Anthropometric Indices in Adults: Which Is the Best Indicator to Identify Alanine Aminotransferase Levels?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Blood Pressure Measurements
2.4. Anthropometric Measurements
2.5. Biochemical Measurements
2.6. Definition of Elevated Serum ALT
2.7. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Study Participants’ Characteristics According to ALT Level
3.3. Correlation of Anthropometric Indices with Serum ALT Levels
3.4. The Logistic Regression Models for Elevated ALT and Each Anthropometric Index
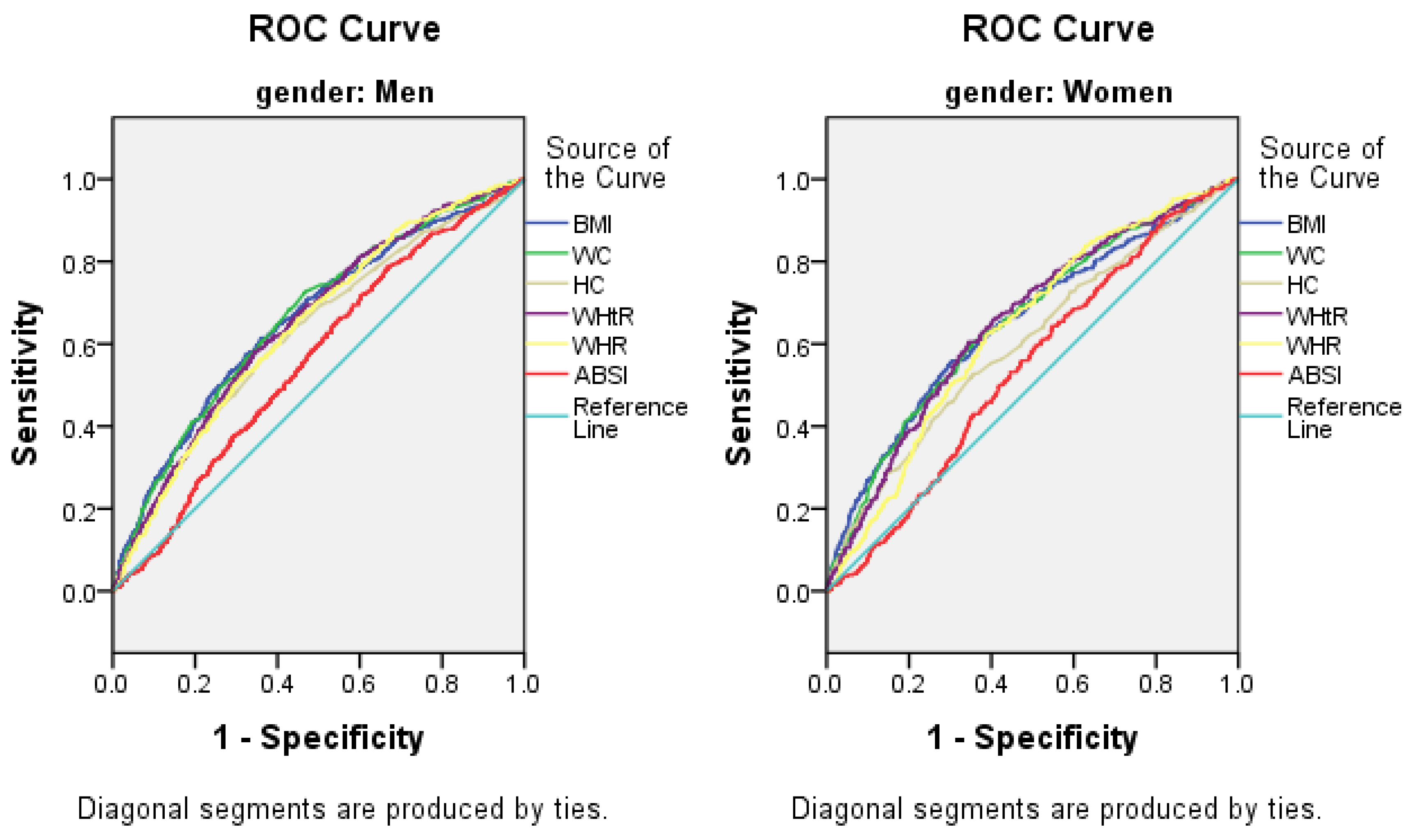
3.5. The AUCs (and 95% CIs) of Anthropometric Measures for the Presence of Elevated ALT
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| WC | waist circumference |
| BMI | body mass index |
| HC | hip circumference |
| WHtR | waist-to-height ratio |
| WHR | waist-to-hip ratio |
| ABSI | A Body Shape Index |
| TC | total cholesterol |
| TG | triacylglycerol |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| FPG | fasting plasma glucose |
| ALT | alanine aminotransferase |
| NAFLD | nonalcoholic fatty liver disease |
| OR | odds ratio |
| CI | confidence interval |
References
- Prati, D.; Taioli, E.; Zanella, A.; Della-Torre, E.; Butelli, S.; Del Vecchio, E.; Vianello, L.; Zanuso, F.; Mozzi, F.; Milani, S.; et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann. Intern. Med. 2002, 137, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.M. Alanine aminotransferase levels: What’s normal? Ann. Intern. Med. 2002, 137, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Goessling, W.; Massaro, J.M.; Vasan, R.S.; D’Agostino, R.B., Sr.; Ellison, R.C.; Fox, C.S. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 2008, 135, 1935.e1–1944.e1. [Google Scholar] [CrossRef] [PubMed]
- Schindhelm, R.K.; Dekker, J.M.; Nijpels, G.; Bouter, L.M.; Stehouwer, C.D.; Heine, R.J.; Diamant, M. Alanine aminotransferase predicts coronary heart disease events: A 10-year follow-up of the Hoorn Study. Atherosclerosis 2007, 191, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.E.; Shin, C.Y.; Yoon, Y.S.; Park, H.S. Elevated alanine aminotransferase levels predict mortality from cardiovascular disease and diabetes in Koreans. Atherosclerosis 2009, 205, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Schindhelm, R.K.; Diamant, M.; Dekker, J.M.; Tushuizen, M.E.; Teerlink, T.; Heine, R.J. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes/Metab. Res. Rev. 2006, 22, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Waters, O.R.; Knuiman, M.W.; Elliott, R.R.; Olynyk, J.K. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: An eleven-year follow-up study. Am. J. Gastroenterol. 2009, 104, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.K.; Nam, H.S.; Rhee, J.A.; Shin, J.H.; Kim, J.M.; Cho, K.H. Metabolic syndrome and ALT: A community study in adult Koreans. