1. Introduction
The cytotoxic evaluation of e-cigarette aerosol is of great importance, although no regulations exist for the testing procedure so far. In vitro tests are a crucial instrument to study toxicological effects of inhaled substances, but along with technical challenges, the choice of the cell model is of great significance. In order to choose the right cell model, profound knowledge of the respiratory tract structure is essential.
The respiratory tract is divided into the upper and lower airways. The upper airways include nose, paranasal sinuses, pharynx, and larynx. The lower airways are further divided into the conducting and respiratory zones, containing about 24 airway generations (divisions) of branching airways, starting at the trachea. The conducting zone includes the trachea (generation “G” 0), bronchi (G 1–3), bronchioles, and terminal bronchioles (G 4–16), whereas the respiratory zone consists of respiratory bronchioles, alveolar ducts, and alveolar sacs (G 17–23) [
1]. All of these zones contain different types of cells.
When airborne substances are inhaled, the site of deposition in the lung is strongly driven by their chemical and physical properties [
2]. Whereas size plays an important role for particles, aerosol deposition is mainly dependent on its solubility. Due to the fact that the conducting airways are lined with mucus, water-soluble components are largely deposited in this area of the respiratory tract. The Henry’s law constant (H) of a substance gives information about its water-solubility and can therefore help to predict the place of deposition. Components with H greater than 1 mol/m³ Pa deposit relatively uniformly over the first 10 generations, those with H around 0.01 mol/m³ Pa between G 10 and G 15, and those with H lower than 10
−3 mol/m³ Pa do not deposit effectively until past G 20 and are therefore still present in the alveolar region [
3].
The main ingredients of e-cigarette liquids are glycerol and propylene glycol as carrier bases, nicotine, and flavors. Glycerol, propylene glycol and nicotine are highly water-soluble (H = 4.7 × 10
6; 7.6 × 10
2; 3.3 × 10
3 mol/m
3 Pa) [
4] and the flavors used are, with a few exceptions, also water-soluble. Therefore, deposition of the aerosol is expected in the conducting zone of the airways, more specifically in the bronchioles and terminal bronchioles, which are composed of goblet cells, club cells, basal cells, and ciliated cells.
For the evaluation of airborne substances, the adenocarcinomic human alveolar basal epithelial cell line A549 represents a prominent cell model [
5,
6,
7]. This cell line is derived from an explanted tumor and expresses some characteristic features of pulmonary alveolar type II cells [
8].
However, taking the deposition characteristics of aerosol compounds into consideration, cells from the conducting zone are a more suitable candidate than alveolar type II cells. Primary bronchial epithelial cells seem to be an adequate cell model because they are able to differentiate into an
in vivo-like epithelium containing mucus-producing and ciliated cells [
9]. However, due to their reduced lifespan, which is limited to four to five passages before losing some characteristic features of their phenotype, their use for routine testing is limited. Therefore, we included an immortalized normal human bronchial epithelial cell line in our experiments, expressing cyclin-dependent kinase (Cdk) 4 and human telomerase reverse transcriptase (hTERT). The combination of both retroviral constructs was chosen since the transfection of either gene alone was shown not to result in immortalization [
10]. The immortalized cell line has a prolonged lifespan, does not have a malignant phenotype, and shows a phenotype comparable to the primary parent cell.
In order to compare the cellular reactions after their contact with e-liquid aerosol, the cell models were exposed directly at the air-liquid interface in a CULTEX® RFS compact module. Two different propylene glycol-based e-liquids (0% nicotine 2.4% nicotine) were vaporized in an e-cigarette and tested for their cytotoxicity. Cigarette smoke and clean air served as positive and negative controls. In order to evaluate the cytotoxic potential of the components, the cells were analyzed 24 h post-exposure for their viability as well as their oxidative stress level.
2. Materials and Methods
2.1. E-Liquids and Cigarettes
The tested refill e-liquids were purchased from Johnsons Creek (Hartland, WI, USA). The e-liquids with the flavor “Tennessee Cured” are propylene glycol-based (75% propylene glycol, 25% glycerol) and have nicotine concentrations of 0.0% and 2.4% (24 mg/mL). The ingredients of the liquids are listed on the bottle and are as follows:
USP grade propylene glycol, USP grade vegetable glycerol, deionized water, natural flavors, artificial flavors, USP grade nicotine (not in 0.0%), USP grade citric acid (as a preservative)
For cigarette smoke exposure, K3R4F research cigarettes (University of Kentucky, Lexington, KY, USA) with a standard cellulose acetate filter tip were used.
