Climate Change Impacts on Environmental and Human Exposure to Mercury in the Arctic
Abstract
:1. Introduction
2. Current Status of Environmental Mercury Contamination
2.1. Major Drivers of Mercury Contamination
2.2. Major Pathways of Mercury to the Arctic with Air Masses and Water Currents
2.3. Concentration Levels of Mercury in Various Components of the Arctic Environment
| Media | Concentration | Unit | Reference |
|---|---|---|---|
| Ambient air * | ≈1.5 | ng/m3 | [20] |
| Ocean | 0.1–3 | pg/L | [36] |
| Ice free surface seawater | 3–52 | pg/L (DMHg) | |
| 15–68 | pg/L (MMHg) | ||
| 15–49 | pg/L (GEM) | ||
| Snow | |||
| Air-25 km flight-rime | 15,500 | ng/L | |
| Air- rime (2005 year) | |||
| Air-rime (2006 year) | 5210 | ng/L | |
| Air-condensate | 1580 | ng/L | |
| Sea-ice surface hoar | 240 | ng/L | |
| Sea-ice frost flowers | 251 | ng/L | |
| Terrestrial ground diamond dust | 140 | ng/L | |
| 476 | ng/L |
2.4. Impacts of Mercury Pollution on Human Health in the Arctic
| Tissue | Species | Location | Year | Unit | Mean | Min | Max | N | Chemical Form | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Spleen | Inuit | Greenland | 1990–1994 | µg/g | 0.10 | 0.031 | 0.31 | 35 | THg | [66] |
| 0.02 | 0.0030 | 0.058 | 32 | MeHg | ||||||
| Brain | Greenland | - | 0.17 | 0.059 | 4.8 | 17 | THg | [67] | ||
| Liver | Greenland | 1990–1994 | 0.54 | 0.087 | 1.5 | 71 | THg | [66] | ||
| 0.10 | 0.022 | 0.21 | 34 | MeHg | ||||||
| Kidney | Greenland | 1990–1994 | 1.4 | 0.024 | 4.9 | 37 | THg | |||
| 0.046 | 0.0080 | 0.11 | 33 | MeHg | ||||||
| Urine | General population | Norway | 2003 | µg/g creatinine | 1.3 | 0.10 | 5.1 | 178 | IHg | [68] |
| Blood | Women | Nunavut-Baffin region, Canada | 1997 | µg/L | 6.7 | 0.10 | 34 | 30 | THg | [69] |
| 6.0 | 0.80 | 29 | 30 | Organic Hg | ||||||
| 2005–2007 | 4.0 | 0.52 | 28 | 99 | THg | |||||
| 2.4 | 0.20 | 23 | 99 | Organic Hg | ||||||
| North Slope, Alaska | 1999–2003 | 1.1 | - | - | 43 | THg | [63] | |||
| Nunavik, Canada | 2007 | 4.0 | 0.70 | 24 | 42 | |||||
| Nunavik, Canada | 2001 | 9.9 | 1.6 | 33 | 19 | |||||
| Nunavik, Canada | 1997 | 11 | 3.8 | 44 | 53 | |||||
| Nunavik, Canada | 1996 | 13 | 4.2 | 29 | 25 | |||||
| Nunavik, Canada | 1992 | 12 | 3.6 | 33 | 11 | |||||
| Disko Bay, Greenland | 2006 | 12 | 3.5 | 33 | 20 | |||||
| Nuuk, Greenland | 2005 | 3.2 | 0.70 | 20 | 10 | |||||
| All Greenland | 1999–2006 | 13 | 0.50 | 160 | 299 | |||||
| Kola Peninsula, Russia | 2001–2003 | 0.89 | 0.50 | 2.4 | 7 | |||||
| Krasnoschelye, Russia | 2001–2003 | 3.3 | 1.0 | 8.2 | 47 | |||||
| Lovosero, Russia | 2001–2003 | 7.3 | 1.3 | 29 | 11 | |||||
| Blood | Men | Krasnoschelye, Russia | 2001–2003 | µg/L | 5.4 | 3.0 | 12 | 4 | THg | [63] |
| Lovosero, Russia | 2001–2003 | 6.6 | 0.50 | 22 | 9 | |||||
| Sisimiut, Greenland | 2002–2003 | 6.5 | 1.4 | 23 | 52 | |||||
| Qaanaag, Greenland | 2003 | 54 | 2.3 | 240 | 43 | |||||
| Nuuk, Greenland | 2005 | 16 | 3.0 | 52 | 16 | |||||
| Quegertarsuaq, Greenland | 2006 | 22 | 3.5 | 79 | 35 | |||||