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Dorn, J.M.; Muti, P.; Freudenheim, J.L.; Farinaro, E.; Russell, M.; Nochajski, T.H.; Trevisan, M. Body fat distribution, relative weight, and liver enzyme levels: A population-based study. Hepatology 2004, 39, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Knuiman, M.W.; Divitini, M.L.; Olynyk, J.K. Body mass index is a stronger predictor of alanine aminotransaminase levels than alcohol consumption. J. Gastroenterol. Hepatol. 2008, 23, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Song, H.R.; Yun, K.E.; Park, H.S. Relation between alanine aminotransferase concentrations and visceral fat accumulation among nondiabetic overweight Korean women. Am. J. Clin. Nutr. 2008, 88, 16–21. [Google Scholar] [PubMed]
- Rocha, R.; Cotrim, H.P.; Carvalho, F.M.; Siqueira, A.C.; Braga, H.; Freitas, L.A. Body mass index and waist circumference in non-alcoholic fatty liver disease. J. Hum. Nutr. Diet. 2005, 18, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Bastien, M.; Poirier, P.; Lemieux, I.; Despres, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; Batsis, J.A.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.; Seidell, J.C.; Meinders, A.E. The value and limitations of the body mass index (BMI) in the assessment of the health risks of overweight and obesity. Ned. Tijdschr. Geneeskd. 2004, 148, 2379–2382. [Google Scholar] [PubMed]
- Prentice, A.M.; Jebb, S.A. Beyond body mass index. Obes. Rev. 2001, 2, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nevill, A.M.; Stewart, A.D.; Olds, T.; Holder, R. Relationship between adiposity and body size reveals limitations of BMI. Am. J. Phys. Anthropol. 2006, 129, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Allison, D.B.; Kotler, D.P.; Ross, R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am. J. Clin. Nutr. 2002, 75, 683–688. [Google Scholar] [PubMed]
- Hsieh, S.D.; Yoshinaga, H. Do people with similar waist circumference share similar health risks irrespective of height? Tohoku J. Exp. Med. 1999, 188, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Kengne, A.P.; Stamatakis, E.; Hamer, M.; Batty, G.D. Body mass index, waist circumference and waist-hip ratio: Which is the better discriminator of cardiovascular disease mortality risk? Evidence from an individual-participant meta-analysis of 82,864 participants from nine cohort studies. Obes. Rev. 2011, 12, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Dobbelsteyn, C.J.; Joffres, M.R.; MacLean, D.R.; Flowerdew, G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjonneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, N.Y.; Krakauer, J.C. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE 2012, 7, e39504. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Petrie, J.; Littler, W.; de Swiet, M.; Padfield, P.L.; Altman, D.G.; Bland, M.; Coats, A.; Atkins, N. An outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J. Hypertens 1993, 11, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Chen, L.Y.; Dai, H.L.; Chen, J.H.; Fang, L.Z. Relationship between alanine aminotransferase levels and metabolic syndrome in nonalcoholic fatty liver disease. J. Zhejiang Univ. Sci. B 2008, 9, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection E. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497.
