In Vitro Evaluation of Genotoxic Effects under Magnetic Resonant Coupling Wireless Power Transfer
Abstract
:1. Introduction
2. Experimental Section
2.1. Exposure System
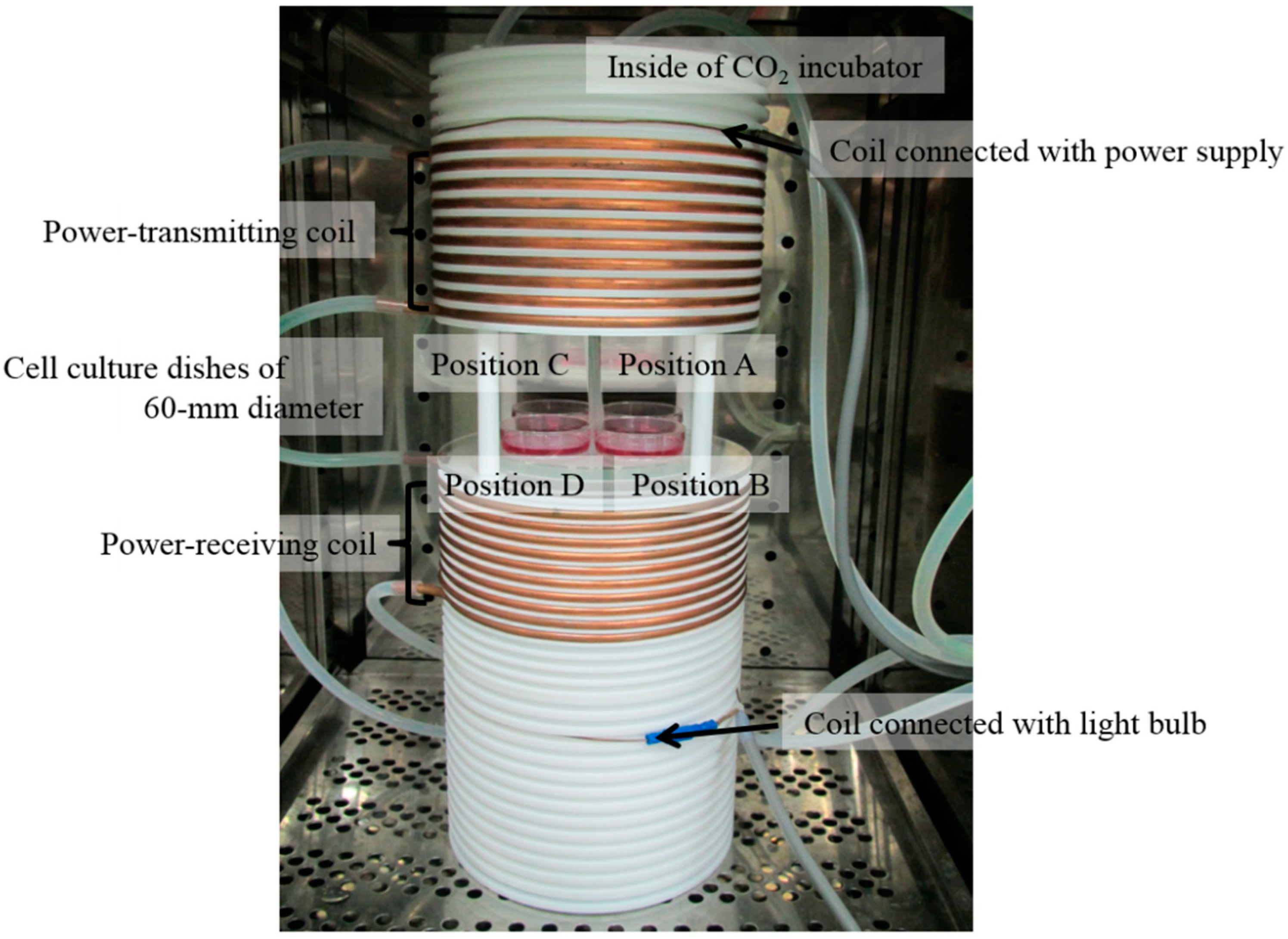
| Type | Position A | Position B | Position C | Position D |