| Narsaq, Greenland | 2006 | 10 | 3.0 | 25 | 29 | |||||
| All Greenland | 1999–2006 | 20 | 1.4 | 240 | 314 | |||||
| Norway | 2003 | 16 | 0.60 | 30 | 184 | [68] | ||||
| Hair | Children | Qaanaaq, Greenland | 1995 | µg/g | 5.5 | - | 18 | 43 | THg | [70] |
| Women | 16 | - | 33 | 31 | ||||||
| 3 year-old children | Nunavut, Canada | 2007–2008 | 1.6 | - | - | 142 | [71] | |||
| Inuit children F | 2007–2008 | 1.3 | - | - | 161 | |||||
| Inuit children M | 2007–2008 | 1.5 | - | - | 196 | |||||
| Inuit children | Kivallig region, Canada | 2007–2008 | 1.1 | - | - | 133 | ||||
| Baffin region, Canada | 2007–2008 | 2.1 | - | - | 158 | |||||
| Kitikmeot region, Canada | 2007–2008 | 0.52 | - | - | 70 |
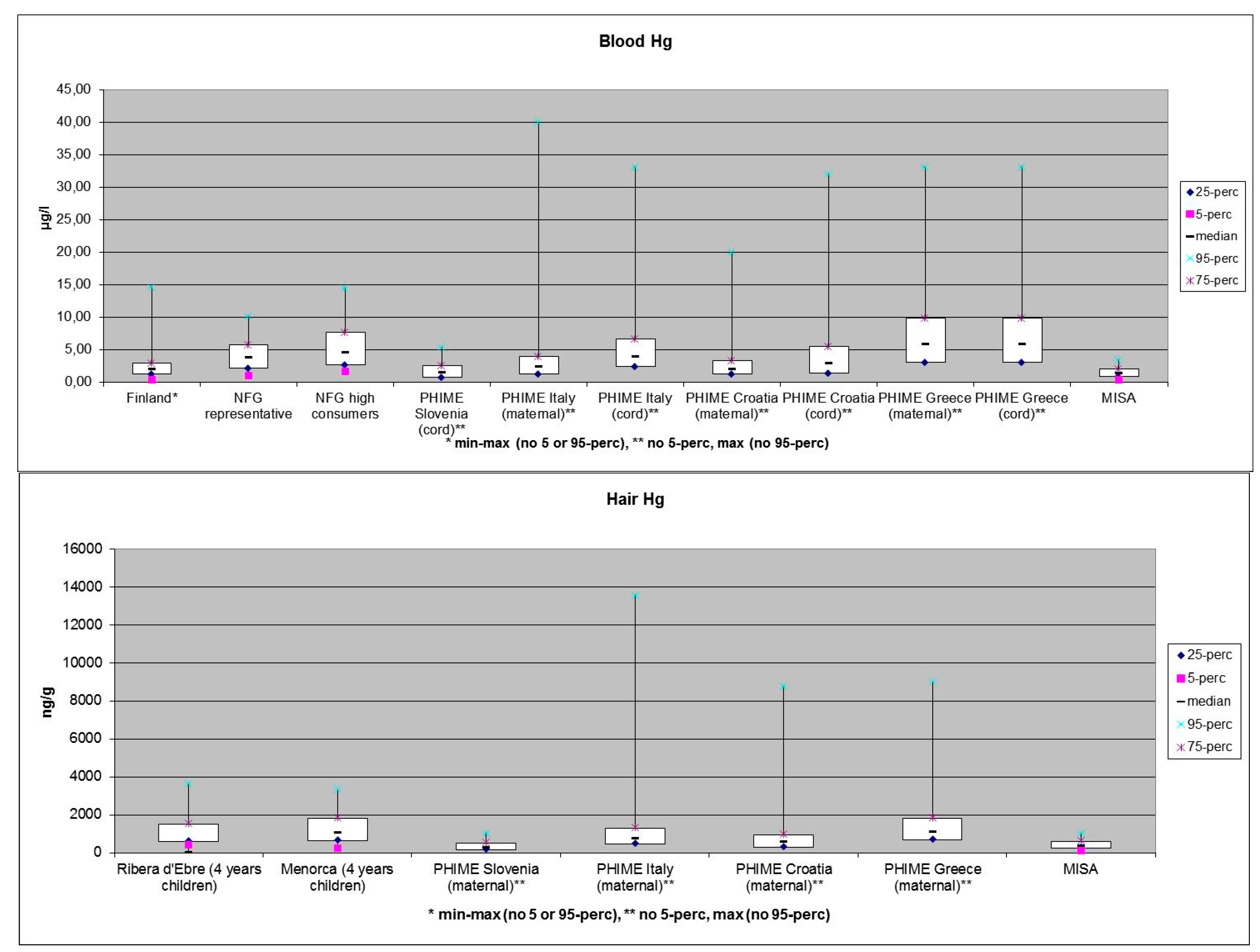
3. What will be the Future Impacts of Energy and Climate Changes on the Contamination of the Arctic Ecosystems?
3.1. Energy and Technology Use Changes
3.2. Climate Change
3.2.1. Effects on Environmental Fate and Behaviour
3.2.2. Effects on Bioaccumulation
3.2.3. Nutritional Transition and Change of Food Supply
4. Final Discussion
4.1. Environmental Fate and Behaviour
4.2. Bioaccumulation
4.3. Nutritional Transition and Change of Food Supply
5. Conclusions
- To assess how climate change may alter the mercury exposure to Arctic populations is a very ambiguous task given the science currently available on the interactions between climate change and mercury cycling in the Arctic. Research indicate that climate change may act in both ways regarding decreasing and increasing effects. As discussed above, there is no linearity in relationships between the future changes of mercury emissions and changes of mercury in fish and seafood. It should be noted that although this paper has reviewed existing information, much more research is needed to further analyse the issue of climate change impact on the effects of mercury on human health in the Arctic.
- New methods would need to be developed or improved for the assessment of emissions reduction impacts, including models integrating mercury transport within the abiotic and biotic parts of the environment, mercury methylation and de-methylation process, dose-response functions for mercury in the populations and cost benefit analysis for selecting the most efficient emission reduction measures. Such knowledge should be further analysed in the context of improving current regulations and possibly definition of new legislation.
- Reductions of mercury emission from anthropogenic sources worldwide would need to be introduced as soon as possible in order to assure lowering the adverse impact of climate change on human health.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- AMBIO. Mercury pollution, special issue. Ambio 2007, 1, 1–65. [Google Scholar]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Munthe, J.; Belhaj, M.; Åström, S. Economic benefits from decreased mercury emissions: Projections for 2020. J. Clean. Product. 2010, 18, 386–394. [Google Scholar] [CrossRef]
