Characterization of the Particle Size Fraction associated with Heavy Metals in Suspended Sediments of the Yellow River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
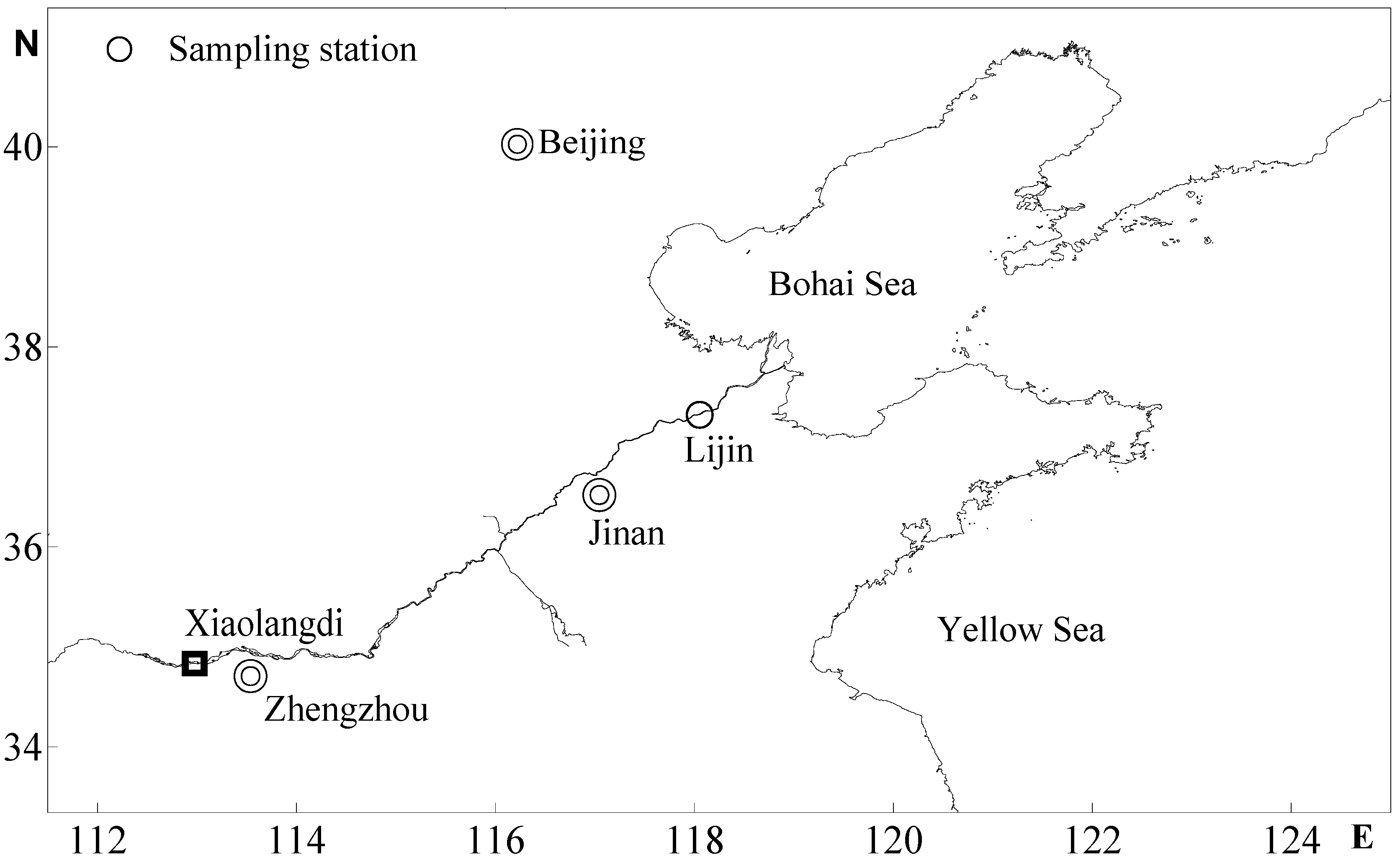
2.2. Analytical Methods
2.3. Assessment Methods
2.3.1. Enrichment Factor (EFx)
2.3.2. Distribution Factor (DFX)
2.3.3. Mass Loading
3. Result and Discussion
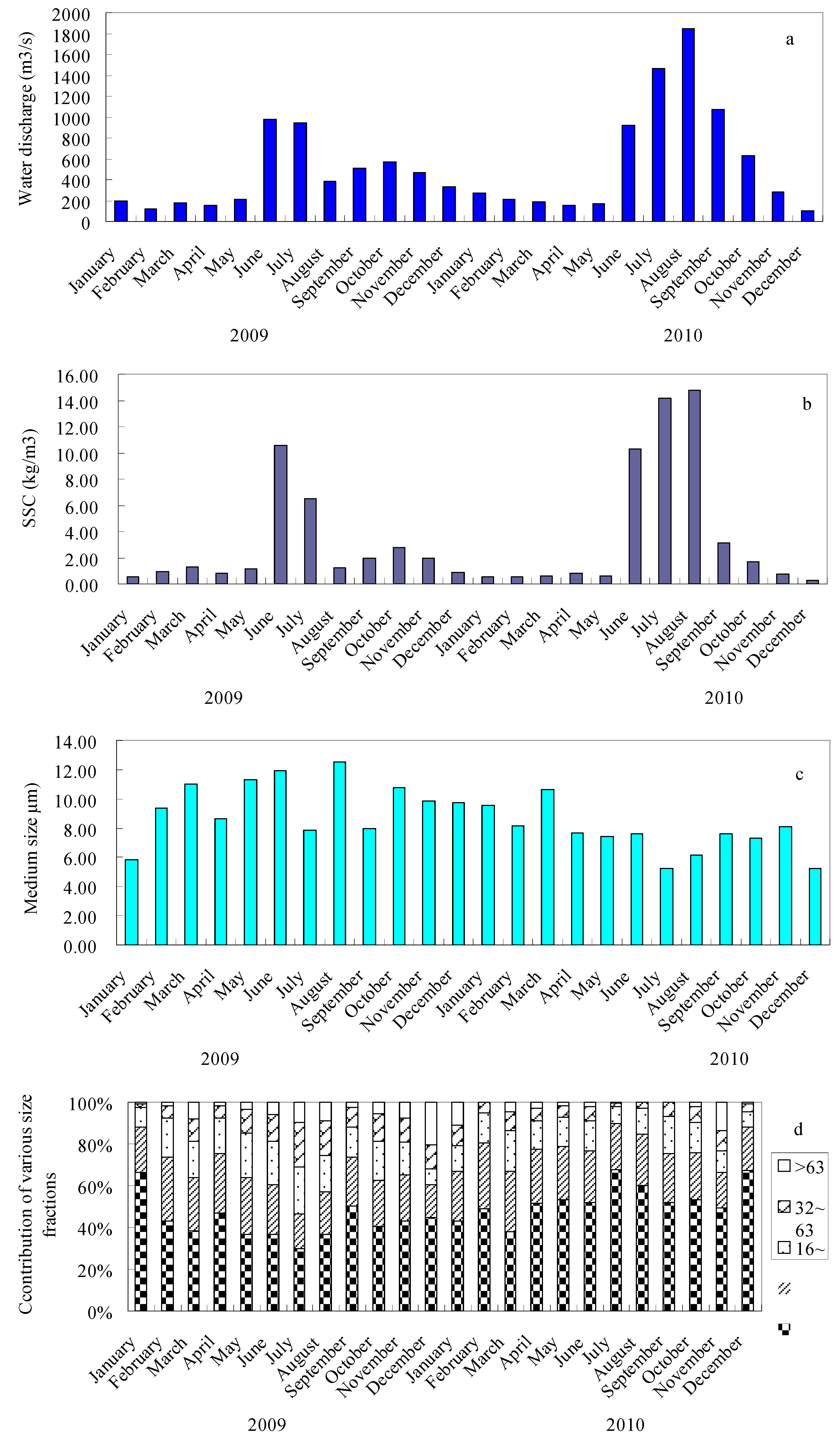
3.1. Particle Size Distribution of Suspended Sediments
3.2. Heavy Metals Variability and Enrichment
| Heavy Metal | Minimum | Maximum | Mean | Background Concentration in Chinese Soils a |
|---|---|---|---|---|
| Cu | 27(1.19) | 43(1.90) | 34 | 22.6 |
| Pb | 21(0.81) | 34(1.31) | 27 | 26 |
| Zn | 86(1.16) | 122(1.64) | 97 | 74.2 |
| Cd | 0.15(1.55) | 0.36(3.71) | 0.23 | 0.097 |
| Cr | 59(0.97) | 99(1.62) | 77 | 61 |
| Mn | 0.058(0.99) | 0.111(1.90) | 0.0912 | 0.0583 |
| Fe | 2.88(0.97) | 4.95(1.67) | 4.13 | 2.97 |
| Sample Time | Cu | Pb | Zn | Cr | Mn | Data Sources |
|---|---|---|---|---|---|---|
| 1980s | 26.7 | 16.4 | 69.8 | 76.9 | 767 | [16,19] |
| 1997–1998 | 17.6 | 29.5 | 60.2 | 65.4 | 431.1 | [33] |
| Nov. 2000 | 22.99 | 19.38 | 76.78 | 600 | [18] | |
| Aug. 2001 | 10.31 | 10.82 | 70.03 | 500 | [18] | |
| Jun. 2009 | 24.0 | 63.1 | 44.7 | 459 | [21] | |
| 2009–2010 | 34.0 | 27.0 | 97.0 | 77.0 | 912 | This study |
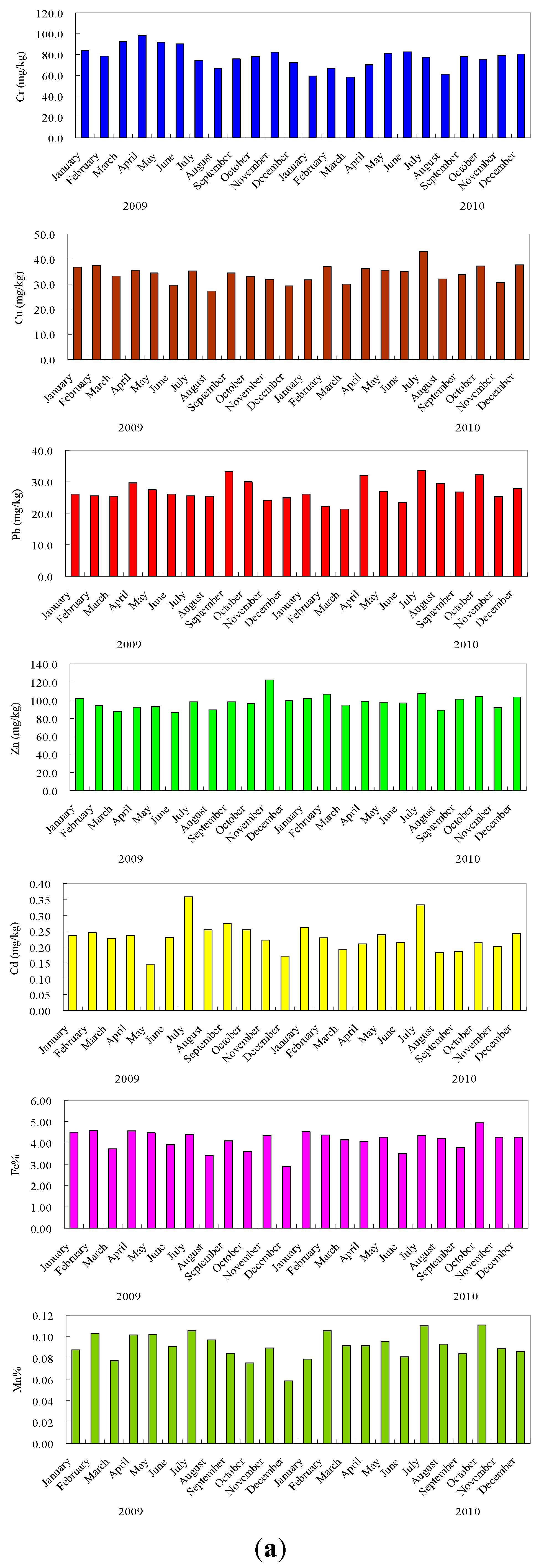
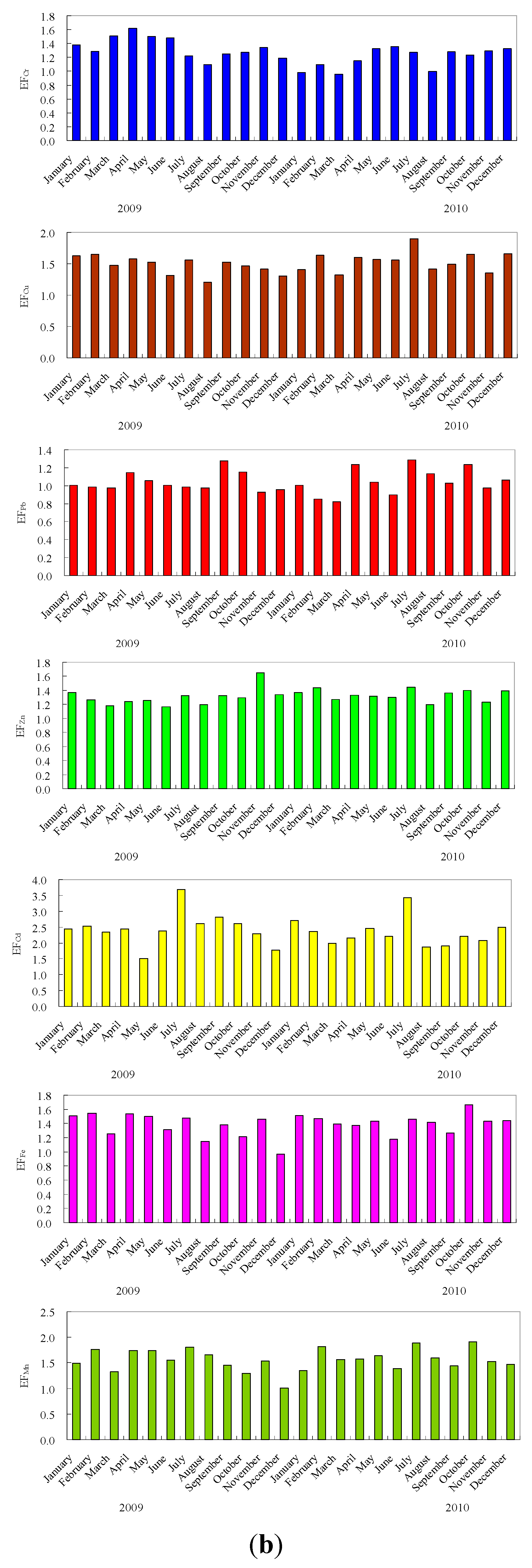
3.3. Distribution of Metals in Particle Size Fractions
| Heavy Metal | <8 µm | 8–16 µm | 16–32 µm | 32–63 µm | >63 µm | |
|---|---|---|---|---|---|---|
| Cu | Concentration | 36–44 | 32–43 | 20–39 | 10–30 | 8-22 |