|---|---|---|---|---|
| Magnetic | 169.2 ± 2.5 A/m | 170.7 ± 2.2 A/m | 167.6 ± 1.7 A/m | 171.5 ± 2.2 A/m |
| Field | (±1.5%) | (±1.3%) | (±1.0%) | (±1.3%) |
| SAR | 21.8 ± 9.5 W/kg | 21.3 ± 10.0 W/kg | 21.6 ± 12.1 W/kg | 20.7 ± 9.7 W/kg |
2.2. Cells and Culture Conditions
2.3. Cell Growth
2.4. Cell Cycle Distribution
2.5. Comet Assay
2.6. Micronucleus Formation
2.7. HPRT Gene Mutation
2.8. Statistical Analysis
3. Results
3.1. Cell Growth
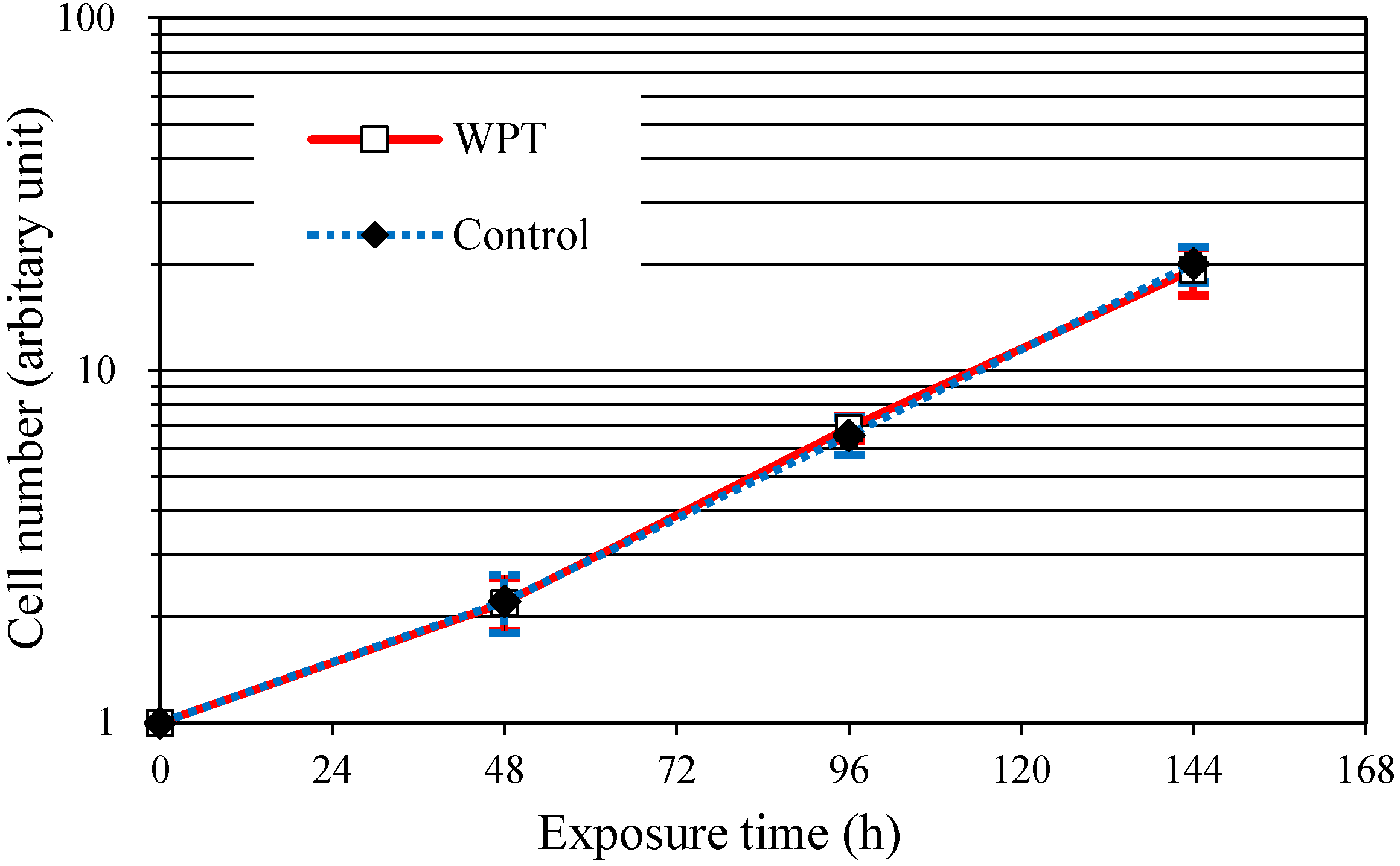
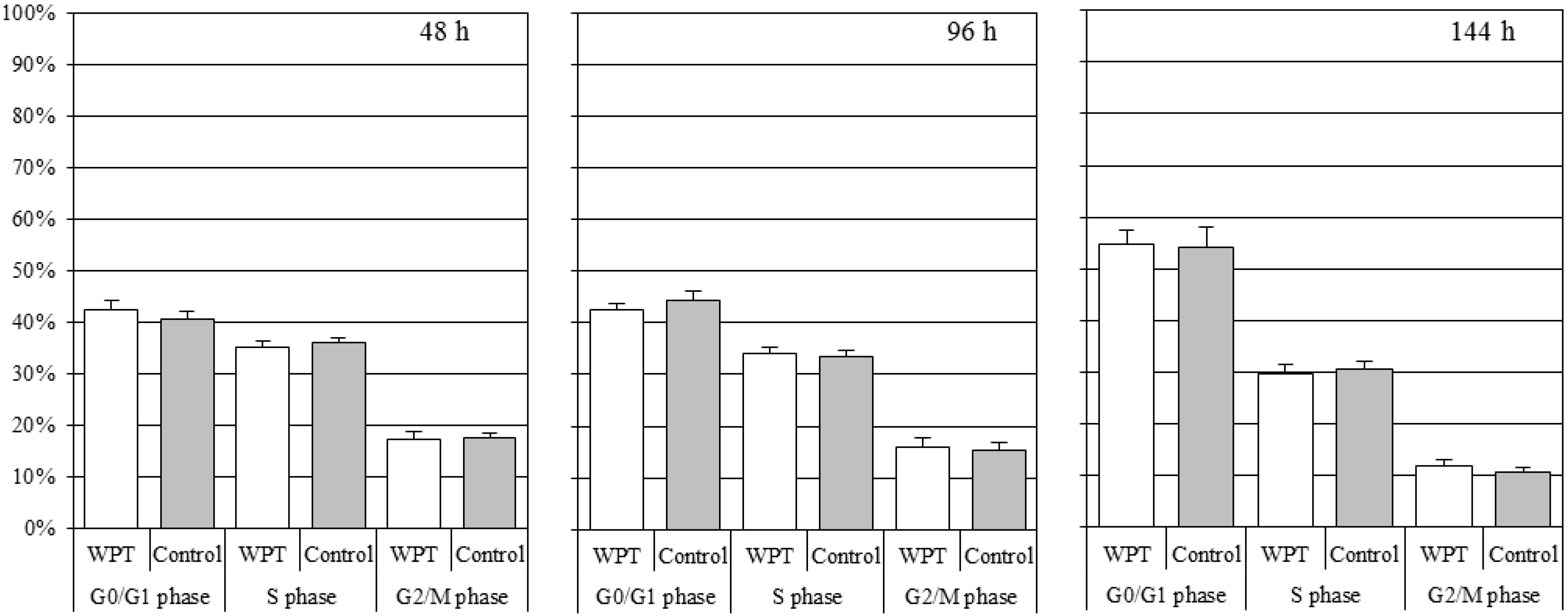