- Macdonald, R.W. Beaufort Sea. In Encyclopedia of the Arctic.1:219–221; Nuttall, M., Ed.; Taylor & Francis: New York, NY, USA, 2005. [Google Scholar]
- Stern, G.; Macdonald, R.W.; Outridge, P.M.; Wilson, S.; Chételat, J.; Cole, A.; Hintelmann, H.; Loseto, L.L.; Steffen, A.; Wang, F.; et al. How does climate change influence arctic mercury? Sci. Total Environ. 2012, 414, 22–42. [Google Scholar] [CrossRef] [PubMed]
- AMAP. AMAP Assessment 2011: Mercury in the Arctic; Arctic Monitoring and Assessment Programme: Oslo, Norway, 2011; p. xiv+193. [Google Scholar]
- Pacyna, J.M. Report on Policy Evaluation and Strategies for Adaptation and Abatement. Deliverable D 51. The EU ArcRisk Project; The Norwegian Institute for Air Research: Kjeller, Norway, 2013. [Google Scholar]
- Sundseth, K.; Pacyna, J.M.; Banel, A.; Pacyna, E.G. ArcRisk Deliverable D48. Case Study on Mercury; Norwegian Institute for Air Research: Kjeller, Norway, 2013. [Google Scholar]
- UNEP. Global Mercury Assessment: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013. [Google Scholar]
- Pacyna, J.M.; Sundseth, K.; Pacyna, E.G.; Jozewicz, W.; Munthe, J.; Belhaj, M.; Astrom, S. An assessment of costs and benefits associated with mercury emission reductions from major anthropogenic sources. J. Air Waste Manag. Assoc. 2010, 60, 302–315. [Google Scholar] [CrossRef] [PubMed]
- AMAP. Updating Historical Global Inventories of Anthropogenic Mercury Emissions to Air. AMAP Technical Report No. 3; Arctic Monitoring and Assessment Programme: Oslo, Norway, 2010. [Google Scholar]
- Lamborg, C.H.; Fitzgerald, W.F.; O’Donnell, J.; Torgersen, T. A non-steady-state compartment model of global-scale mercury biochemistry with inter-hemispheric atmospheric gradients. Geochim. Cosmochim. Acta 2002, 66, 1105–1118. [Google Scholar] [CrossRef]
- Bergan, T.; Gallardo, L.; Rodhe, H. Mercury in the global troposphere: A three-dimensional model study. Atmos. Environ. 1999, 33, 1575–1585. [Google Scholar] [CrossRef]
- Mason, R.P.; Sheu, G.R. Role of the ocean in the global mercury cycle. Glob. Biogeo. Cycles 2002, 16, 40/1–40/14. [Google Scholar] [CrossRef]
- Seigneur, C.; Vijayaraghavan, K.; Lohman, K.; Karamchandani, P.; Scott, C. Global source attribution for mercury deposition in the United States. Environ. Sci. Technol. 2004, 38, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Mason, R. Mercury emissions from natural sources and their importance in the global mercury cycle. In Mercury Fate and Transport in the Global Atmosphere; Pirrone, N., Mason, R., Eds.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Selin, N.E.; Jacob, D.J.; Park, R.J.; Yantosca, R.M.; Strode, S.; Jaeglé, L.; Jaffe, D. Chemical cycling and deposition of atmospheric mercury: Global constraints from observations. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Mason, R.P. Human impacts on open ocean mercury concentrations. Glob. Biogeochem. Cycles 2007, 21. [Google Scholar] [CrossRef]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Cinirella, S. GMOS (Global Mercury Observation System) deliverable D2.1. In Report and Database for Current Emissions and Emission Factors for the 2005, Including Mapping of Total Mercury Emissions and Its Chemical Forms; Norwegian Institute for Air Research: Kjeller, Norway, 2011. [Google Scholar]