2.2. Cell Cultivation
Normal human bronchial epithelial (NHBE) cells were isolated from a healthy tissue sample derived from a 75-year-old patient with a non-small cell lung cancer (NSCLC) after lobectomy (Bielefeld Evangelical Hospital, Bielefeld, Germany). The cells received were named NHBE48 [
11]. In accordance with the Declaration of Helsinki, the subject gave his informed consent to the research use of the lung tissue samples removed. After the first passage, NHBE cells were cultivated in collagen IV-coated culture flasks using AEGM medium. After reaching 80%–90% confluence, the cells were seeded on collagen IV-coated cell culture inserts (seeding density: 2.1 × 10
5/cm
2).
For the exposure experiments, the cells were cultivated under submerged conditions and supplied with AEGM medium for one day before the apical medium was removed and the cells were transferred to the exposure module. The exposure experiments were performed with cells of passages 2–4.
For immortalization, the NHBE48 cells were transduced with third generation state-of-the-art lentiviral constructs containing cyclin-dependent kinase (Cdk) 4 and human telomerase reverse transcriptase (hTERT). The immortalized cell line was named CL-1548 (SIRION Biotech GmbH, Martinsried, Germany). CL-1548 cells were cultivated in regular cell culture flasks using AEGM medium incl. supplements (as supplied by the manufacturer), G418 (50 µg/mL) and puromycin (0.3 µg/mL). After reaching 80%–90% confluence, the cells were seeded on collagen IV-coated cell culture inserts (12 mm diameter, seeding density: 2.5 × 105/cm2). For the exposure experiments, the cells were cultivated under submerged conditions for one day before the apical medium was removed and the cells were transferred to the exposure module. Cells of passages 10–11 were used for the experiments.
In order to compare their differentiation capacity, NHBE48 and CL-1548 cells were additionally cultivated air-lifted over a time period of 24 days. They were seeded on collagen IV-coated cell culture inserts after reaching 80%–90% confluence, as described above. The apical medium was removed after obtaining 100% confluence and the basal cell culture medium was replaced by PneumaCult™ ALI medium. The medium was changed daily. The cells were cultivated air-lifted for a further 21 days and then analyzed for their differentiation capacity based on histological sections stained with hematoxylin and eosin (H&E stain).
A549 cells were obtained from the American Type Culture Collection (ATCC, USA, CCL-185). A549 cells were cultivated in regular cell culture flasks using DMEM medium incl. 10% FCS and 0.05% gentamycin. After reaching 80%–90% confluence, the cells were seeded on cell culture inserts (12 mm diameter, seeding density: 2.5 × 105/cm2). The cells were cultivated under submerged conditions for one day before the apical medium was removed and the cells were transferred to the exposure module. Cells of passages 13–17 were used for the experiments.
For all cell types, DMEM medium + 0.05% gentamycin was used during the exposure experiments. Post-incubation of the cells was carried out using the cell culture medium described above for each cell line.
DMEM medium was obtained from Life Technologies (Carlsbad, CA, USA), G418 and puromycin from Applichem (Darmstadt, Germany), gentamycin and FCS from Biochrom (Cambridge, UK), PneumaCult™ ALI medium from STEMCELL Technologies (Vancouver, Canada) and AEGM medium from PromoCell (Heidelberg, Germany).
2.3. Exposure
All exposure experiments were performed in a CULTEX® RFS compact module (Cultex Laboratories GmbH, Hannover, Germany), exposing six cell culture inserts at a time in each experimental run. For e-cigarette experiments, a Reevo Mini-S e-cigarette (In-Smoke, Winnenden, Germany) was used, equipped with a 3.3V/900 mAh battery and a vaporizer with a resistance of 2.2 Ohm. The e-cigarette was connected to the piston pump of a smoking robot and 200 puffs were taken with a puff volume of 35 mL, a puff duration of 2 s, a blow-out time of 7 s and an interpuff interval of 10 s. The smoking robot was operated in asynchronous mode, meaning that puffs were taken successively, so that the cells were exposed to aerosol during the whole experimental phase. For better distribution, the e-liquid aerosol was diluted with synthetic air (1 L/min) before being sucked into the CULTEX® RFS compact via a vacuum pump with a flow rate of 5 mL/min/insert.