- Tamura, S.; Shimomura, I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Degrace, P.; Demizieux, L.; Du, Z.Y.; Gresti, J.; Caverot, L.; Djaouti, L.; Jourdan, T.; Moindrot, B.; Guilland, J.C.; Hocquette, J.F.; et al. Regulation of lipid flux between liver and adipose tissue during transient hepatic steatosis in carnitine-depleted rats. J. Biol. Chem. 2007, 282, 20816–20826. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, C.E.; Everhart, J.E. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin. Gastroenterol. Hepatol. 2005, 3, 1260–1268. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Sattar, N.; Smith, G.D.; Ebrahim, S. The associations of physical activity and adiposity with alanine aminotransferase and gamma-glutamyltransferase. Am. J. Epidemiol. 2005, 161, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Cole, T.J.; Dixon, A.K. Ratio of waist circumference to height is strong predictor of intra-abdominal fat. BMJ Clin. Res. Ed. 1996, 313, 559–560. [Google Scholar] [CrossRef]
- Kim, J.; Jo, I. Relationship between body mass index and alanine aminotransferase concentration in non-diabetic Korean adults. Eur. J. Clin. Nutr. 2010, 64, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; McGreal, N.; Deutsch, R.; Finegold, M.J.; Lavine, J.E. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics 2005, 115, e561–e565. [Google Scholar] [CrossRef] [PubMed]
- Sabir, N.; Sermez, Y.; Kazil, S.; Zencir, M. Correlation of abdominal fat accumulation and liver steatosis: Importance of ultrasonographic and anthropometric measurements. Eur. J. Ultrasound 2001, 14, 121–128. [Google Scholar] [CrossRef]
- Chan, D.F.; Li, A.M.; Chu, W.C.; Chan, M.H.; Wong, E.M.; Liu, E.K.; Chan, I.H.; Yin, J.; Lam, C.W.; Fok, T.F.; et al. Hepatic steatosis in obese Chinese children. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
| Variables | Men | Women | p Value | Total |
|---|---|---|---|---|
| (n = 5243) | (n = 6088) | (N = 11,331) | ||
| Age, year | 54.4 ± 10.8 | 53.4 ± 10.3 | <0.001 * | 53.8 ± 10.6 |
| Race (Han), % | 4966 (94.7) | 5779 (94.9) | 0.324 | 10,745 (94.8) |
| Clinical characteristics | ||||
| Current smoking status, % | 2995 (57.1) | 1004 (16.5) | <0.001 * | 3999 (35.3) |
| Current drinking status, % | 2385 (45.5) | 176 (2.9) | <0.001 * | 2561 (22.6) |
| Education, % | <0.001 * | |||
| Primary school or below | 2191 (41.8) | 3456 (56.8) | 5647 (49.8) | |
| Middle school | 2454 (46.8) | 2162 (35.5) | 4616 (40.7) | |
| High school or above | 598 (11.4) | 470 (7.7) | 1068 (9.4) | |
| Physical activity, % | <0.001 * | |||