| Mean | 40 (1.18) | 37 (1.09) | 33 (0.97) | 15 (0.44) | 11 (0.32) | |
| Pb | Concentration | 22–38 | 21–39 | 14–35 | 10–25 | 9–17 |
| Mean | 30 (1.15) | 30(1.15) | 25 (0.96) | 16 (0.62) | 13 (0.50) | |
| Zn | Concentration | 98–150 | 80–127 | 66–126 | 37–73 | 31–65 |
| Mean | 114(1.16) | 100 (1.02) | 88 (0.90) | 52 (0.53) | 41 (0.42) | |
| Cd | Concentration | 0.17–0.36 | 0.15–0.35 | 0.13–0.34 | 0.07–0.24 | 0.06–0.22 |
| Mean | 0.26 (1.13) | 0.24 (1.04) | 0.21 (0.91) | 0.13 (0.57) | 0.13 (0.57) | |
| Cr | Concentration | 62–114 | 54–111 | 51–90 | 32–95 | 23–72 |
| Mean | 85 (1.10) | 81 (1.05) | 71 (0.92) | 53 (0.69) | 45 (0.58) | |
| Mn | Concentration | 0.078–0.111 | 0.076–0.111 | 0.039–0.106 | 0.030–0.082 | 0.030–0.072 |
| Mean | 0.092 (1.12) | 0.091 (1.11) | 0.084 (1.02) | 0.048 (0.059) | 0.038 (0.46) | |
| Fe | Concentration | 3.71–4.90 | 3.21–4.48 | 2.01–4.06 | 1.63–3.37 | 1.60–3.07 |
| Mean | 4.49 (1.14) | 4.03 (1.02) | 3.06 (0.77) | 2.41 (0.61) | 2.02 (0.51) | |
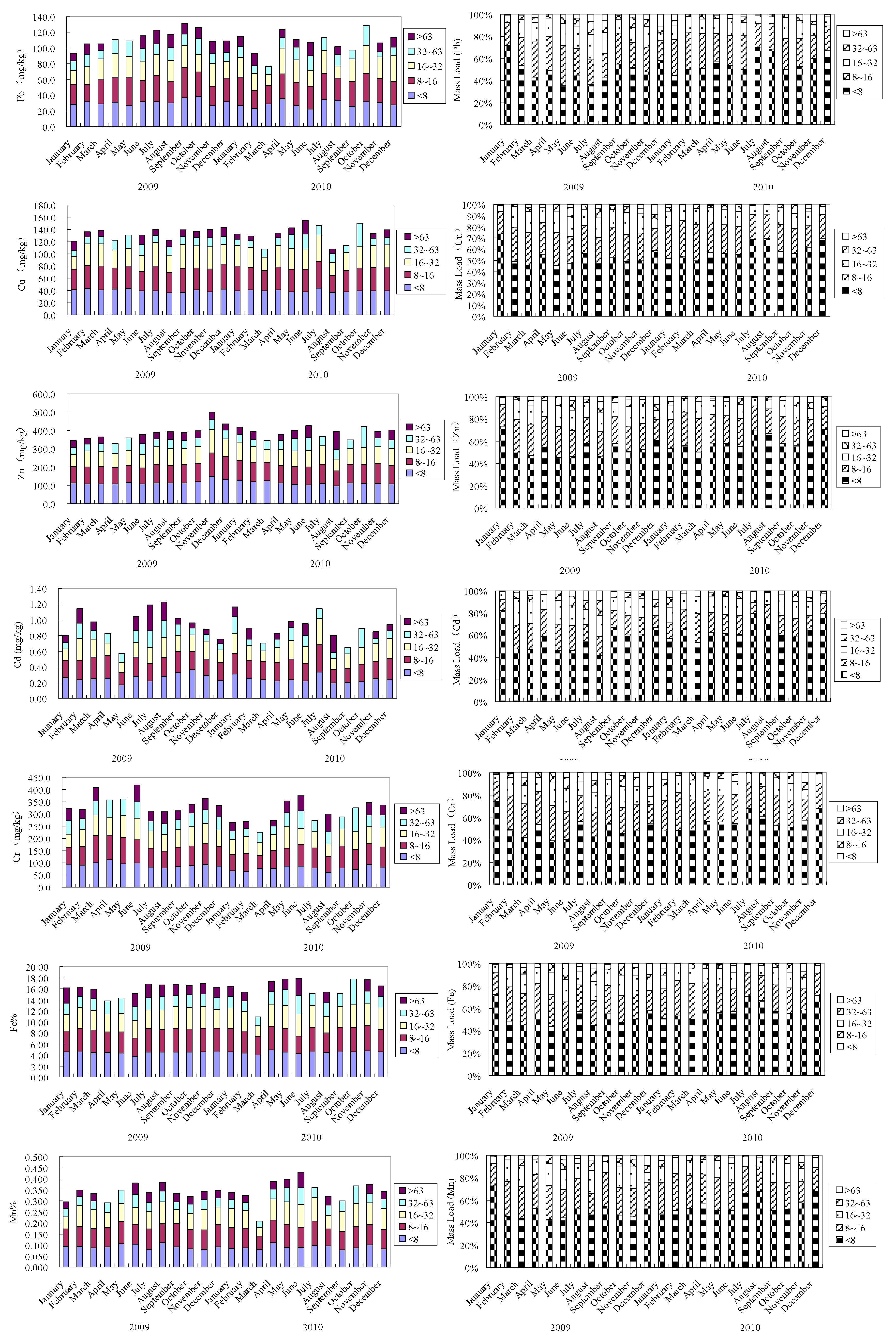
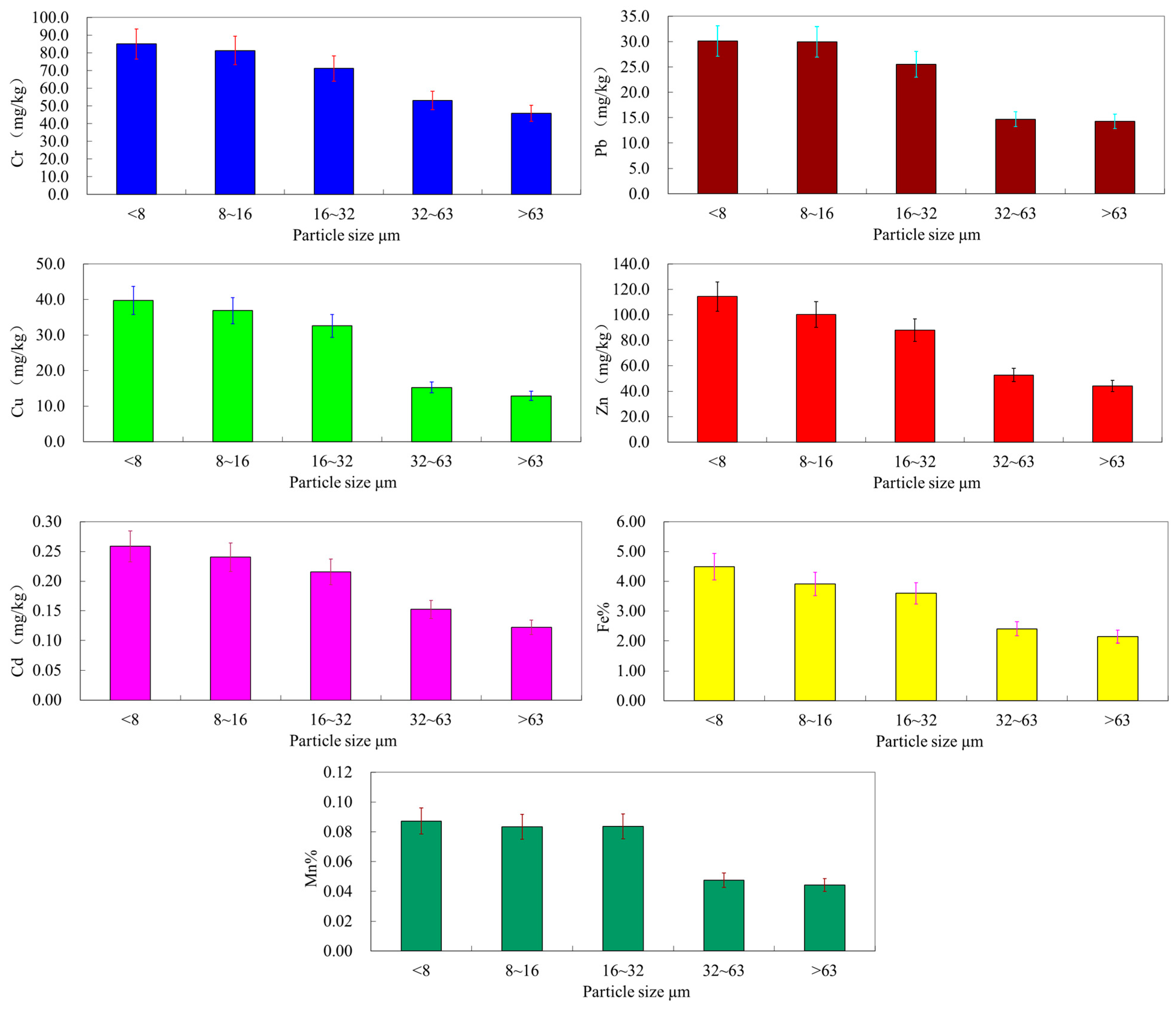
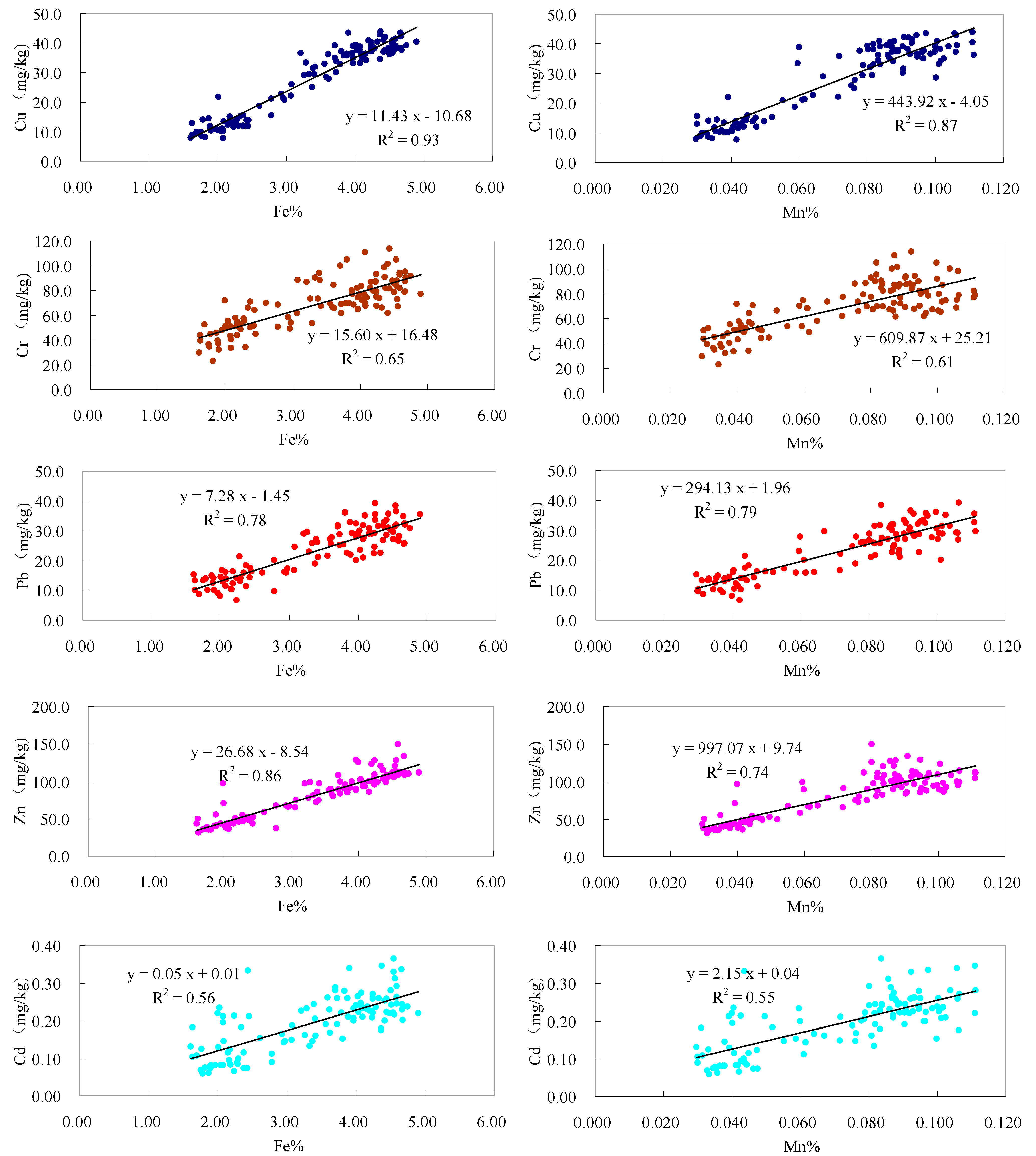
3.4. Importance of Particle Size Fractions in the Transport of Heavy Metal to the Bohai Sea
| Heavy Metal | <8µm | 8~16µm | 16~32µm | 32~63µm | >63µm | Total | |
|---|---|---|---|---|---|---|---|
| Cu | 2009 | 949 | 434 | 236 | 113 | 60 | 1792 |
| 2010 | 2414 | 1104 | 601 | 287 | 151 | 4557 | |
| Pb | 2009 | 787 | 360 | 196 | 94 | 49 | 1486 |
| 2010 | 1923 | 880 | 479 | 229 | 120 | 3631 | |
| Zn | 2009 | 2761 | 1263 | 687 | 329 | 173 | 5213 |
| 2010 | 6505 | 2976 | 1620 | 774 | 407 | 12282 | |