For mainstream smoke exposure, 10 K3R4F cigarettes were smoked by the smoking robot using the same parameters as described for the e-cigarette. Each cigarette was puffed six times. The freshly generated mainstream smoke was equally diluted with synthetic air (1 L/min) and also entered the CULTEX® RFS compact with a rate of 5 mL/min/insert.
The clean air exposure (clean air control) was performed for 30 min (corresponding to the exposure time for 200 puffs of e-liquid aerosol) and with the flow rates described above.
As a second control, cell cultures were used that remained air-lifted in the incubator for the exposure time (incubator control).
The flow rates were controlled by mass flow controllers (IQ + Flow and EL-Flow Select, Bronckhorst, Ruurlo, The Netherlands). The exhaust air was directed back to the fume hood.
2.4. Cell Viability Assay
The analyses were done 24 h after the exposure, allowing the cells to respond to the exposure. In order to analyze cell viability and oxidative stress in the same cell, two cell-based assays were combined. The oxidative stress was analyzed first, using the ROS-Glo™ H2O2 Assay (Promega, Madison, WI, USA). Afterwards, cell viability was measured using the CellTiter-Blue® Assay (Promega).
For the ROS-Glo™ H2O2 Assay, AEGM medium (200 µL) and H2O2 substrate solution (50 µL) were added on the surface of the cells. After 3 h incubation at 37 °C/5% CO2, 75 µL of the solution was transferred into a white 96-well plate. Detection solution (75 µL) was added and after 20 min incubation at room temperature, the relative luminescence was measured.
In order to measure the cell viability in the same cell culture insert, the remaining medium was removed from the cells, and 300 µL AEGM medium as well as 60 µL CellTiter-Blue® solution were added. The cultures were then incubated for 2 h at 37 °C/5% CO2. Afterwards, 100 mL of the solution were pipetted into a black 96-well plate to measure the fluorescence at 544Ex/590Em nm.
2.5. Histology
For histological investigations, the cultures were fixed with 10% formalin for one hour. Following the fixation, the membranes were washed twice with water, released from the inserts and embedded in paraffin. Using a microtome, sections of 5 µm thickness were prepared, beginning at the middle of the membrane. After deparaffinization, sections were stained with hematoxylin and eosin.
2.6. Statistical Analysis
