The MARINA Risk Assessment Strategy: A Flexible Strategy for Efficient Information Collection and Risk Assessment of Nanomaterials
Abstract
:1. Introduction
1.1. Present Initiatives and Views
1.2. Preconditions for A Risk Assessment Strategy for ENMs
- Identification of all relevant exposure scenarios during the life cycle of an ENM. This may for instance relate to scenarios with a specific exposure route and the highest potential of exposure or to a specific life-cycle stage of concern, depending on the assessment goals of the user. It is noted that relevance of an exposure scenario can be indicated by information on exposure, fate/kinetics or hazard or any combination of these domains. These initial considerations shall be based on all available data; it is acknowledged that these data will be limited for an ENM under development.
- Definition of a minimum base set of obligatory information, in order to be able to identify relevant exposure scenarios.
- Verification whether the exposure scenarios need further consideration, i.e., that it cannot be ruled out that a human health and/or environmental risk may be present.
- If risk cannot be ruled out, the type of information that best serves the risk assessment process needs to be determined and guidance should be provided how to obtain the required information. Data gaps should be addressed in a stepwise process starting with relatively simple/inexpensive methods or tools and moving to more sophisticated tools, as needed further down the risk assessment process. Only data should be generated that benefits the risk assessment process.
2. The MARINA Risk Assessment Strategy
2.1. Outline
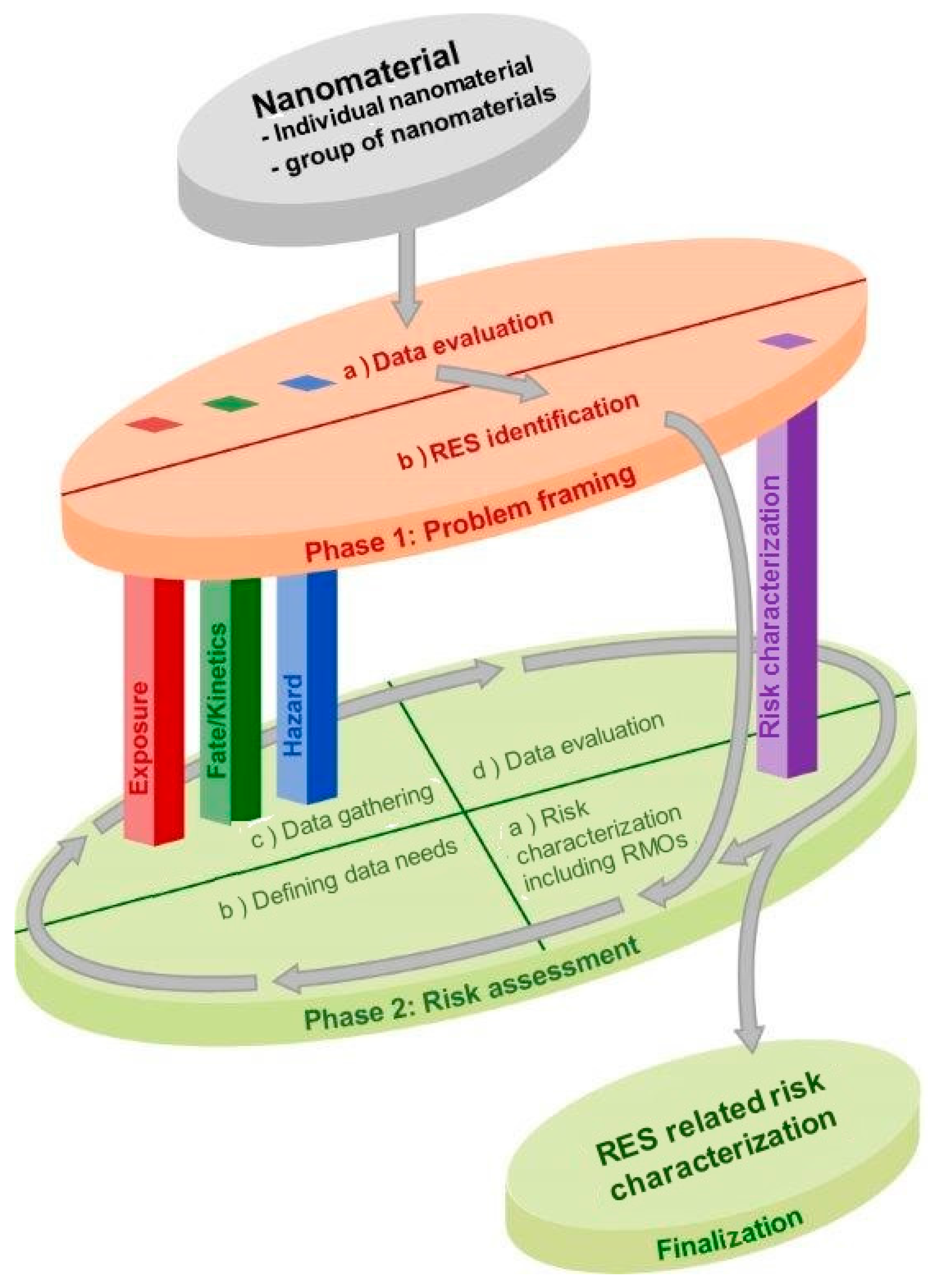
2.2. Phase 1: Problem Framing
2.2.1. Step a: Data Evaluation
2.2.2. Step b: RES Identification
2.3. Phase 2: Risk Assessment
2.3.1. Iterative Risk Assessment Process
- Step (a)
- Risk characterization including risk management options. This step focuses on whether the question on potential health and/or environmental risks can be sufficiently addressed. If yes, the risk can be characterized by using tools from the risk characterization pillar. If no, go to step (b) (Defining data needs).