| Cd | 2009 | 8.0 | 3.7 | 2.0 | 1.0 | 0.4 | 15.1 |
| 2010 | 15.8 | 7.2 | 3.9 | 1.9 | 1.0 | 29.8 | |
| Cr | 2009 | 2466 | 1128 | 614 | 293 | 154 | 4655 |
| 2010 | 4841 | 2215 | 1205 | 576 | 302 | 9139 | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Azimi, S.; Rocher, V.; Muller, M.; Moilleron, R.; Thevenot, D.R. Sources, distribution and variability of hydrocarbons and metals in atmospheric deposition in an urban area (Paris, France). Sci. Total Environ. 2005, 337, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Waeles, M.; Riso, R.D.; Le Corre, P. Distribution and seasonal changes of lead in an estuarine system affected by agricultural practices: The Penzé Estuary, NW France. Estuar. Coast. Shelf Sci. 2007, 74, 570–578. [Google Scholar] [CrossRef]
- Milliman, J.D.; Syvitski, J.P. Geomorphic/tectonic control of sediment discharge to the ocean: The importance of small mountainous rivers. J. Geol. 1992, 100, 525–544. [Google Scholar] [CrossRef]
- Zhang, J. Heavy metal compositions of suspended sediments in the Changjiang (Yangtze River) estuary: Significance of riverine transport to the ocean. Cont. Shelf Res. 1999, 19, 1521–1543. [Google Scholar] [CrossRef]
- Feng, H.; Han, X.; Zhang, W.; Yu, L. A preliminary study of heavymetal contamination in Yangtze River intertidal zone due to urbanization. Mar. Pollut. Bull. 2004, 49, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Lin, F.J. Elemental characteristics of surface suspended particulates off the Changjiang estuary during the 1998 flood. J. Mar. Syst. 2010, 81, 323–334. [Google Scholar] [CrossRef]