| Low | 1180 (22.5) | 2188 (35.9) | 3368 (29.7) | |
| Moderate | 3775 (72.0) | 3555 (58.4) | 7330 (64.7) | |
| High | 288 (5.5) | 345 (5.7) | 633 (5.6) | |
| Family income, CNY/year, % | 0.024 # | |||
| ≤5000 | 696 (13.3) | 707 (11.6) | 1403 (12.4) | |
| 5000–20,000 | 2819 (53.8) | 3366 (55.3) | 6185 (54.6) | |
| >20,000 | 1728 (33.0) | 2015 (33.1) | 3743 (33.0) | |
| SBP, mm·Hg | 143.6 ± 22.6 | 140.5 ± 24.0 | <0.001 * | 141.7 ± 23.4 |
| DBP, mm·Hg | 83.7 ± 11.8 | 80.5 ± 11.5 | <0.001 * | 82.0 ± 11.8 |
| Anthropometric indices | ||||
| Weight, kg | 68.6 ± 11.1 | 60.3 ± 10.1 | <0.001 * | 64.1 ± 11.4 |
| Height, m | 166.4 ± 6.3 | 155.6 ± 6.1 | <0.001 * | 160.6 ± 8.2 |
| HC, cm | 96.2 ± 7.1 | 95.4 ± 7.5 | <0.001 * | 95.8 ± 7.3 |
| WC, cm | 83.7 ± 9.7 | 81.2 ± 9.7 | <0.001 * | 82.4 ± 9.8 |
| BMI, kg/m2 | 24.7 ± 3.5 | 24.9 ± 3.8 | 0.059 | 24.8 ± 3.7 |
| WHtR | 0.50 ± 0.06 | 0.52 ± 0.06 | <0.001 * | 0.51 ± 0.06 |
| WHR | 0.87 ± 0.08 | 0.85 ± 0.07 | <0.001 * | 0.86 ± 0.08 |
| ABSI | 0.0766 ± 0.0048 | 0.0767 ± 0.0055 | 0.525 | 0.0767 ± 0.0052 |
| Laboratory data | ||||
| TC, mmol/L | 5.2 ± 1.0 | 5.3 ± 1.1 | <0.001 * | 5.2 ± 1.1 |
| TG, mmol/L | 1.7 ± 1.6 | 1.6 ± 1.3 | 0.185 | 1.6 ± 1.5 |
| HDL-C, mmol/L | 1.4 ± 0.4 | 1.4 ± 0.3 | 0.722 | 1.4 ± 0.4 |
| LDL-C, mmol/L | 2.9 ± 0.8 | 3.0 ± 0.8 | <0.001 * | 2.9 ± 0.8 |
| FPG, mmol/L | 6.0 ± 1.7 | 5.9 ± 1.6 | 0.004 # | 5.9 ± 1.6 |
| Serum uric acid, umol/L | 333.7 ± 83.5 | 255.7 ± 67.7 | <0.001 * | 291.8 ± 84.4 |
| ALT, U/L | 25.6 ± 22.4 | 19.7 ± 13.0 | <0.001 * | 22.4 ± 18.2 |
| Variables | Men (n = 5243) | p Value | Women (n = 6088) | p Value | ||
|---|---|---|---|---|---|---|
| ALT ≤ 40 | ALT > 40 | ALT ≤ 40 | ALT > 40 | |||
| (n = 3375) | (n = 1432) | (n = 3375) | (n = 1432) | |||
| Age, year | 54.9 ± 10.8 | 49.7 ± 9.4 | <0.001 * | 54.4 ± 10.4 | 53.3 ± 9.0 | 0.936 |
| Race (Han), % | 4443 (94.7) | 523 (95.1) | 0.763 | 5505 (94.9) | 274 (95.8) | 0.581 |
| Clinical characteristics | ||||||
| Current smoking status, % | 2726 (58.1) | 269 (48.9) | <0.001 * | 957 (16.5) | 47 (16.4) | 0.528 |
| Current drinking status, % | 2116 (45.1) | 269 (48.9) | 0.094 | 167 (2.9) | 9 (3.1) | 0.718 |
| Education, % | <0.001 * | 0.111 | ||||
| Primary school or below | 200 (42.8) | 182 (33.1) | 3277 (56.5) | 179 (62.6) | ||
| Middle school | 2162 (46.1) | 292 (53.1) | 2072 (35.7) | 90 (31.5) | ||
| High school or above | 522 (11.1) | 76 (13.8) | 453 (7.8) | 17 (5.9) | ||
| Physical activity, % | 0.580 | 0.049 # | ||||
| Low | 1061 (22.6) | 119 (21.6) | 2066 (35.6) | 122 (42.7) | ||