- Cole, A.S.; Steffen, A.; Pfaffhuber, K.A.; Berg, T.; Pilote, M.; Poissant, L.; Tordon, R.; Hung, H. Ten-year trends of atmospheric mercury in the high Arctic compared to Canadian sub-Arctic and mid-latitude sites. Atmos. Chem. Phys. 2013, 13, 1535–1545. [Google Scholar] [CrossRef]
- Carrie, J.; Stern, G.; Sanei, H.; Macdonald, R.W.; Wang, F. Determination of mercury biogeochemical fluxes in the remote Mackenzie River Basin, northwest Canada, using speciation of sulphur and organic carbon. Appl. Geochem. 2012, 27, 815–824. [Google Scholar] [CrossRef]
- Coquery, M.; Cossa, D.; Martin, J.M. The distribution of dissolved and particulate matter in three Siberian estuaries and adjacent coastal waters. Water Air Soil Pollut. 1995, 80, 653–664. [Google Scholar] [CrossRef]
- Graydon, J.A.; Emmerton, C.A.; Lesack, L.F.W.; Kelly, E.N. Mercury in the Mackenzie River delta and estuary: Concentrations and fluxes during open-water conditions. Sci. Total Environ. 2009, 407, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Leitch, D.R.; Carrie, J.; Lean, D.; Macdonald, R.W.; Stern, G.A.; Wang, F. The delivery of mercury to the Beaufort Sea of the Arctic Ocean by the Mackenzie River. Sci. Total Environ. 2007, 373, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Outridge, P.M.; Macdonald, R.W.; Wang, F.; Stern, G.A.; Dastoor, A.P. A mass balance inventory of mercury in the Arctic Ocean. Environ. Chem. 2008, 5, 89–111. [Google Scholar] [CrossRef]
- Schuster, P.F.; Striegl, R.G.; Aiken, G.R.; Krabbenhoft, D.P.; Dewild, J.F.; Butler, K.; Kamark, B.; Dornblaser, M. Mercury export from Yukon River Basin and potential response to a changing climate. Environ. Sci. Technol. 2011, 45, 9262–9267. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.A.; Jacob, D.J.; Soerensen, A.L.; Amos, H.M.; Steffen, A.; Sunderland, E.M. Riverine source of Arctic Ocean mercury inferred from atmospheric observations. Nat. Geosci. 2012, 5, 499–504. [Google Scholar] [CrossRef]
- Kirk, J.L.; Lehnherr, I.; Andersson, M.; Braune, B.M.; Chan, L.; Dastoor, A.P.; Durnford, D.; Gleason, A.L.; Loseto, L.L.; Steffen, A.; et al. Mercury in Arctic marine ecosystems: Sources, pathways and exposure. Environ. Res. 2012, 119, 64–87. [Google Scholar] [CrossRef] [PubMed]
- Sonke, J.E.; Heimburger, L.E. Mercury in flux. Nat. Geosci. 2012, 5, 447–448. [Google Scholar] [CrossRef]
- Goodsite, M.E.; Outridge, P.M.; Christensen, J.H.; Dastoor, A.; Muir, D.; Travnikov, O.; Wilson, S. 2013 How well do environmental archives of atmospheric mercury deposition in the Arctic reproduce rates and trends depicted by atmospheric models and measurements? Sci. Total Environ. 2013, 452–453, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Slemr, F.; Brunke, E.-G.; Ebinghaus, R.; Kuss, J. Worldwide trend of atmospheric mercury since 1995. Atmos. Chem. Phys. 2011, 11, 4779–4787. [Google Scholar] [CrossRef]