- Song, Y.; Ji, J.; Mao, C.; Yang, Z.; Yuan, X.; Ayoko, G.A.; Frost, R.L. Heavymetal contamination in suspended solids of Changjiang River—Environmental implications. Geoderma 2009, 159, 286–295. [Google Scholar] [CrossRef]
- Weber, F.A.; Voegelin, A.; Kretzschmar, R. Multi-metal contaminant dynamics in temporarily flooded soil under sulfate limitation. Geochim. Cosmochim. Acta 2009, 73, 5513–5527. [Google Scholar] [CrossRef]
- Rocha, L.; Rodrigues, S.M.; Lopes, I.; Soares, A.M.V.M.; Duarte, A.C.; Pereira, E. The water-soluble fraction of potentially toxic elements in contaminated soils: Relationships between ecotoxicity, solubility and geochemical reactivity. Chemosphere 2011, 84, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Cabrera, J.C.; Georgiadis, M.; Jayachandran, K. Assessment of arsenic mobility in the soils of some golf courses in South Florida. Sci. Total Environ. 2002, 291, 123–134. [Google Scholar] [CrossRef]
- Ljung, K.; Selinus, O.; Otabbong, E.; Berglund, M. Metal and arsenic distribution in soil particle sizes relevant to soil ingestion by children. Appl. Geochem. 2006, 21, 1613–1624. [Google Scholar] [CrossRef]
- Semlali, R.M.; van Oort, F.; Denaix, L.M.L. Estimating distributions of endogenous and exogenous Pb in soils by using Pb isotopic ratios. Environ. Sci. Technol. 2001, 35, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.Z. Water Resources of Yellow River; Yellow River Water Conservancy Press: Zhengzhou, China, 1996. (In Chinese) [Google Scholar]
- Dai, Y.L.; Wen, T.M. The soil and water conservation, ecology and environmental construction on the Loess Plateau. Inf. Work Soil Water Conversat. 2000, 2, 32–34. (In Chinese) [Google Scholar]