| Moderate | 3370 (71.8) | 405 (73.6) | 3407 (58.7) | 148 (51.7) | ||
| High | 262 (5.6) | 26 (4.7) | 329 (5.7) | 16 (5.6) | ||
| Family income, CNY/year, % | <0.001 * | 0.715 | ||||
| ≤5000 | 654 (13.9) | 42 (7.6) | 677 (11.7) | 30 (10.5) | ||
| 5000–20,000 | 2548 (54.3) | 271 (49.3) | 3210 (55.3) | 156 (54.5) | ||
| >20,000 | 1491 (31.8) | 237 (43.1) | 1915 (33.0) | 100 (35.0) | ||
| SBP, mm·Hg | 143.4 ± 22.8 | 145.5 ± 20.3 | 0.021 # | 139.9 ± 24.0 | 142.6 ± 24.2 | 0.063 |
| DBP, mm·Hg | 83.8 ± 11.7 | 87.5 ± 11.9 | <0.001 * | 80.4 ± 11.5 | 82.6 ± 11.4 | 0.002 # |
| Anthropometric indices | ||||||
| Weight, kg | 67.9 ± 10.7 | 74.5 ± 12.7 | <0.001 * | 60.0 ± 9.9 | 65.6 ± 12.1 | <0.001 * |
| Height, m | 166.3 ± 6.4 | 167.5 ± 6.1 | <0.001 * | 155.6 ± 6.1 | 155.9 ± 6.0 | 0.455 |
| HC, cm | 95.9 ± 7.0 | 98.7 ± 7.2 | <0.001 * | 95.3 ± 7.4 | 98.1 ± 7.9 | <0.001 * |
| WC, cm | 83.2 ± 9.5 | 88.8 ± 9.8 | <0.001 * | 81.0 ± 9.6 | 86.4 ± 10.1 | <0.001 * |
| BMI, kg/m2 | 24.5 ± 3.4 | 26.5 ± 4.0 | <0.001 * | 24.7 ± 3.7 | 26.9 ± 4.4 | <0.001 * |
| WHtR | 0.50 ± 0.06 | 0.53 ± 0.06 | <0.001 * | 0.52 ± 0.06 | 0.55 ± 0.06 | <0.001 * |
| WHR | 0.87 ± 0.08 | 0.90 ± 0.06 | <0.001 * | 0.85 ± 0.08 | 0.88 ± 0.06 | <0.001 * |
| ABSI | 0.0765 ± 0.0049 | 0.0773 ± 0.0043 | <0.001 * | 0.0767 ± 0.0056 | 0.0772 ± 0.0045 | <0.001 * |
| Laboratory data | ||||||
| TC, mmol/L | 5.1 ± 1.0 | 5.4 ± 1.2 | <0.001 * | 5.3 ± 1.1 | 5.8 ± 1.4 | <0.001 * |
| TG, mmol/L | 1.6 ± 1.5 | 2.4 ± 2.2 | <0.001 * | 1.6 ± 1.3 | 2.2 ± 1.9 | <0.001 * |
| HDL-C, mmol/L | 1.4 ± 0.4 | 1.4 ± 0.3 | 0.002 # | 1.4 ± 0.3 | 1.3 ± 0.4 | <0.001 * |
| LDL-C, mmol/L | 2.9 ± 0.8 | 3.0 ± 0.9 | <0.001 * | 3.0 ± 0.8 | 3.3 ± 1.0 | <0.001 * |
| FPG, mmol/L | 5.9 ± 1.7 | 6.1 ± 1.5 | 0.118 | 5.8 ± 1.6 | 6.2 ± 1.6 | 0.001 # |
| Serum uric acid, umol/L | 329.7 ± 81.2 | 368.4 ± 94.1 | <0.001 * | 254.3 ± 66.8 | 283.3 ± 78.2 | <0.001 * |
| ALT, U/L | 20.7 ± 7.6 | 67.2 ± 48.5 | <0.001 * | 17.6 ± 6.9 | 61.6 ± 28.6 | 0.036 # |
| Anthropometric Measurements | Men (n = 5243) | Women (n = 6088) | ||
|---|---|---|---|---|
| Coefficient (r) | p Value | Coefficient (r) | p Value | |
| BMI, kg/m2 | 0.330 | <0.001 * | 0.269 | <0.001 * |
| WC, cm | 0.310 | <0.001 * | 0.269 | <0.001 * |
| HC, cm | 0.276 | <0.001 * | 0.198 | <0.001 * |
| WHtR | 0.346 | <0.001 * | 0.282 | <0.001 * |
| WHR | 0.253 | <0.001 * | 0.236 | <0.001 * |
| ABSI | 0.101 | <0.001 * | 0.093 | <0.001 * |
| Quintile (Men) | BMI | WC | HC | WHtR | WHR | ABSI |