- Step (b)
- Defining data needs. Identification of the data gap(s) with the highest priority to be addressed for the purpose of risk characterization, and definition of data or information that is required to optimally fill in this gap.
- Step (c)
- Data gathering. A compilation of data (including data generation) from one or more of the three information-gathering pillars, based on defined guidance, guidelines and other tools to ensure reliability and relevance.
- Step (d)
- Data evaluation. Evaluation of collected data in coherence with the data already available, including consideration of possibilities for read-across, grouping and data-sharing.
- (i)
- The available data are insufficient to draw a final conclusion on risks with a sufficient level of certainty. The process proceeds to the next step of the evaluation cycle, i.e., step (b) Defining data needs.
- (ii)
- The available data are sufficient and negligible risks are expected. The iterative process ends and the results will be passed through to the finalization step (lower green disc in Figure 1, see below (Finalization process) for description).
- (iii)
- The available data are sufficient and risks are present, but no adequate risk management options (RMOs), i.e., identification of the best (regulatory) option to manage the risk, are available. The iterative process ends and the results will be passed through to the finalization step (lower green disc in Figure 1, see below (Finalization process) for description). It is not expected that gathering additional information would further refine the risk assessment. This conclusion may include that the exposure scenario considered should be avoided, which might imply that a specific application/use should be restricted or that the development of the ENM should be reconsidered.
- (iv)
- The available data are sufficient and health/environmental risks are present and adequate RMOs are available. RMOs will be considered for their applicability and appropriate risk management measures (RMMs), if available, will be taken. The adequacy of the RMMs will be evaluated, and, if risks can be adequately controlled by RMMs taken, in principle no further evaluation is needed. It may, however, also be concluded that additional information is needed for the evaluation of the appropriateness of the RMMs, in which case another iteration of the evaluation cycle is needed.
2.3.2. Finalization Process
2.4. Pillars

3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arts, J.H.; Hadi, M.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; Warheit, D.; et al. A critical appraisal of existing concepts for the grouping of nanomaterials. Regul. Toxicol. Pharmacol. 2014, 70, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.H.; Hadi, M.; Irfan, M.A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanogrouping). Regul. Toxicol. Pharmacol. 2015, 71, S1–S27. [Google Scholar] [CrossRef] [PubMed]
- Oomen, A.G.; Bos, P.M.; Fernandes, T.F.; Hund-Rinke, K.; Boraschi, D.; Byrne, H.J.; Aschberger, K.; Gottardo, S.; von der Kammer, F.; Kuhnel, D.; et al. Concern-driven integrated approaches to nanomaterial testing and assessment—Report of the nanosafety cluster working group 10. Nanotoxicology 2014, 8, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.; Fabian, E.; Ma-Hock, L.; van Ravenzwaay, B.; Wohlleben, W.; Wiench, K.; Oesch, F. Toxico-/biokinetics of nanomaterials. Arch. Toxicol. 2012, 86, 1021–1060. [Google Scholar] [CrossRef] [PubMed]
- Pietroiusti, A.; Campagnolo, L.; Fadeel, B. Interactions of engineered nanoparticles with organs protected by internal biological barriers. Small 2013, 9, 1557–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council. Toxicity Testing in the 21st Century—A Vision and a Strategy; National Research Council: Washington, DC, USA, 2007. [Google Scholar]
- Krewski, D.; Andersen, M.E.; Mantus, E.; Zeise, L. Toxicity testing in the 21st century: Implications for human health risk assessment. Risk Anal. 2009, 29, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.E.; Krewski, D. Toxicity testing in the 21st century: Bringing the vision to life. Toxicol. Sci. 2009, 107, 324–330. [Google Scholar] [CrossRef] [PubMed]
- European Union. Addressing the New Challenges for Risk Assessment; SCHER (Scientific Committee on Health and Environmental Risks), SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks), SCCS (Scientific Committee on Consumer Safety): Brussels, Belgium, 2013. [Google Scholar]
- Tantra, R.; Oksel, C.; Puzyn, T.; Wang, J.; Robinson, K.N.; Wang, X.Z.; Ma, C.Y.; Wilkins, T. Nano(Q)SAR: Challenges, pitfalls and perspectives. Nanotoxicology 2015, 9, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Emerging and Newly-Identified Health Risks. The Appropriateness of the Risk Assessment Methodology in Accordance with the Technical Guidance Documents for New and Existing Substances for Assessing the Risks of Nanomaterials; Scientific Committee on Emerging and Newly-Identified Health Risks: Brussels, Belgium, 2007. [Google Scholar]
- Organisation for Economic Co-operation and Development. Important Issues on Risk Assessment of Manufactured Nanomaterials; Organisation for Economic Co-operation and Development: Paris, France, 2012. [Google Scholar]
- Organisation for Economic Co-operation and Development. Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials. Series on the Safety of Manufactured Nanomaterials No. 36. Available online: Http://www.Oecd.Org/officialdocuments/publicdisplaydocumentpdf/?Cote=env/jm/mono%282012%2940&doclanguage=en (accessed on 15 April 2015).
