Survival of Salmonella enterica in Aerated and Nonaerated Wastewaters from Dairy Lagoons
Abstract
:1. Introduction
2. Experimental Section
2.1. Dairy Manure Flush Wastewater
| Chemical component a | Summer Sampling b | Winter Sampling c | |||||
|---|---|---|---|---|---|---|---|
| Dairy A-CirCulators | Dairy A-Settling Lagoon | Dairy B-Settling Lagoon 2 | Dairy A-CirCulators | Dairy A-Settling Lagoon | Dairy B-Settling Lagoon 1 | Dairy B-Settling Lagoon 2 | |
| N | 278 | 318 | 512 | 1284 | 1476 | 1022 | 1035 |
| P | 89 | 82 | 53 | 67 | 105 | 107 | 130 |
| K | 465 | 395 | 363 | 695 | 813 | 811 | 481 |
| S | 67 | 30 | 7.0 | 69 | 90 | 36 | 37 |
| Mg | 90 | 95 | 102 | 129 | 158 | 120 | 126 |
| Ca | 96 | 123 | 140 | 255 | 332 | 182 | 194 |
| Na | 194 | 194 | 151 | 342 | 394 | 185 | 160 |
| Fe | 8.4 | 34 | 4.8 | 9.1 | 8.8 | 11 | 18 |
| Al | 3.6 | 5.1 | 1.7 | 5.6 | 7.9 | 9.8 | 5.4 |
| Mn | 0.3 | 0.8 | 0.6 | 1.5 | 2.3 | 1.3 | 1.3 |
| Cu | <0.1 | <0.1 | <0.1 | 0.5 | 0.6 | 0.5 | 0.3 |
| Zn | 0.1 | <0.1 | <0.1 | 1.8 | 2.2 | 1.4 | 1.1 |
| EC | 3.8 | 4.2 | 4.9 | 8.0 | 9.3 | 5.6 | 4.9 |
| OM | 500 | 1500 | 1800 | 3873 | 5701 | 2298 | 2554 |
| NO3− | <0.5 | <0.5 | <0.5 | <0.5 | 0.6 | <0.5 | <0.5 |
| NH4+ | 181 | 255 | 270 | 396 | 642 | 332 | 345 |
| BOD | 506 | 453 | 578 | 2163 | 2410 | 927 | 476 |
| COD | 1541 | 1785 | 1825 | 1980 | 2500 | 460 | 762 |
| TSS | 280 | 335 | 230 | 1867 | 2765 | 393 | 840 |
| C:N ratio | 1:1 | 3:1 | 2:1 | 2:1 | 2:1 | 1:1 | 1:1 |
2.2. Isolation of Salmonella from Manure and Wastewaters
2.3. Fate of Salmonella in Wastewater
| Organism a | Strain No. | Source | Details b |
|---|---|---|---|
| Salmonella enterica serovar Enteritidis | MM155 | Almond outbreak | RM2970; original isolation from soil drag swab, 10/01, LJH 620; PT30; University of California (UC), Davis |
| S. enterica serovar Montevideo | MM156 | Almond outbreak | RM2977; LJH 627, UC, Davis |
| S. enterica serovar Thompson | MM157 | Stool from 14 year old girl from Pennsylvania | RM2270; original source, CDC, the Salmonella Reference Laboratory |