Significant differences between the two groups were evaluated by Student’s unpaired t-test, whereas the asterisks are defined as followed: **** p < 0.0001; *** p = 0.0001–0.001; ** p = 0.001–0.01; * p = 0.01–0.05.
3. Results and Discussion
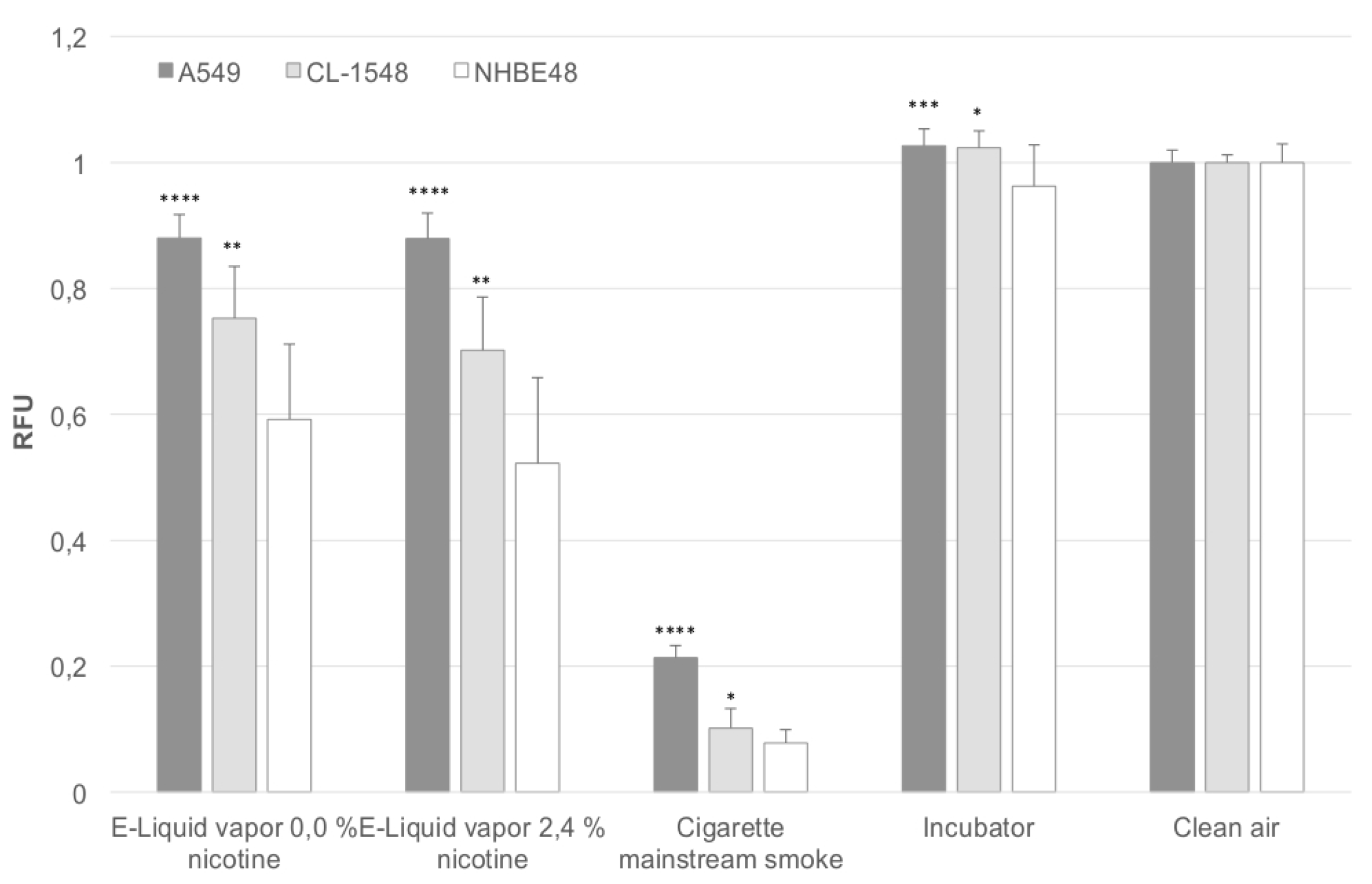
Figure 1 shows the relative viability of cells after direct exposure to aerosol of e-liquids and mainstream cigarette smoke. The results are normalized to the clean air control. In addition, the results obtained after mainstream smoke exposure are adjusted to the number of puffs taken. During e-cigarette exposure, 200 puffs were taken, in the case of regular combustible cigarettes, 60 puffs were taken. Due to the fact that the cell viability decreases with an increasing number of puffs, the cell viability was divided by 3.33 (200 divided by 60) in the case of mainstream smoke-exposed cells. This adjustment could be done, since the cell viability decreases linearly with an increasing number of puffs, as determined in previous experiments. Primary NHBE48 cells represent the most sensitive cells, responding to e-liquid aerosol exposure with a decrease in viability up to 60% and 52% compared to clean air-exposed cells. Cigarette mainstream smoke-exposed cells only show 7% viability of clean air-exposed cells.
Immortalized CL-1548 cells are less sensitive to e-liquid aerosol (75% and 70% viability) and mainstream smoke exposure (10% viability) compared to primary NHBE48 cells, but are still significantly more sensitive than A549 cells (88% viability for e-liquid aerosol, 21% for mainstream smoke exposure). In all cell types, no significant differences were seen after the exposure to nicotine-containing and nicotine-free aerosol.
Figure 1.
Puff-adjusted values for cell viability after exposure. The results are normalized to the clean air control and are given as a mean of five (NHBE48) and three (CL-1548, A549) independent experiments with three samples each + standard deviation. The asterisks indicate the statistical significance compared to NHBE48 cells. The relevance of the significance is explained in
Section 2.6.
Figure 1.
Puff-adjusted values for cell viability after exposure. The results are normalized to the clean air control and are given as a mean of five (NHBE48) and three (CL-1548, A549) independent experiments with three samples each + standard deviation. The asterisks indicate the statistical significance compared to NHBE48 cells. The relevance of the significance is explained in
Section 2.6.