- Yang, Z.; Cheng, Y.; Wang, H. The Geology of China; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Zhang, J.; Huang, W.W.; Liu, M.G.; Zhou, Q. Drainage basin weathering and major element transport of two large Chinese rivers (Huanghe and Changjiang). J. Geophys. Res. 1990, 95, 13277–13288. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, J. Effect of particle size on transition metal concentrations in the Changjiang (Yangtze River) and the Huanghe (Yellow River), China. Sci. Total Environ. 1990, 94, 187–207. [Google Scholar]
- Qiao, S.; Yang, Z.; Pan, Y.; Guo, Z. Metals in suspended sediments from the Changjiang (Yangtze River) and Huanghe (Yellow River) to the sea, and their comparison. Estuar. Coast. Shelf Sci. 2007, 74, 539–548. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, J.; Zhou, Z. Particulate element inventory of the Huanghe (Yellow River): A large, high-turbidity river. Geochim. Cosmochim. Acta 1992, 56, 3669–3680. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C. Riverine composition and estuarine geochemistry of particulate metals in China—Weathering features, anthropogenic impact and chemical fluxes. Estuar. Coast. Shelf Sci. 2002, 54, 1051–1070. [Google Scholar] [CrossRef]
- Bi, N.; Yang, Z.; Wang, H.; Xu, C.; Guo, Z. Impact of artificial water and sediment discharge regulation in the Huanghe (Yellow River) on the transport of particulate heavy metals to the sea. Catena 2014, 121, 232–240. [Google Scholar] [CrossRef]
- Walling, D.E.; Woodward, J.C. Use of a field-based water elutriation system for monitoring the in situ particle size characteristics of fluvial suspended sediment. Water Res. 1993, 23, 1413–1421. [Google Scholar] [CrossRef]