- Stone, V.; Pozzi-Mucelli, S.; Tran, L.; Aschberger, K.; Sabella, S.; Vogel, U.; Poland, C.; Balharry, D.; Fernandes, T.; Gottardo, S.; et al. ITS-NANO—Prioritising nanosafety research to develop a stakeholder driven intelligent testing strategy. Part. Fibre Toxicol. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Hristozov, D.; Gottardo, S.; Semenzin, E.; Oomen, A.; Bos, P.; Peijnenburg, W.; van Tongeren, M.; Nowack, B.; Hunt, N.; Brunelli, A.; et al. Frameworks and tools for risk assessment and management of manufactured nanomaterials. Environ. Int. Submitted.
- Oomen, A.G.; Bleeker, E.; Bos, P.; van Broekhuizen, F.; Gottardo, S.; Groenewold, M.; Hristozov, D.; Hund-Rinke, K.; Irfan, M.-A.; Marcomini, A.; et al. Grouping and read-across approaches for risk assessment of nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 13415–13434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeire, T.; van de Bovenkamp, M.; de Bruin, Y.B.; Delmaar, C.; van Engelen, J.; Escher, S.; Marquart, H.; Meijster, T. Exposure-based waiving under reach. Regul. Toxicol. Pharmacol. 2010, 58, 408–420. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bos, P.M.J.; Gottardo, S.; Scott-Fordsmand, J.J.; Van Tongeren, M.; Semenzin, E.; Fernandes, T.F.; Hristozov, D.; Hund-Rinke, K.; Hunt, N.; Irfan, M.-A.; et al. The MARINA Risk Assessment Strategy: A Flexible Strategy for Efficient Information Collection and Risk Assessment of Nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 15007-15021. https://doi.org/10.3390/ijerph121214961
Bos PMJ, Gottardo S, Scott-Fordsmand JJ, Van Tongeren M, Semenzin E, Fernandes TF, Hristozov D, Hund-Rinke K, Hunt N, Irfan M-A, et al. The MARINA Risk Assessment Strategy: A Flexible Strategy for Efficient Information Collection and Risk Assessment of Nanomaterials. International Journal of Environmental Research and Public Health. 2015; 12(12):15007-15021. https://doi.org/10.3390/ijerph121214961
Chicago/Turabian StyleBos, Peter M. J., Stefania Gottardo, Janeck J. Scott-Fordsmand, Martie Van Tongeren, Elena Semenzin, Teresa F. Fernandes, Danail Hristozov, Kerstin Hund-Rinke, Neil Hunt, Muhammad-Adeel Irfan, and et al. 2015. "The MARINA Risk Assessment Strategy: A Flexible Strategy for Efficient Information Collection and Risk Assessment of Nanomaterials" International Journal of Environmental Research and Public Health 12, no. 12: 15007-15021. https://doi.org/10.3390/ijerph121214961
APA StyleBos, P. M. J., Gottardo, S., Scott-Fordsmand, J. J., Van Tongeren, M., Semenzin, E., Fernandes, T. F., Hristozov, D., Hund-Rinke, K., Hunt, N., Irfan, M.-A., Landsiedel, R., Peijnenburg, W. J. G. M., Sánchez Jiménez, A., Van Kesteren, P. C. E., & Oomen, A. G. (2015). The MARINA Risk Assessment Strategy: A Flexible Strategy for Efficient Information Collection and Risk Assessment of Nanomaterials. International Journal of Environmental Research and Public Health, 12(12), 15007-15021. https://doi.org/10.3390/ijerph121214961








