A Survey of Soil Enzyme Activities along Major Roads in Beijing: The Implications for Traffic Corridor Green Space Management
Abstract
:1. Introduction
2. Experimental Section
2.1. Soil Collection and Sampling
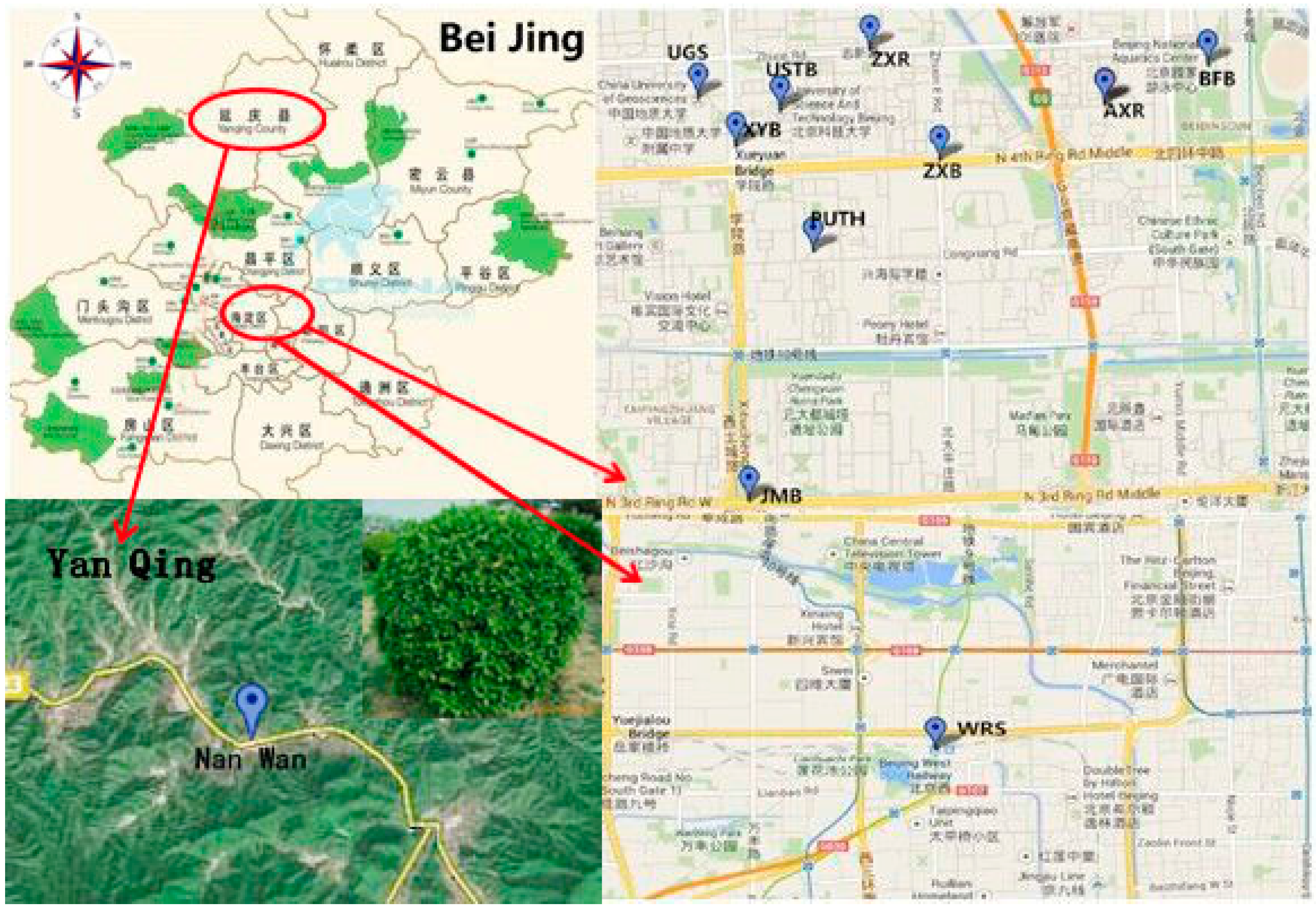
2.2. Measurement of Enzyme Activities
2.3. Traffic-Related Air Pollution
3. Statistical Analysis
| Sample | Bulk Density (g/cm3) | Porosity % | Permeability mm/min | pH | Organic Matter (mg/kg) | Soil Nutrients | |
|---|---|---|---|---|---|---|---|
| Total Nitrogen (mg/kg) | Available Phosphorus (mg/kg) | ||||||
| WRS | 1.28 | 49.66 | 20.35 | 8.21 | 10.97 | 0.62 | 16.82 |
| PUTH | 1.47 | 46.52 | 10.34 | 7.44 | 13.14 | 0.71 | 17.34 |
| AXR | 1.35 | 48.26 | 18.96 | 8.35 | 10.05 | 0.85 | 16.53 |
| ZXR | 1.52 | 45.39 | 8.93 | 7.82 | 14.27 | 0.67 | 18.68 |
| BFB | 1.24 | 52.15 | 21.37 | 8.26 | 9.75 | 0.75 | 14.60 |
| XYB | 1.44 | 47.36 | 10.74 | 8.38 | 13.46 | 0.56 | 20.46 |
| UGS | 1.18 | 54.38 | 12.36 | 7.67 | 10.53 | 0.92 | 18.61 |