3.2. Cell Cycle Distribution
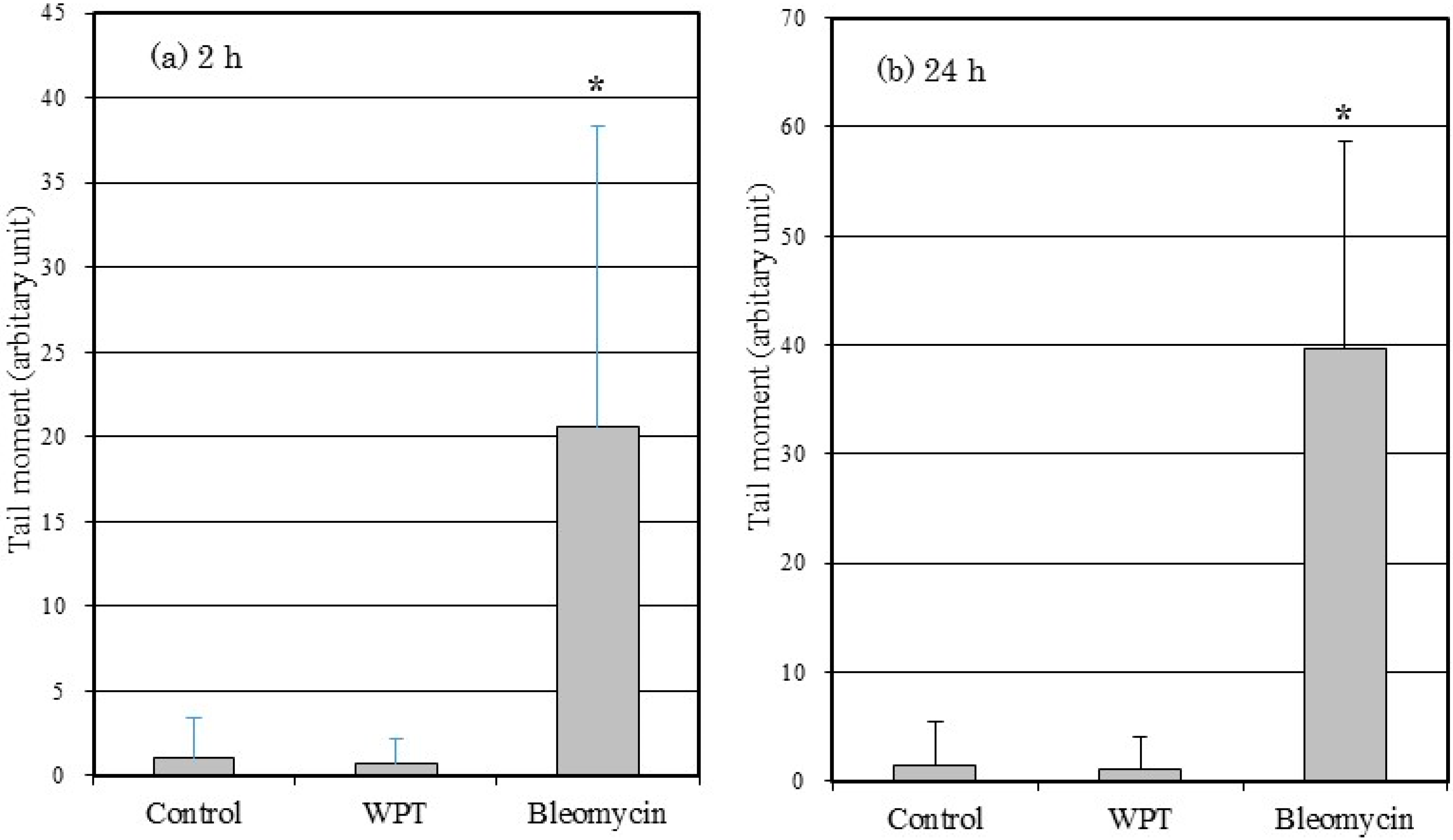
3.3. Comet Assay
3.4. Micronucleus Formation
3.5. Mutation of the HPRT Gene
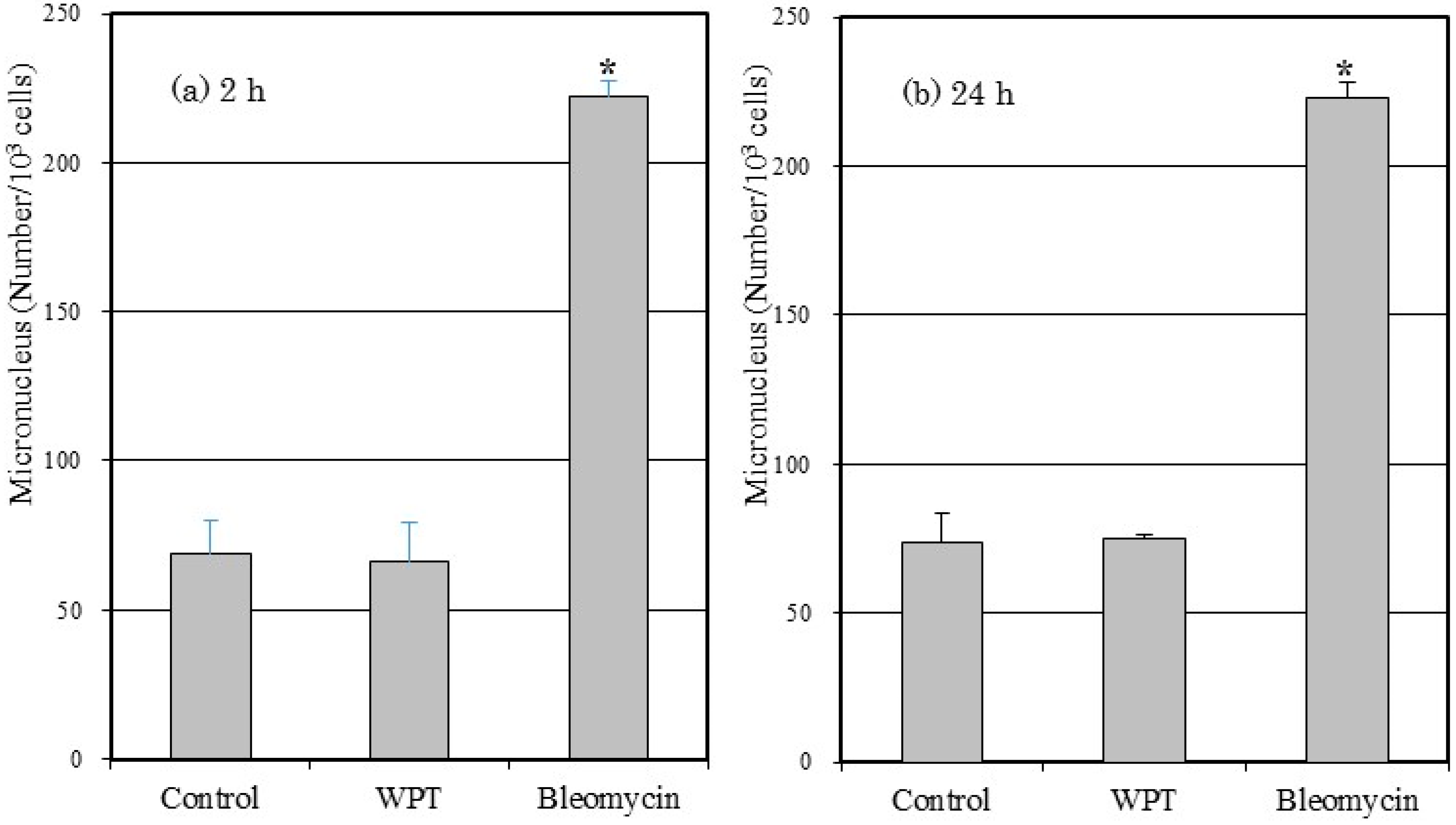
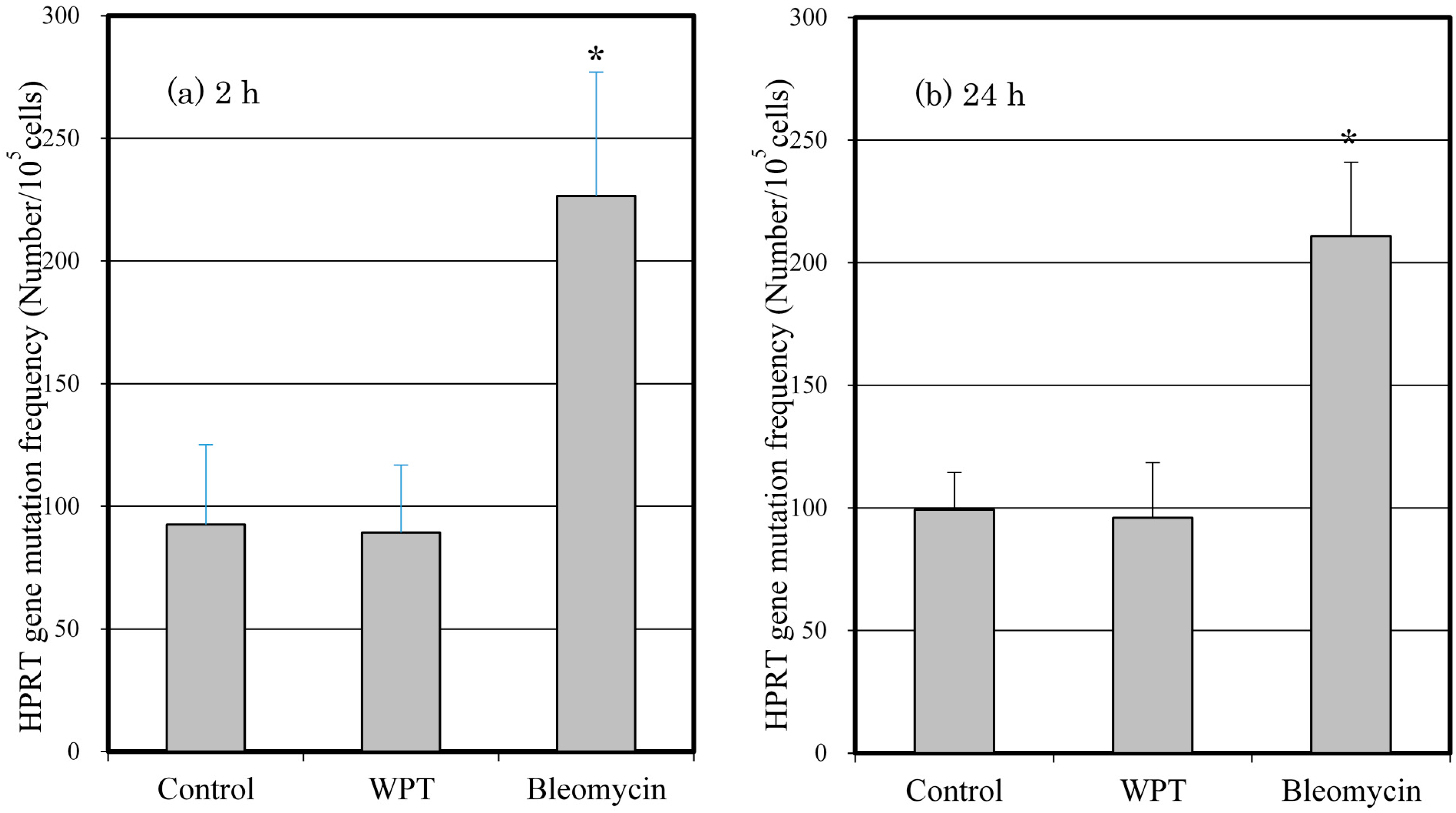
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). Electromagnetic Fields (300Hz-300GHz); Environmental Health Criteria Monograph No. 137. WHO Press: Geneva, Switzerland, 1993. Available online: http://www.inchem.org/documents/ehc/ehc/ehc137.htm (accessed on 11 January 2015).
- World Health Organization (WHO). Extremely Low Frequency Fields; Environmental Health Criteria Monograph No. 238. WHO Press: Geneva, Switzerland, 2007. Available online: http://www.who.int/peh-emf/publications/Complet_DEC_2007.pdf (accessed on 11 January 2015).