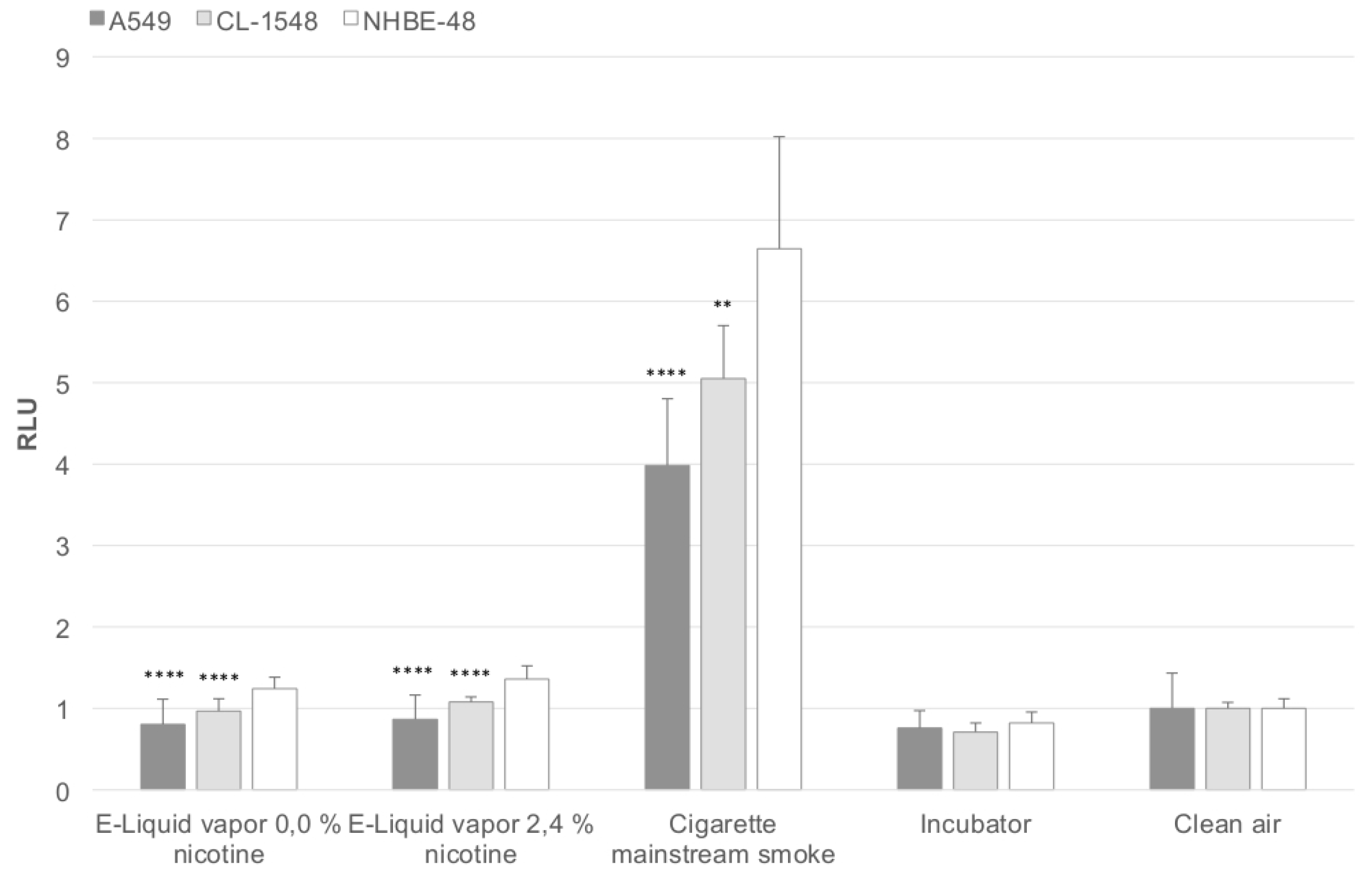
In
Figure 2, the corresponding oxidative stress levels are shown, reflecting the results obtained after viability measurements. The oxidative stress level is elevated in CL-1548 cells compared to A549 cells, but lower than those of primary NHBE48 cells.
Figure 2.
Puff-adjusted values for the oxidative stress level after exposure. The results are normalized to the clean air control and are given as a mean of five (NHBE48) and three (CL-1548, A549) independent experiments with three samples each + standard deviation. The asterisks indicate the statistical significance compared to NHBE48 cells. The relevance of the significance is explained in
Section 2.6.