| JMB | 1.21 | 52.75 | 29.26 | 8.29 | 11.52 | 0.65 | 21.69 |
| ZXB | 1.38 | 46.53 | 18.62 | 8.32 | 13.61 | 0.78 | 17.73 |
| USTB | 1.26 | 50.48 | 25.41 | 8.16 | 10.44 | 0.69 | 18.37 |
| YQ | 1.15 | 57.86 | 31.69 | 6.89 | 32.27 | 1.06 | 8.32 |
4. Results and Discussion
4.1. Physical and Chemical Parameters
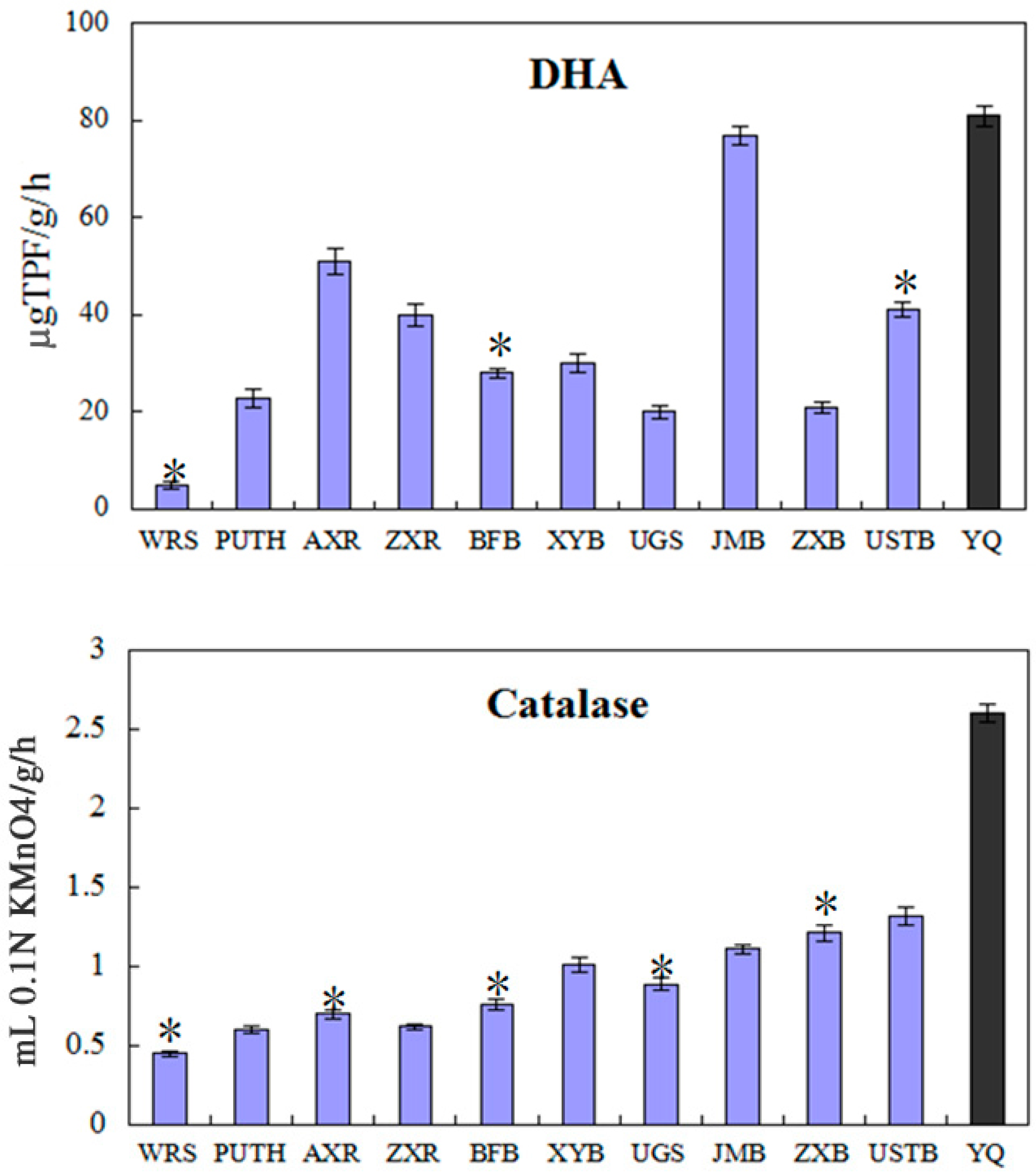
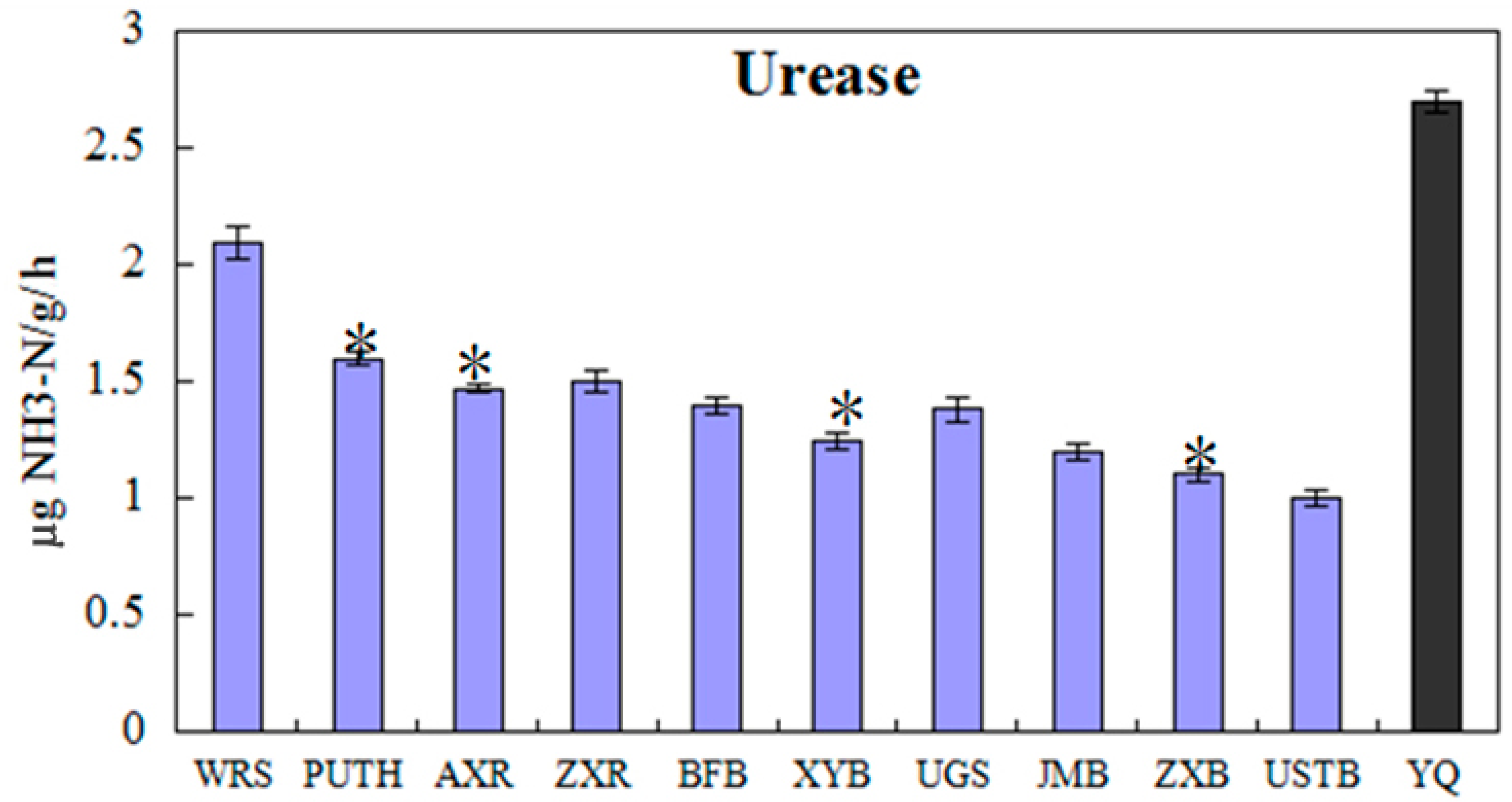
4.2. Dehydrogenase, Catalase and Urease Activities
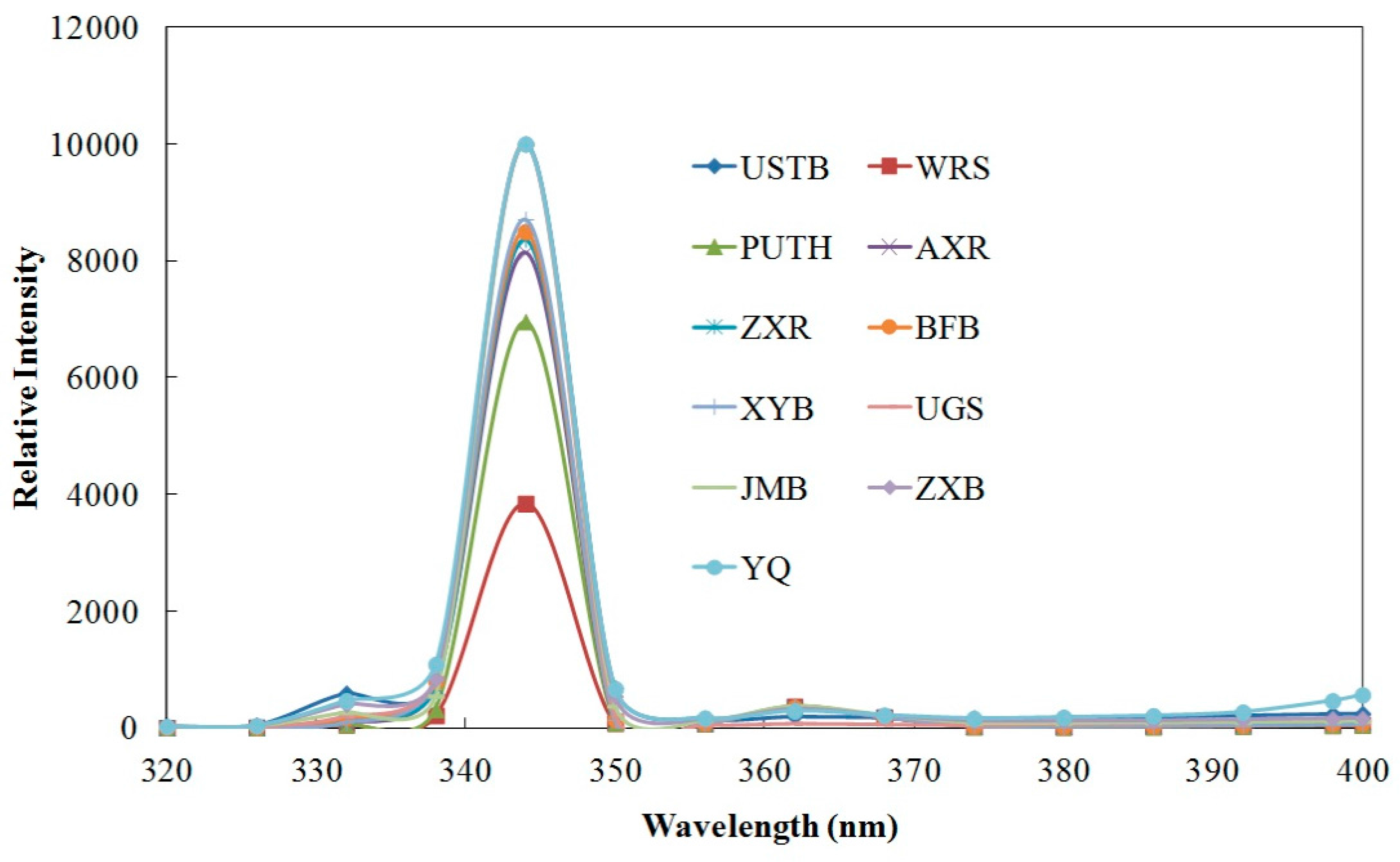
4.3. Fluorescence Activity Analysis
4.4. Traffic-Related Air Pollutions
4.5. The Impact of Traffic-Related Air Pollution on Soil Enzyme Activities
| Category | YQ | Average of the Ten Urban Sample Sites |
|---|---|---|
| The increase in PM10 exposure during the haze day (µg/m3) | 63.5 | 205.5 |
| The increase in PM2.5 exposure during the haze day (µg/m3) | 57.0 | 179.5 |
| DHA (µg TPF/g/h) | 82.52 | 34.00 |
| Catalase (mL 0.1 N KMnO4/g /h) | 2.64 | 0.84 |
| Urease (µgNH3-N/g/h) | 2.70 | 1.39 |