- Li, Y.; Gou, X.; Wang, G.; Zhang, Q.; Su, Q.; Xiao, G. Heavy metal contamination and source in arid agricultural soil in central Gansu Province, China. J. Environ. Sci. 2008, 20, 607–612. [Google Scholar] [CrossRef]
- Chan, L.S.; Ng, S.L.; Davis, A.M.; Yim, W.W.S.; Yeung, C.H. Magnetic properties and heavy-metal contents of contaminated seabed sediments of Penny’s Bay, Hong Kong. Pollut. Bull. 2001, 42, 569–583. [Google Scholar] [CrossRef]
- Wei, F.; Chen, J.; Wu, Y.; Zheng, C. Study the background contents on 61 elements of soils in China. Chin. J. Environ. Sci. 1991, 12, 12–19. [Google Scholar]
- Acosta, J.A.; Faz, A.; Kalbitz, K.; Jansen, B.; Martinez-Martinez, S. Heavy metal concentrations in particle size fractions from street dust of Murcia (Spain) as the basis for risk assessment. J. Environ. Monit. 2011, 13, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.A.; Cano, A.F.; Arocena, J.M.; Debela, F.; Martínez-Martínez, S. Distribution of metals in soil particle size fractions and its implication to risk assessment of playgrounds in Murcia City (Spain). Geoderma 2009, 149, 101–109. [Google Scholar] [CrossRef]
- Sutherland, R.A. Lead in grain size fractions of road-deposited sediment. Environ. Pollut. 2003, 121, 229–237. [Google Scholar] [CrossRef]
- Davis, H.T.; Aelion, C.M.; McDermott, S.; Lawson, A.B. Identifying natural and anthropogenic sources of metals in urban and rural soils using GIS-based data, PCA, and spatial interpolation. Environ. Pollut. 2009, 157, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Manta, D.S.; Angelone, M.; Bellanca, A.; Neri, R.; Sprovieri, M. Heavy metals in urban soils: A case study from the city of Palermo (Sicily), Italy. Sci. Total Environ. 2002, 300, 229–243. [Google Scholar] [CrossRef]