- Streets, D.; Devane, M.K.; Lu, Z.; Bond, T.C.; Sunderland, E.M.; Jacob, D.J. All-time Releases of Mercury to the atmosphere from human activities. Environ. Sci. Technol. 2011, 45, 10485–10491. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.E.; Brooks, S.; Lin, C.J.; Scott, K.J.; Landis, M.S.; Stevens, R.K.; Goodsite, M.; Richter, A. Dynamic oxidation of gaseous mercury in the Arctic troposphere at polar sunrise. Environ. Sci. Technol. 2002, 36, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.A.; Sturm, M.; Simpson, W.R.; Brooks, S.; Lindberg, S.E.; Perovich, D.K. Elevated mercury measured in snow and frost flowers near Arctic sea ice leads. Geophys. Res. Lett. 2005, 2. [Google Scholar] [CrossRef]
- Dommergue, A.; Larose, C.; Fain, X.; Clarisse, O.; Foucher, D.; Hintelmann, H.; Schneider, D.; Ferrari, C.P. Deposition of mercury species in the Ny-Ålesund area (79° N) and their transfer during snowmelt. Environ. Sci. Technol. 2010, 44, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, O.; Halsall, C. ArcRisk Deliverable D12 Database of Spatially Resolved Contemporary Contaminant Concentrations Measured in the Arctic; Lancaster University (ULANC) Department of Environmental Sciences: Lancaster, UK, 2010. [Google Scholar]
- Ferriss, B.E.; Essington, T.E. Regional patterns in mercury and selenium concentrations of yellow fin tuna (Thunnus albacares) and bigeye tuna (Thunnus obesus) in the Pacific Ocean. Can. J. Fish. Aquat. Sci. 2011, 68, 2046–2056. [Google Scholar] [CrossRef]
- Vo, A.T.E.; Bank, M.S.; Shine, J.P.; Edwards, S.V. Temporal increase in organic mercury in an endangered pelagic seabird assessed by century-old museum specimens. Proc. Natl. Acad. Sci. USA 2011, 108, 7466–7471. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.A.; Popp, B.N.; Kaneko, J.J.; Drazen, J.C. The influence of depth on mercury levels in pelagic fishes and their prey. Proc. Natl. Acad. Sci. USA 2009, 106, 13865–13869. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, M.C.; Costa, V.; Menezes, G.M.; Pinho, M.R.; Santos, R.S.; Monteiro, L.R. Intra- and inter-specific variability in total and methylmercury bioaccumulation by eight marine fish species from the Azores. Mar. Pollut. Bull. 2007, 54, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Costa, V.; Furness, R.; Santos, R. Mercury concentrations in prey fish indicate enhanced bioaccumulation in mesopelagic environments. Mar. Ecol. Prog. Ser. 1996, 141, 21–25. [Google Scholar] [CrossRef]
- Monteiro, L.R.; Furness, R.W. Accelerated increase in mercury contamination in North Atlantic mesopelagic food chains as indicated by time series of seabird feathers. Environ. Toxicol. Chem. 1997, 16, 2489–2493. [Google Scholar] [CrossRef]