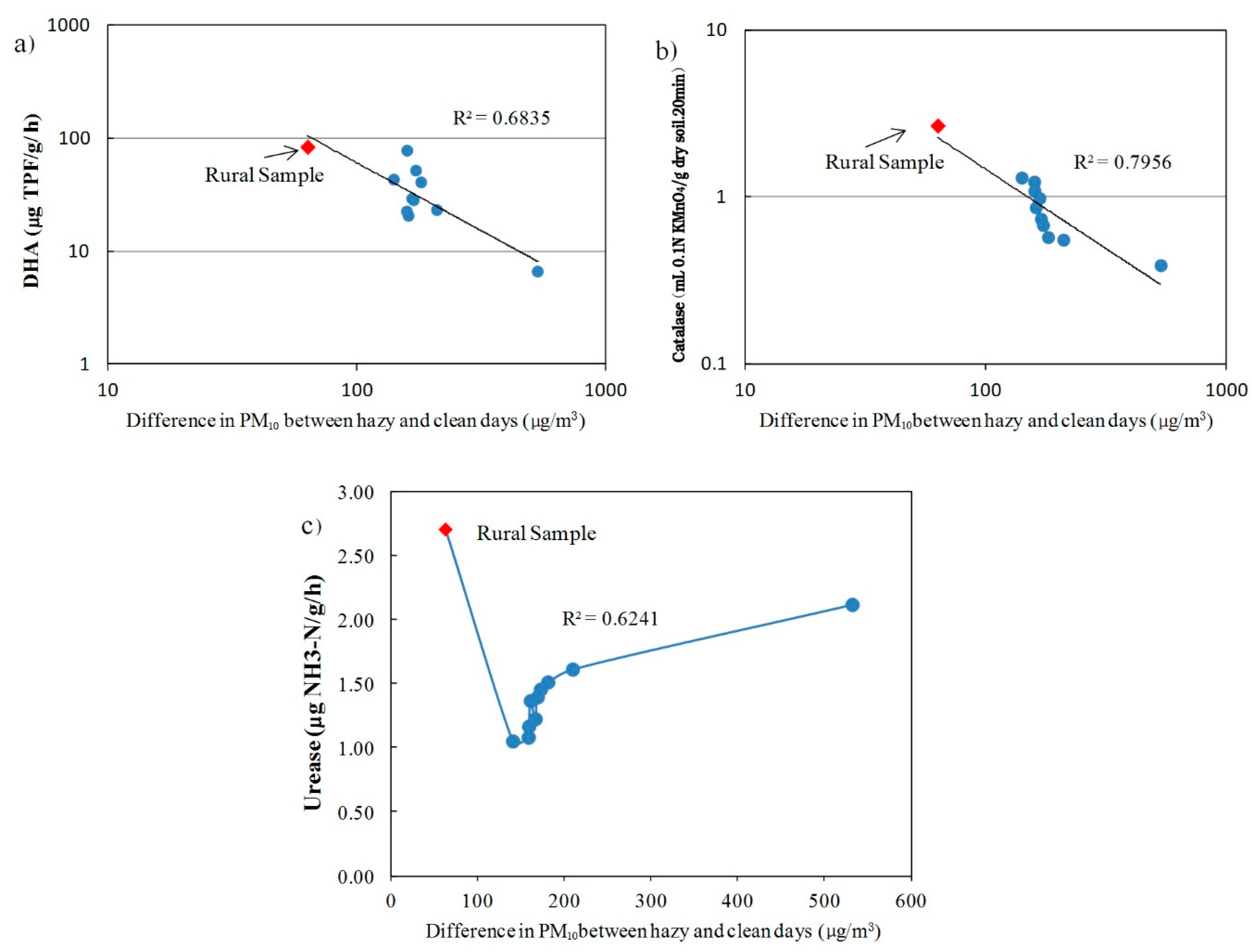
4.6. Enzyme Activities and Traffic Pollution
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taleghani, M.; Sailor, D.J.; Tenpierik, M.; van den Dobbelsteen, A. Thermal assessment of heat mitigation strategies: The case of Portland State University, Oregon, USA. Build. Environ. 2014, 73, 138–150. [Google Scholar] [CrossRef]
- Weber, F.; Kowarik, I.; Saumel, I. Herbaceous plants as filters: Immobilization of particulates along urban street corridors. Environ. Pollut. 2014, 186, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.; Horner, R.R. Performance Assessment of a Street-Drainage Bioretention System. Water Environ. Res. 2010, 82, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Rhea, L.; Shuster, W.; Shaffer, J.; Losco, R. Data proxies for assessment of urban soil suitability to support green infrastructure. J. Soil Water Conserv. 2014, 69, 254–265. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Lipinska, A.; Kucharski, J.; Wyszkowska, J. The Effect of Polycyclic Aromatic Hydrocarbons on the Structure of Organotrophic Bacteria and Dehydrogenase Activity in Soil. Polycycl. Aromat. Compd. 2014, 34, 35–53. [Google Scholar] [CrossRef]
- Dick, R.P. Soil enzyme-activities as indicators of soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Springer Berlin Heidelberg: Berlin, German, 1994; pp. 107–124. [Google Scholar]
- Ma, J.; Shen, J.L.; Liu, Q.X.; Fang, F.; Cai, H.S.; Guo, C.H. Risk assessment of petroleum-contaminated soil using soil enzyme activities and genotoxicity to Vicia faba. Ecotoxicology 2014, 23, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Ciarkowska, K.; Solek-Podwika, K.; Wieczorek, J. Enzyme activity as an indicator of soil-rehabilitation processes at a zinc and lead ore mining and processing area. J. Environ. Manag. 2014, 132, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Gao, R.T.; Xi, B.D.; Zhang, Y.; Zhang, H. Long-term effects of irrigation using water from the river receiving treated industrial wastewater on soil organic carbon fractions and enzyme activities. Agric. Water Manag. 2014, 135, 100–108. [Google Scholar] [CrossRef]
- Miller, C.A.; Hidy, G.; Hales, J.; Kolb, C.E.; Werner, A.S.; Haneke, B.; Parrish, D.; Frey, H.C.; Rojas-Bracho, L.; Deslauriers, M.; et al. Air emission inventories in North America: A critical assessment. J. Air Waste Manag. Assoc. 2006, 56, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Dao, L.G.; Morrison, L.; Zhang, H.X.; Zhang, C.S. Influences of traffic on Pb, Cu and Zn concentrations in roadside soils of an urban park in Dublin, Ireland. Environ. Geochem. Health 2014, 36, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.I.; Brooks, R.R.; Roberts, E.; Boswell, C.R. Heavy-metal pollution from automotive emissions and its effect on roadside soils and pasture species in New Zealand. Environ. Sci. Technol. 1977, 11, 917–920. [Google Scholar] [CrossRef]