- Shinohara, N. Power without wire. IEEE Microw. Mag. 2011, 12, S64–S73. [Google Scholar] [CrossRef]
- Kurs, A.; Karalis, A.; Moffatt, R.; Joannpoilos, J.D.; Fisher, P.; Soljacic, M. Wireless power transfer via strongly coupled magnetic resonances. Sci. Mag. 2007, 317, 83–86. [Google Scholar]
- Karalis, A.; Joannopoulos, J.D.; Soljacic, M. Efficient wireless non-radiative mid-range energy transfer. Ann. Phys. 2008, 323, 34–48. [Google Scholar] [CrossRef]
- Shinohara, N. Wireless power transmission progress for electric vehicle in Japan. IEEE Radio Wirel. Symp. (RWS) 2013. [Google Scholar] [CrossRef]
- Park, S.W.; Wake, K.; Watanabe, S. Incident electric field effect and numerical dosimetry for a wireless power transfer system using magnetically coupled resonances. IEEE Trans. Microw. Theory Tech. 2013, 61, 3461–3469. [Google Scholar] [CrossRef]
- Christ, A.; Douglas, M.G.; Cooper, E.B.; Sample, A.P.; Waters, B.H.; Smith, J.R.; Kuster, N. Evaluation of wireless resonant power transfer systems with human electromagnetic exposure limits. IEEE Trans. Electromagn. Compat. 2013, 55, 265–274. [Google Scholar]
- Chun, Y.; Park, S.; Kim, J.; Kim, J.; Kim, H.; Kim, J.; Kim, N.; Ahn, S. Electromagnetic compatibility of resonance coupling wireless power transfer in on-line electric vehicle system. IEICE Trans. Commun. 2014, 97-B, 416–423. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Non-ionizing radiation, Part 1: Static and extremely low frequency (ELF) electric and magnetic fields. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2002; Volume 80. [Google Scholar]
- International Agency for Research on Cancer (IARC). Non-ionizing radiation, Part 2: Radiofrequency electromagnetic fields. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2013; Volume 102. [Google Scholar]
- Mizuno, K.; Miyakoshi, J.; Shinohara, N. In vitro exposure system using magnetic resonant coupling wireless power transfer. Wirel. Power Transf. 2014, 1, 97–107. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz–100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 74, 494–522. [Google Scholar]
- Fairbairn, D.W.; Olive, P.L.; O’Neill, K.L. The comet assay: A comprehensive review. Mutat. Res. 1995, 339, 37–59. [Google Scholar] [CrossRef]
- Kirsch-Volders, M. Towards a validation of the micronucleus test. Mutat. Res. 1997, 392, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.T.; Caskey, C.T. HPRT: Gene structure, expression, and mutation. Ann. Rev. Genet. 1985, 19, 127–148. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, K.; Shinohara, N.; Miyakoshi, J. In Vitro Evaluation of Genotoxic Effects under Magnetic Resonant Coupling Wireless Power Transfer. Int. J. Environ. Res. Public Health 2015, 12, 3853-3863. https://doi.org/10.3390/ijerph120403853
Mizuno K, Shinohara N, Miyakoshi J. In Vitro Evaluation of Genotoxic Effects under Magnetic Resonant Coupling Wireless Power Transfer. International Journal of Environmental Research and Public Health. 2015; 12(4):3853-3863. https://doi.org/10.3390/ijerph120403853
Chicago/Turabian StyleMizuno, Kohei, Naoki Shinohara, and Junji Miyakoshi. 2015. "In Vitro Evaluation of Genotoxic Effects under Magnetic Resonant Coupling Wireless Power Transfer" International Journal of Environmental Research and Public Health 12, no. 4: 3853-3863. https://doi.org/10.3390/ijerph120403853
APA StyleMizuno, K., Shinohara, N., & Miyakoshi, J. (2015). In Vitro Evaluation of Genotoxic Effects under Magnetic Resonant Coupling Wireless Power Transfer. International Journal of Environmental Research and Public Health, 12(4), 3853-3863. https://doi.org/10.3390/ijerph120403853