- Gehrke, G.E.; Blum, J.D.; Slotton, D.G.; Greenfield, B.K. Mercury isotopes link mercury in San Francisco Bay forage fish to surface sediments. Environ. Sci. Technol. 2011, 45, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Senn, D.B.; Chesney, E.J.; Blum, J.D.; Bank, M.S.; Maage, A.; Shine, J.P. Stable isotope (N, C, Hg) study of methylmercury sources and trophic transfer in the northern gulf of Mexico. Environ. Sci. Technol. 2010, 44, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, I.; Hope, H.; Gabrielsen, G.W. Biomagnification of mercury in selected species from an Arctic marine food web in Svalbard. Sci. Total Environ. 2009, 407, 4744–4751. [Google Scholar] [CrossRef] [PubMed]
- Loseto, L.L.; Stern, G.A.; Deibel, D.; Connelly, T.L.; Prokopowicz, A.; Lean, D.R.S.; Fortier, L.; Ferguson, S.H. Linking mercury exposure to habitat and feeding behaviour in Beaufort Sea beluga whales. J. Mar. Syst. 2008, 74, 1012–1024. [Google Scholar] [CrossRef]
- Dietz, R.; Riget, F.; Born, E.W.; Sonne, C.; Grandjean, P.; Kirkegaard, M.; Olsen, M.T.; Asmund, G.; Renzoni, A.; Baagoe, H.; et al. Trends in mercury in hair of Greenlandic polar bears (Ursus maritimus) during 1892–2001. Environ. Sci. Technol. 2006, 40, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Brookens, T.J.; Harvey, J.T.; O’Hara, T.M. Trace element concentrations in the Pacific harbour seal (Phoca vitulina richardii) in central and northern California: Influence of age, sex, and trophic level. Sci. Totol Environ. 2007, 372, 676–692. [Google Scholar] [CrossRef]
- Brookens, T.J.; O’Hara, T.M.; Taylor, R.J.; Bratton, G.R.; Harvey, J.T. Total mercury body burden in Pacific harbour seal, Phoca Witulina richardii, pups from central California. Mar. Pollut. Bull. 2008, 56, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Marek, T.; Knott, K.K.; Meyer, B.E.; O’Hara, T.M. Mercury concentrations in southern Beaufort Sea polar bears: Variation based on stable isotopes of carbon and nitrogen. Environ. Toxicol. Chem. 2009, 28, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Braune, B.M.; Mallory, M.L.; Gilchrist, H.G. Elevated mercury levels in a declining population of ivory gulls in the Canadian Arctic. Mar. Pollut. Bull. 2006, 52, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Tartu, S.; Goutte, A.; Bastamante, P.; Angelier, F.; Moe, B.; Clement-Chastler, C.; Bech, C.; Gabrielsen, G.W.; Bustnes, J.O.; Chasel, O. To breed or not to breed: Endocrine response to mercury contamination by an Arctic seabird. Biol. Lett. 2013, 9. [Google Scholar] [CrossRef]
- Evans, M.S.; Muir, D.; Lockhart, W.L.; Stern, G.; Ryan, M.; Roach, P. Persistent organic pollutants and metals in the freshwater biota of the Canadian Subarctic and Arctic: An overview. Sci. Totol Environ. 2005, 351, 94–147. [Google Scholar] [CrossRef]
- Baird, C.; Cann, M. Environmental Chemistry; W.H. Freeman: New York, NY, USA, 2004. [Google Scholar]