- Chen, J.H.; He, F.; Zhang, X.H.; Sun, X.; Zheng, J.F.; Zheng, J.W. Heavy metal pollution decreases microbial abundance, diversity and activity within particle-size fractions of a paddy soil. Fems Microbiol. Ecol. 2014, 87, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liang, D.L.; Liu, J.J.; Lei, L.M.; Yu, D.S. Transformation of heavy metal fractions on soil urease and nitrate reductase activities in copper and selenium co-contaminated soil. Ecotoxicol. Environ. Saf. 2014, 110, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Friedel, J.K.; Molter, K.; Fischer, W.R. Comparison and improvement of methods for determing soil dehydrogenase activity using triphenyl chloride and iodonitrotetrazolium chloride. Biol. Fertil. Soils 1994, 18, 291–296. [Google Scholar] [CrossRef]
- Goldblith, S.A.; Proctor, B.E. Photometric determination of catalase activity. J. Biol. Chem. 1950, 187, 705–709. [Google Scholar] [PubMed]
- Van Slyke, D.D.; Archibald, R.M. Manometric, titrimetric, and colorimetric methods for measurement of urease activity. J. Biol. Chem. 1944, 154, 623–642. [Google Scholar]
- Rezacova, V.; Gryndler, M. Fluorescence spectroscopy: A tool to characterize humic substances in soil colonized by microorganisms? Folia Microbiol. 2006, 51, 215–221. [Google Scholar] [CrossRef]
- Song, S.J.; Wu, Y.; Jiang, J.K.; Yang, L.; Cheng, Y.; Hao, J.M. Chemical characteristics of size-resolved PM2.5 at a roadside environment in Beijing, China. Environ. Pollut. 2012, 161, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Huang, G.; Buyantuev, A.; Wu, J.; Luo, S.; Ma, K. Spatial heterogeneity of urban soils: The case of the Beijing metropolitan region, China. Ecol. Process 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Martins, Z.; Chan, H.S.; Sephton, M.A. Fluorescence spectroscopy as a life detection technique. In Proceedings of the European Planetary Science Congress, Rome, Italy, 19–24 September 2010; Volume 1, p. 600.