2.4. Monitoring Salmonella
2.5. Statistical analysis.
3. Results
3.1. Chemistry of Wastewaters
3.2. Salmonella in Wastewater from Dairies
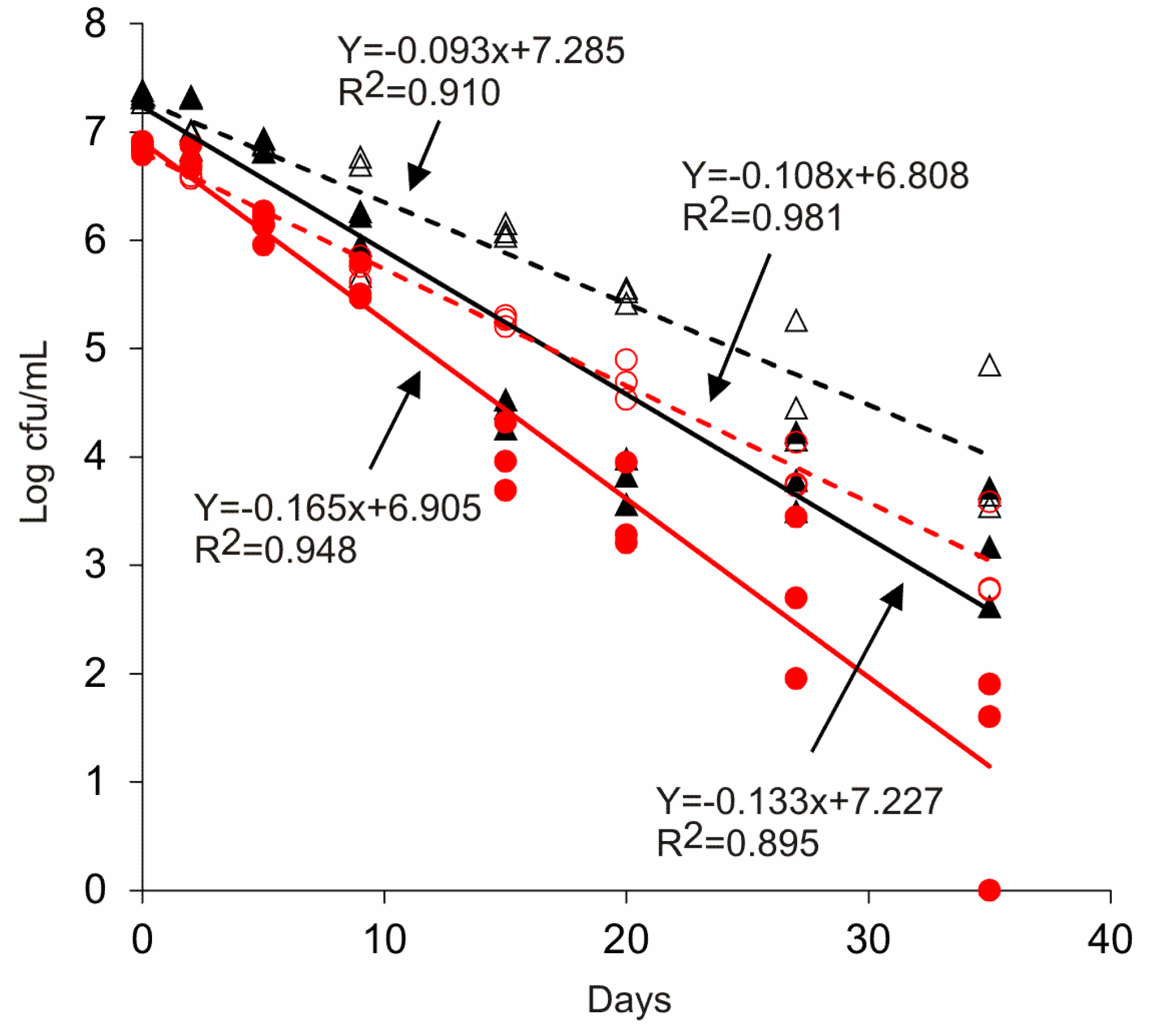
3.3. Survival of Salmonella in Wastewater
| S. enterica Serovars | D-value a (Fastest/Slowest) | Wastewater Source |
|---|---|---|
| Enteritidis | 1.4 | Circulated—dairy A |
| 6.1 | Settling—dairy A | |
| Montevideo | 1.7 | Circulated—dairy A |
| 11.1 | Settling lagoon 1—dairy B | |
| Thompson | 1.8 | Circulated—dairy A |
| 9.4 | Settling—dairy A |
| Serovar a | Strain | D-value, d | Mean | p-value | α at 0.05 | |
|---|---|---|---|---|---|---|
| Aerated | Not Aerated | (Serovars) | ||||
| Enteritidis | MM155 | 2.3 b | 5.3 b | 3.8 | 0.084 | 0.316 |
| Montevideo | MM156 | 5.4 a | 9.0 a | 7.2 | ||
| Thompson | MM157 | 4.8 ab | 8.0 a | 6.4 | ||
| Mean (aeration) | 4.2 B | 7.4 A | ||||
| p-value | 0.005 | |||||
| α at 0.05 | 0.793 | |||||
| Manure Water Source | Aeration in Microcosms | D-value, Days a | Mean (Water Source) | α at 0.05 | p-value | |
|---|---|---|---|---|---|---|
| Montevideo | Thompson | |||||
| Dairy A—CirCulators | Aerated | 7.6 b | 6.1 b | 6.9 C | 0.987 | <0.001 |
| Dairy A—settling lagoon | Nonaerated | 11.0 a | 9.4 a | 10.2 A | ||
| Dairy B—settling lagoon 1 | Nonaerated | 11.1 a | 9.4 a | 10.2 A | ||
| Dairy B—settling lagoon 2 | Nonaerated | 8.2 b | 7.9 ab | 8.0 B | ||
| Mean (serovar) | 9.5 A | 8.2 B | ||||
| α at 0.05 | 0.552 | |||||
| p-value | 0.026 | |||||
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef] [PubMed]
- DeWaal, C.S.; Glassman, M.S. Outbreak Alert! 2014. Center for Science in the Public Interest. Available online: http://cspinet.org/reports/outbreakalert2014.pdf (accessed on 4 June 2014).