- Sanfeliu, C.; Sebastia, J.; Cristofol, R.; Rodriguez-Farre, E. Neurotoxicity of organomercurial compounds. Neurotox Res. 2003, 5, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Lopez, M.E.; de Sa, A.L.; Herculano, A.M.; Burbano, R.R.; do Nascimento, J.L.M. Methylmercury genotoxicity: A novel effect in human cell lines of the central nervous system. Environ. Int. 2007, 3, 141–146. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003, 18, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.C.N.; Oikawa, T.; Vieira, J.L.; Gomes, M.S.; Guimaraes, G.A.; Crespo-Lopez, M.E. Comparative study of human exposure to mercury in riverside communities in the Amazon region. Braz. J. Med. Biol. Res. 2006, 39, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.F.; Johansson, C.; Onishchenko, N.; Coccini, T.; Roda, E.; Vahter, M.; Ceccatelli, S.; Manzo, L. Human developmental neurotoxicity of methylmercury: Impact of variables and risk modifiers. Regul. Toxicol. Pharm. 2008, 51, 201–214. [Google Scholar] [CrossRef]
- AMAP. AMAP Assessment 2002: Human Health in the Arctic. pp xiii+137; Arctic Monitoring and Assessment Programme: Oslo, Norway, 2003. [Google Scholar]
- Mergler, D.; Anderson, H.A.; Chan, H.M.; Mahaffey, K.R.; Murray, M.; Sakamoto, M.; Stern, A.H. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio 2007, 36, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rae, D.; Graham, L. Benefits of Reducing Mercury in Saltwater Ecosystems, a Case Study; US EPA, Office of Wetlands, Oceans, and Watersheds: Washington, DC, USA, 2004.
- AMAP. AMAP Assessment 2009: Human Health in the Arctic; Arctic Monitoring and Assessment Programme: Oslo, Norway, 2009. [Google Scholar]
- PHIME, Public health impact of long-term, low-level mixed element exposure in susceptible population strata. Publishable Final report. Publishable Final report. Lund University, August 2011.
- Bandtsaeter, A.; Knutsen, H. ArcRisk Deliverable D39. Effects of Climate/Global Change on Future Dietary Exposure in ArcRisk Study Groups; Norwegian Institute of Public Health: Oslo, Norway, 2013. [Google Scholar]
- Johansen, P.; Mulvad, G.; Pedersen, H.S.; Hansen, J.C.; Riget, F. Human accumulation of mercury in Greenland. Sci. Total Environ. 2007, 377, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.B.; Hansen, J.C.; Mulvad, G.; Pedersen, H.S.; Gregersen, M.; Danscher, G. Mercury accumulations in brains from populations exposed to high and low dietary levels of methyl mercury. Circumpolar Health 1999, 58, 96–107. [Google Scholar]
- Jenssen, M.T.S.; Brantsæter, A.L.; Haugen, M.; Meltzer, H.M.; Larssen, T.; Kvalem, H.E.; Birgisdottir, B.E.; Thomassen, Y.; Ellingsen, D.; Alexander, J.; et al. Dietary mercury exposure in a population with a wide range of fish consumption—Self-capture of fish and regional differences are important determinants of mercury in blood. Environ. Sci. Technol. 2012, 439, 220–229. [Google Scholar]
- Donaldson, S.G.; van Oostdam, J.; Tikhonov, C.; Feeley, M.; Armstrong, B.; Ayotte, P.; Boucher, O.; Bowers, W.; Chan, L.; Dallaire, F.; et al. Environmental contaminants and human health in the Canadian Arctic. Sci. Total Environ. 2010, 408, 5165–5234. [Google Scholar] [CrossRef] [PubMed]