- Nielsen, M.N.; Winding, A.; Binnerup, S. Microorganisms as Indicators of Soil Health; National Environmental Research Institute: Copenhagen, Denmark, 2002. [Google Scholar]
- Makoi, J.; Ndakidemi, P.A. Selected soil enzymes: Examples of their potential roles in the ecosystem. Afr. J. Biotechnol. 2008, 7, 181–191. [Google Scholar]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, W.T.; Dick, W.A. Relationships between enzyme activities and microbial growth and activity indexes in soil. Soil Sci. Soc. Am. J. 1983, 47, 945–951. [Google Scholar] [CrossRef]
- Mobley, H.; Hausinger, R. Microbial Ureases: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85108. [Google Scholar]
- Turer, D.; Maynard, J.B.; Sansalone, J.J. Heavy metal contamination in soils of urban highways: Comparison between runoff and soil concentrations at Cincinnati, Ohio. Water Air Soil Pollut. 2001, 132, 293–314. [Google Scholar] [CrossRef]
- Luo, N.N.; Zhao, W.J.; Yan, X.; Guo, X.Y.; Guang, H.; Xiong, G.L. The spatial coupling relationship between atmoshperic particulates and heavy metal of surface soil in Beijing urban area. Ecol. Environ. Sci. 2013, 22, 1025–1031. [Google Scholar]
- Chen, X.; Xia, X.H.; Zhao, Y.; Zhang, P. Heavy metal concentrations in roadside soils and correlation with urban traffic in Beijing, China. J. Hazard. Mater. 2010, 181, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Gulser, F.; Erdogan, E. The effects of heavy metal pollution on enzyme activities and basal soil respiration of roadside soils. Environ. Monit. Assess. 2008, 145, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Liu, S.Q.; Zheng, D.W.; Feng, S.D. Effects of cadium, zinc and lead on soil enzyme activities. J. Environ. Sci. China 2006, 18, 1135–1141. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kucharski, J.; Lajszner, W. Enzymatic activities in different soils contaminated with copper. Polish J. Environ. Stud. 2005, 14, 659–664. [Google Scholar]
- Durrani, G.F.; Hassan, M.; Baloch, M.K.; Hameed, G. Effect of traffic pollution on plant photosynthesis. J. Chem. Soc. Pak. 2004, 26, 176–179. [Google Scholar]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leiros, M.C.; Seoane, S.; Gil-Sotres, F. Limitations of soil enzymes as indicators of soil pollution. Soil Biol. Biochem. 2000, 32, 1867–1875. [Google Scholar] [CrossRef]
- Koyama, A.; Wallenstein, M.D.; Simpson, R.T.; Moore, J.C. Carbon-Degrading Enzyme Activities Stimulated by Increased Nutrient Availability in Arctic Tundra Soils. PLoS ONE 2013, 8, e77212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, Y.; Miao, C.Y.; Xia, J.; Mao, L.; Wang, Y.F.; Zhou, P. Plant diversity reduces the effect of multiple heavy metal pollution on soil enzyme activities and microbial community structure. Front. Environ. Sci. Eng. 2012, 6, 213–223. [Google Scholar] [CrossRef]
- Marano, V.; Rizzoni, G. Energy and economic evaluation of PHEVs and their interaction with renewable energy sources and the power grid. In Proceedings of the IEEE International Conference on Vehicular Electronics and Safety, 2008, ICVES 2008. 22–24 September 2008; IEEE: New York, NY, USA, 2008; pp. 84–89. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Meng, L.; Herman, U.; Lu, Z.; Crittenden, J. A Survey of Soil Enzyme Activities along Major Roads in Beijing: The Implications for Traffic Corridor Green Space Management. Int. J. Environ. Res. Public Health 2015, 12, 12475-12488. https://doi.org/10.3390/ijerph121012475
Li T, Meng L, Herman U, Lu Z, Crittenden J. A Survey of Soil Enzyme Activities along Major Roads in Beijing: The Implications for Traffic Corridor Green Space Management. International Journal of Environmental Research and Public Health. 2015; 12(10):12475-12488. https://doi.org/10.3390/ijerph121012475
Chicago/Turabian StyleLi, Tianxin, Linglong Meng, Uwizeyimana Herman, Zhongming Lu, and John Crittenden. 2015. "A Survey of Soil Enzyme Activities along Major Roads in Beijing: The Implications for Traffic Corridor Green Space Management" International Journal of Environmental Research and Public Health 12, no. 10: 12475-12488. https://doi.org/10.3390/ijerph121012475
APA StyleLi, T., Meng, L., Herman, U., Lu, Z., & Crittenden, J. (2015). A Survey of Soil Enzyme Activities along Major Roads in Beijing: The Implications for Traffic Corridor Green Space Management. International Journal of Environmental Research and Public Health, 12(10), 12475-12488. https://doi.org/10.3390/ijerph121012475





