini, S. (2016). BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. International Journal of Environmental Research and Public Health, 13(6), 544. https://doi.org/10.3390/ijerph13060544