- Kylander, M.E.; Weiss, D.J.; Martínez Cortízas, A.; Spiro, B.; Garcia-Sanchez, R.; Coles, B.J. Refining the pre-industrial atmospheric Pb isotope evolution curve in Europe using an 8000 year old peat core from NW Spain. Earth Planet Sci. Lett. 2005, 240, 467–485. [Google Scholar] [CrossRef]
- Zhang, W.G.; Feng, H.; Chang, J.N.; Qu, J.; Yu, L.Z. Lead (Pb) isotopes as a tracer of Pb origin in Yangtze River intertidal zone. Chem. Geol. 2008, 257, 257–263. [Google Scholar] [CrossRef]
- Yang, S.; Li, C.; Jung, H.; Lee, H. Discrimination of geochemical compositions between the Changjiang and the Huanghe sediments and its application for the identification sediment source in the Jiangsu coastal plain, China. Mar. Geol. 2002, 186, 229–241. [Google Scholar] [CrossRef]
- Yellow River Water Resources Conservation Commission. Yellow River Water Resources Bulletin 2010. Available online: http://www.yellowriver.gov.cn/other/hhgb/2010/index.html (accessed on 1 March 2015).
- Jain, C.K.; Harish, G.; Chakrapani, G.J. Enrichment and fractionation of heavy metals in bed sediments of River Narmada, India. Environ. Monit. Assess. 2008, 141, 35–47. [Google Scholar] [CrossRef] [PubMed]
- ATSDR—Agency for Toxic Substances and Diseases Registry. Division of Toxicology and Environmental Medicine, Public Health Statement of Cadmium; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2008.