Figure 2.
Puff-adjusted values for the oxidative stress level after exposure. The results are normalized to the clean air control and are given as a mean of five (NHBE48) and three (CL-1548, A549) independent experiments with three samples each + standard deviation. The asterisks indicate the statistical significance compared to NHBE48 cells. The relevance of the significance is explained in
Section 2.6.
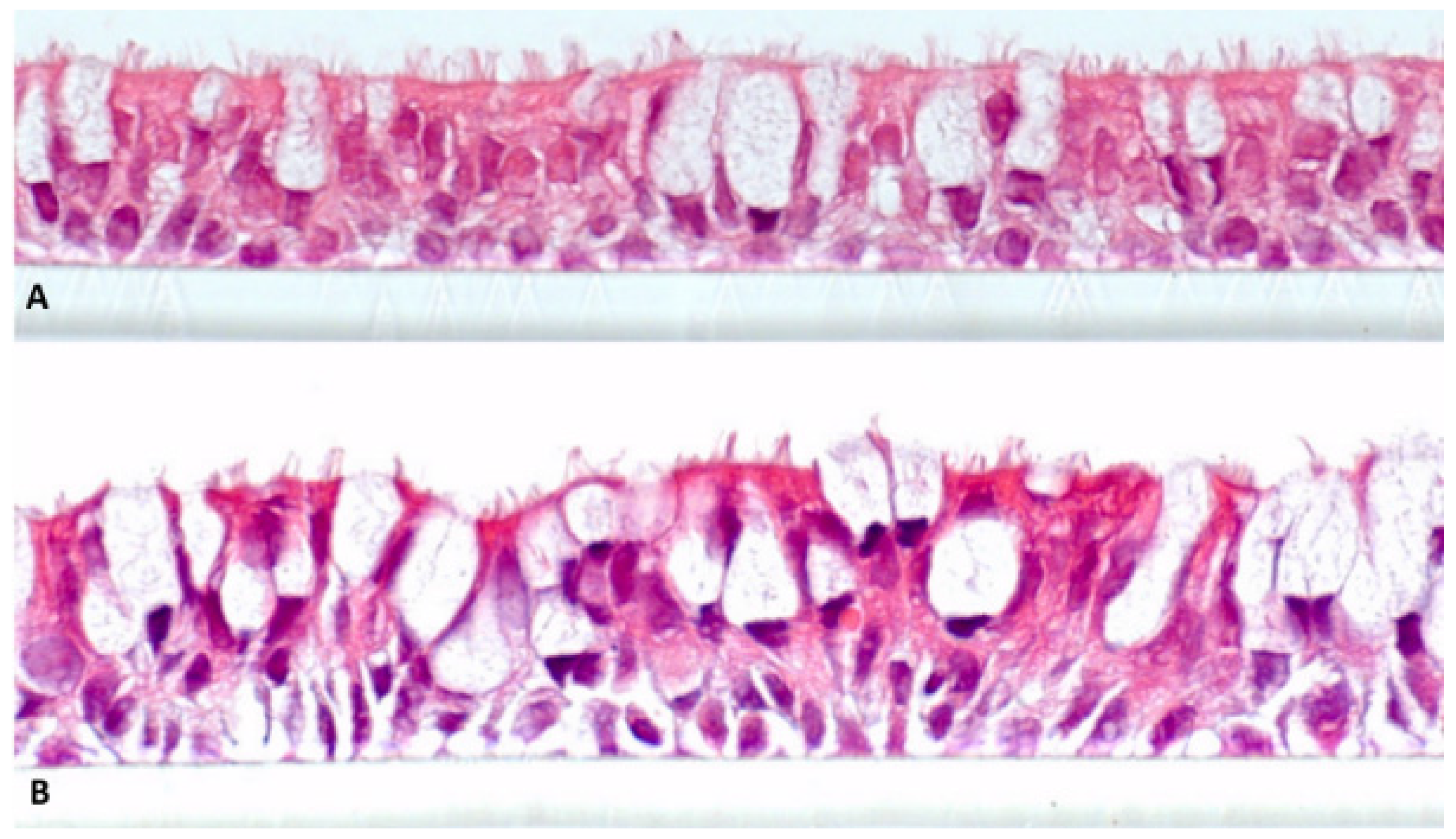
Figure 3 shows H&E-stained sections of NHBE48 and CL-1548 cells, cultivated for 21 days under air-lifted conditions (total cultivation time: 24 days). It is obvious that both cell cultures show a pseudo-stratified structure and differentiation characteristics of the lung epithelium
in vivo. The basal layers consist of basal cells, and at the luminal cell layer, ciliated and mucus-producing cells are detectable.