| 1 (Reference) | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1.79 (1.34, 2.40) * | 1.09 (0.67, 1.77) | 1.51 (1.14, 2.01) # | 1.82 (1.27, 2.61) # | 1.27 (0.92, 1.76) | 1.15 (0.85, 1.56) |
| 3 | 2.69 (2.03, 3.57) * | 2.01 (1.31, 3.08) # | 1.94 (1.47, 2.56) * | 2.53 (1.79, 3.58) * | 2.06 (1.52, 2.79) * | 1.61 (1.21, 2.13) # |
| 4 | 4.17 (3.15, 5.54) * | 3.69 (2.47, 5.53) * | 2.72 (2.06, 3.58) * | 4.38 (3.15, 6.08) * | 3.40 (2.54, 4.53) * | 2.51 (1.93, 3.27) * |
| Quintile (Women) | BMI | WC | HC | WHtR | WHR | ABSI |
| 1 (Reference) | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 2.08 (1.38, 3.16) # | 1.16 (0.83, 1.62) | 1.19 (0.81, 1.75) | 1.58 (1.02, 2.45) # | 1.06 (0.68, 1.63) | 1.26 (0.87, 1.81) |
| 3 | 3.47 (2.34, 5.14) * | 2.33 (1.74, 3.11) * | 1.46 (1.03, 2.08) # | 2.17 (1.43, 3.30) * | 1.55 (1.04, 2.32) # | 1.78 (1.26, 2.53) # |
| 4 | 4.15 (2.78, 6.19) * | 3.66 (2.77, 4.83) * | 2.32 (1.64, 3.27) * | 4.29 (2.91, 6.33) * | 3.39 (2.38, 4.85) * | 1.41 (0.96, 2.06) |
| Anthropometric Measurements | Men (n = 5243) | Women (n = 6088) | ||
|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | |
| BMI, kg/m2 | 0.658 | 0.633–0.683 | 0.651 | 0.616–0.685 |
| WC, cm | 0.651 | 0.627–0.674 | 0.653 | 0.621–0.685 |
| HC, cm | 0.626 | 0.601–0.651 | 0.602 | 0.568–0.637 |
| WHtR | 0.664 | 0.640–0.688 | 0.655 | 0.622–0.688 |
| WHR | 0.641 | 0.618–0.665 | 0.635 | 0.604–0.665 |
| ABSI | 0.561 | 0.537–0.585 | 0.542 | 0.511–0.573 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Guo, X.; Yu, S.; Zhou, Y.; Li, Z.; Sun, Y. Anthropometric Indices in Adults: Which Is the Best Indicator to Identify Alanine Aminotransferase Levels? Int. J. Environ. Res. Public Health 2016, 13, 226. https://doi.org/10.3390/ijerph13020226
Chen S, Guo X, Yu S, Zhou Y, Li Z, Sun Y. Anthropometric Indices in Adults: Which Is the Best Indicator to Identify Alanine Aminotransferase Levels? International Journal of Environmental Research and Public Health. 2016; 13(2):226. https://doi.org/10.3390/ijerph13020226
Chicago/Turabian StyleChen, Shuang, Xiaofan Guo, Shasha Yu, Ying Zhou, Zhao Li, and Yingxian Sun. 2016. "Anthropometric Indices in Adults: Which Is the Best Indicator to Identify Alanine Aminotransferase Levels?" International Journal of Environmental Research and Public Health 13, no. 2: 226. https://doi.org/10.3390/ijerph13020226
APA StyleChen, S., Guo, X., Yu, S., Zhou, Y., Li, Z., & Sun, Y. (2016). Anthropometric Indices in Adults: Which Is the Best Indicator to Identify Alanine Aminotransferase Levels? International Journal of Environmental Research and Public Health, 13(2), 226. https://doi.org/10.3390/ijerph13020226