- Yellow River Conservancy Commission. Review of Yellow River Basin; Henan People’s Press: Henan, China, 1998. (In Chinese) [Google Scholar]
- Krishna, A.K.; Govil, P.K. Heavy metal contamination of soil around Pali industrial area, Rajasthan, India. Environ. Geol. 2004, 47, 38–44. [Google Scholar] [CrossRef]
- Kulahci, F.; Sen, Z. Multivariate statistical analyses of artificial radionuclides and heavy metals contaminations in deep mud of Keban Dam Lake, Turkey. Appl. Radiat. Isot. 2008, 66, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, K.; Ramasamy, V.; Suresh, G. Impact of sediment characteristics on the heavy metal concentration and their ecological risk level of surface sediments of Vaigai river, Tamilnadu, India. Spectrochim. Acta. A 2015, 137, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Kampunzu, H.A.B.; Modisi, M.P.; Totolo, O. Distribution of heavy metals in Gaborone urban soils (Botswana) and its relationship to soil pollution and bedrock composition. Environ. Geol. 2003, 45, 171–180. [Google Scholar] [CrossRef]
- Saeedi, M.; Hosseinzadeh, M.; Jamshidi, A.; Pajooheshfar, S.P. Assessment of heavy metals contamination and leaching characteristics in highway side soils, Iran. Environ. Monit. Assess. 2009, 151, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Bibi, M.H.; Ahmed, F.; Ishiga, H. Assessment of metal concentrations in lake sediments of southwest Japan based on sediment quality guidelines. Environ. Geol. 2007, 52, 625–639. [Google Scholar] [CrossRef]
- Ajmone-Marsan, F.; Biasioli, M.; Kralj, T.; Grcman, H.; Davidson, C.M.; Hursthouse, A.S.; Madrid, L.; Rodrigues, S. Metals in particle-size fractions of the soils of five European cities. Environ. Pollut. 2008, 152, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Madrid, F.; Biasioli, M.; Ajmone-Marsan, F. Availability and bioaccessibility of metals in fine particles of some urban soils. Arch. Environ. Contam. Toxicol. 2008, 55, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, L.; Lu, M.; Hutchinson, S.M.; Feng, H. Magnetic approach to normalizing heavy metal concentrations for particle size effects in intertidal sediments in the Yangtze Estuary, China. Environ. Poll. 2007, 147, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yu, S.; Li, X. Distribution, availability, and sources of trace metals in different particle size fractions of urban soils in Hong Kong: Implications for assessing the risk to human health. Environ. Pollut. 2001, 159, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.; Cornu, S. Location of natural trace elements in silty soils using particle-size fractionation. Geoderma 2006, 133, 295–308. [Google Scholar] [CrossRef]
- Gibbs, R.J.; Tshudy, D.M.; Konwar, L.; Martin, J.M. Coagulation and transport of sediments in the Gironde estuary. Sedmentology 1989, 36, 987–999. [Google Scholar] [CrossRef]
- Alber, M. Settleable and non-settleable suspended sediments in the Ogeechee River estuary, Georgia, USA. Estuar. Coast. Shelf Sci. 2000, 50, 805–816. [Google Scholar] [CrossRef]
- Shulkin, V.M.; Bogdanova, N.N. Mobilization of metals from riverine suspended matter in seawater. Mar. Chem. 2003, 83, 157–167. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liu, C.Q. Distribution and partition behavior of heavy metals between dissolved and acid-soluble fractions along a salinity gradient in the Changjiang Estuary, Eastern China. Chem. Geol. 2003, 202, 383–396. [Google Scholar] [CrossRef]
- Waeles, M.; Tanguy, V.; Lespes, G.; Riso, R.D. Behaviour of colloidal trace metals (Cu, Pb and Cd) in estuarine waters: An approach using frontal ultrafiltration (UF) and stripping chronopotentiometric methods (SCP). Estuar. Coast. Shelf Sci. 2008, 80, 538–544. [Google Scholar] [CrossRef]
- Oursel, B.; Garnier, C.; Pairaud, I.; Omanovic, D.; Durrieu, G.; Syakti, A.D.; Le Poupon, C.; Thouvenin, B.; Lucas, Y. Behaviour and fate of urban particles in coastal waters: Settling rate, size distribution and metals contamination characterization. Estuar. Coast. Shelf Sci. 2014, 138, 14–26. [Google Scholar] [CrossRef]
- Hatje, V.; Payne, T.E.; Hill, D.M.; McOrist, G.; Birch, G.F.; Szymczak, R. Kinetics of trace element uptake and release by particles in estuarine waters: Effects of pH, salinity, and particle loading. Environ. Int. 2003, 29, 619–629. [Google Scholar] [CrossRef]
- Cantwell, M.G.; Burgess, R.M.; King, J.W. Resuspension of contaminated field and formulated reference sediments part I: Evaluation of metal release under controlled laboratory conditions. Chemosphere 2008, 73, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Q.; Wang, X.; Jian, H.; Chen, H.; Yu, Z. Characterization of the Particle Size Fraction associated with Heavy Metals in Suspended Sediments of the Yellow River. Int. J. Environ. Res. Public Health 2015, 12, 6725-6744. https://doi.org/10.3390/ijerph120606725
Yao Q, Wang X, Jian H, Chen H, Yu Z. Characterization of the Particle Size Fraction associated with Heavy Metals in Suspended Sediments of the Yellow River. International Journal of Environmental Research and Public Health. 2015; 12(6):6725-6744. https://doi.org/10.3390/ijerph120606725
Chicago/Turabian StyleYao, Qingzhen, Xiaojing Wang, Huimin Jian, Hongtao Chen, and Zhigang Yu. 2015. "Characterization of the Particle Size Fraction associated with Heavy Metals in Suspended Sediments of the Yellow River" International Journal of Environmental Research and Public Health 12, no. 6: 6725-6744. https://doi.org/10.3390/ijerph120606725
APA StyleYao, Q., Wang, X., Jian, H., Chen, H., & Yu, Z. (2015). Characterization of the Particle Size Fraction associated with Heavy Metals in Suspended Sediments of the Yellow River. International Journal of Environmental Research and Public Health, 12(6), 6725-6744. https://doi.org/10.3390/ijerph120606725