Figure 3.
Cell cultures differentiated at the air-liquid interface for 21 days. (a) Primary normal human bronchial epithelial cells NHBE48; (b) Immortalized normal human bronchial epithelial cells (CL-1548). Magnification 630 × 10.
Figure 3.
Cell cultures differentiated at the air-liquid interface for 21 days. (a) Primary normal human bronchial epithelial cells NHBE48; (b) Immortalized normal human bronchial epithelial cells (CL-1548). Magnification 630 × 10.
4. Discussion
Primary normal human bronchial epithelial cells are an important tool for the in vitro toxicity analysis of e-liquids, but their short lifespan makes them unfavorable for routine testing. Therefore, an immortalized cell line generated by transducing primary normal human bronchial epithelial cells with cyclin-dependent kinase (Cdk) 4 and human telomerase reverse transcriptase (hTERT) was included in our investigations. The cell line CL-1548, exhibiting a mucociliary differentiation capacity, was tested in a direct acute toxicity approach using e-liquid aerosol and cigarette mainstream smoke.
The differentiation capacity of the immortalized cell line CL-1548 is comparable to its parent cells NHBE48. After 21 days of cultivation at the air-liquid interface, both cell cultures show a pseudostratified morphology consisting of basal, mucus-producing, and ciliated cells (
Figure 2). This property is a crucial factor for toxicity tests dealing with prolonged and repeated exposure to study chronic effects. Further characterizations have been carried out using immunohistological staining. Several cell types have been detected, including mucus-producing, ciliated, and basal cells. A detailed immunohistological characterization will be released in an independent publication.
Regarding
Figure 1 and
Figure 2, it is obvious that the sensitivity of CL-1548 cells to e-cigarette aerosol and cigarette mainstream smoke is slightly reduced compared to primary cells, but still higher than that of A549 cells. Furthermore, the relation between the viability of e-cigarette aerosol (0.0% and 2.4% nicotine) and mainstream smoke-exposed cells remains similar (factor 7.45 and 6.94 for immortalized cells, 7.68 and 6.77 for primary cells) in the case of CL-1548 and NHBE48 cells. In the case of A549 cells, the viability difference between e-liquid aerosol and cigarette mainstream smoke-exposed cells is significantly lower (4.1 for both liquids), clarifying that the cells have a different response characteristic. The oxidative stress levels reflect these results. Oxidative stress is highest in primary NHBE48 cells. A549 and CL-1548 cells show lower oxidative stress levels, whereas those of CL-1548 cells are still higher than those of A549 cells.
5. Conclusions
In conclusion, we present an immortalized normal human bronchial epithelial cell line with a phenotype comparable to the primary parent cell, which is suitable for use in the in vitro testing of e-liquid aerosols. Although the immortalized cell line shows slightly lower sensitivity to e-liquid aerosol and mainstream smoke exposure than the parent primary cell, it is still preferable to the widely used adenocarcinoma cell line A549. Besides the higher sensitivity compared to A549 cells, the tested immortalized cell line CL-1548 is capable of differentiating into an in vivo-like epithelium with basal, mucus-producing, and ciliated cells. This property is crucial for toxicological evaluations with regard to long-term (chronic) effects. In this case, fully differentiated cell cultures are used to represent the healthy epithelium in vivo. Furthermore, A549 cells represent a model for alveolar type II cells, which are not the main target of e-liquid aerosol. Due to its water solubility, it is likely be deposited in the conducting zone of the airways, and does not reach the alveolar region. Therefore, a bronchial epithelial cell is more suitable. Although primary NHBE cells represent the “gold standard” for in vitro toxicity testing, the immortalized cell line CL-1548 might be a good replacement for routine testing due to its prolonged lifespan and the resulting higher reproducibility of results. The differentiation capacity of the cell line CL-1548 presented here further allows its use for analyzing chronic effects after prolonged and repeated e-liquid aerosol exposure.