- Weihem, P.; Hansenm, J.C.; Muratam, K.; Debesm, F.; Jørgensenm, P.; Steuerwaldm, U.; Whitem, R.F.; Grandjeanm, P. Neurobehavioral performance of Inuit children with increased prenatal exposure to methylmercury. Int. J. Circumpolar Health 2002, 61, 41–49. [Google Scholar] [PubMed]
- Tian, W.; Egeland, G.M.; Sobol, I.; Chan, H.M. Mercury hair concentrations and dietary exposure among Inuit preschool children in Nunavut, Canada. Environ. Int. 2011, 37, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, E.G.; Pacyna, J.M.; Sundseth, K.; Munthe, J.; Kindbom, K.; Wilson, S.; Steinhuisen, F.; Maxon, P. Global emissions of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos. Environ. 2010, 44, 2487–2499. [Google Scholar] [CrossRef]
- Rafaj, P.; Cofala, J.; Kuenen, J.; Wyrwa, A.; Zysk, J. Benefits of European climate policies for mercury air pollution. Atmosphere 2014, 5, 45–59. [Google Scholar] [CrossRef]
- Goodsite, M.E.; Plane, J.M.C.; Skov, H. A theoretical study of the oxidation of Hg-0 to HgBr2 in the troposphere. Environ. Sci. Technol. 2004, 38, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.H.; Brandt, J.; Frohn, L.M.; Skov, H. Modelling of mercury in the Arctic with the Danish eulerian hemispheric model. Atmos. Chem. Phys. 2004, 4, 2251–2257. [Google Scholar] [CrossRef]
- Cain, A.; Jacobson, D. Great Lakes Mercury Emission Reduction Strategy. Available online: http://www.glrppr.org/glmst/Mercury-Emissions-Reduction-Strategy.pdf (accessed on 12 July 2010).
- Wayland, M.; Hobson, K.A.; Sirois, J. Environmental contaminants in colonial water birds from Great Slave Lake, NWT: Spatial, temporal and food-chain considerations. Arctic 2000, 53, 221–233. [Google Scholar] [CrossRef]
- Riget, F.; Braune, B.; Bignert, A.; Wilson, S.; Aars, J.; Born, E.; Dam, M.; Dietz, R.; Evans, M.; Evans, T.; et al. Temporal trends of Hg in Arctic biota, an update. Sci. Total Environ. 2011, 409, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundseth, K.; Pacyna, J.M.; Banel, A.; Pacyna, E.G.; Rautio, A. Climate Change Impacts on Environmental and Human Exposure to Mercury in the Arctic. Int. J. Environ. Res. Public Health 2015, 12, 3579-3599. https://doi.org/10.3390/ijerph120403579
Sundseth K, Pacyna JM, Banel A, Pacyna EG, Rautio A. Climate Change Impacts on Environmental and Human Exposure to Mercury in the Arctic. International Journal of Environmental Research and Public Health. 2015; 12(4):3579-3599. https://doi.org/10.3390/ijerph120403579
Chicago/Turabian StyleSundseth, Kyrre, Jozef M. Pacyna, Anna Banel, Elisabeth G. Pacyna, and Arja Rautio. 2015. "Climate Change Impacts on Environmental and Human Exposure to Mercury in the Arctic" International Journal of Environmental Research and Public Health 12, no. 4: 3579-3599. https://doi.org/10.3390/ijerph120403579
APA StyleSundseth, K., Pacyna, J. M., Banel, A., Pacyna, E. G., & Rautio, A. (2015). Climate Change Impacts on Environmental and Human Exposure to Mercury in the Arctic. International Journal of Environmental Research and Public Health, 12(4), 3579-3599. https://doi.org/10.3390/ijerph120403579