- Pell, A.N. Manure and microbes: Public and animal health problem? J. Dairy Sci. 1997, 80, 2673–2681. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Walters, L.D.; Avery, S.M.; Munro, F.; Moore, A. Analyses of livestock production, waste storage, and pathogen levels and prevalences in farm manures. Appl. Environ. Microbiol. 2005, 71, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, M.L.; Walters, L.D.; Avery, S.M.; Synge, B.A.; Moore, A. Levels of zoonotic agents in British livestock manures. Lett. Appl. Microbiol. 2004, 39, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Fossler, C.P.; Wells, S.J.; Kaneene, J.B.; Ruegg, P.L.; Warnick, L.D.; Bender, J.B.; Godden, S.M.; Halbert, L.W.; Campbell, A.M.; Zwald, A.M.; et al. Prevalence of Salmonella spp. on conventional and organic dairy farms. J. Amer. Vet. Med. Assn. 2004, 225, 567–573. [Google Scholar] [CrossRef]
- Fossler, C.P.; Wells, S.J.; Kaneene, J.B.; Ruegg, P.L.; Warnick, L.D.; Bender, J.B.; Eberly, L.E.; Godden, S.M.; Halbert, L.W. Herd-level factors associated with isolation of Salmonella in a multi-state study of conventional and organic dairy farms I Salmonella shedding in cows. Prev. Vet. Med. 2005, 70, 257–277. [Google Scholar] [CrossRef]
- Cooley, M.B.; Quinones, B.; Oryang, D.; Mandrell, R.E.; Gorski, L. Prevalence of shiga toxin producing Escherichia coli, Salmonella enterica, and Listeria monocytogenes at public access watershed sites in a California Central Coast agricultural region. Front. Cell. Infect. Microbiol. 2014. [Google Scholar] [CrossRef]
- Gorski, L.; Parker, C.T.; Liang, A.; Cooley, M.B.; Jay-Russell, M.T.; Gordus, A.G.; Atwill, E.R.; Mandrell, R.E. Prevalence, distribution, and diversity of Salmonella enterica in a major produce region of California. Appl. Environ. Microbiol. 2011, 77, 2734–2748. [Google Scholar] [CrossRef] [PubMed]
- Kinde, H.; Castellan, D.M.; Kass, P.H.; Ardans, A.; Cutler, G.; Breitmeyer, R.E.; Bell, D.D.; Ernst, R.A.; Kerr, D.C.; Little, H.E.; et al. The occurrence and distribution of Salmonella Enteritidis and other serovars on California egg laying premises: A comparison of two sampling methods and two culturing techniques. Avian Dis. 2004, 48, 590–594. [Google Scholar] [CrossRef]
- Watabe, M.; Rao, J.R.; Stewart, T.A.; Xu, J.; Millar, B.C.; Xiao, L.; Lowery, C.J.; Dooley, J.S.; Moore, J.E. Prevalence of bacterial faecal pathogens in separated and unseparated stored pig slurry. Lett. Appl. Microbiol. 2003, 36, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.O.; Malin, C.; Gstraunthaler, G.; Illmer, P. Survival of selected pathogens in diluted sludge of a thermophilic waste treatment plant and in NaCl-solution under aerobic and anaerobic conditions. Waste Manage. 2009, 29, 425–429. [Google Scholar] [CrossRef]
- Uesugi, A.R.; Danyluk, M.D.; Mandrell, R.E.; Harris, L.J. Isolation of Salmonella Enteritidis phage type 30 from a single almond orchard over a 5-year period. J. Food Prot. 2007, 70, 1784–1789. [Google Scholar]
- Himathongkham, S.; Riemann, H.; Bahari, S.; Nuanualsuwan, S.; Kass, P.; Cliver, D.O. Survival of Salmonella typhimurium and Escherichia coli O157:H7 in poultry manure and manure slurry at sublethal temperatures. Avian Dis. 2000, 44, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Himathongkham, S.; Bahari, S.; Riemann, H.; Cliver, D. Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol. Lett. 1999, 178, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Ceustermans, A.; de Clercq, D.; Aertsen, A.; Michiels, C.; Geeraerd, A.; van Impe, J.; Coosemans, J.; Ryckeboer, J. Inactivation of Salmonella Senftenberg strain W 775 during composting of biowastes and garden wastes. J. Appl. Microbiol. 2007, 103, 53–64. [Google Scholar] [CrossRef]
- Grewal, S.K.; Rajeev, S.; Sreevatsan, S.; Michel, F.C., Jr. Persistence of Mycobacterium avium subsp. paratuberculosis and other zoonotic pathogens during simulated composting, manure packing, and liquid storage of dairy manure. Appl. Environ. Microbiol. 2006, 72, 565–574. [Google Scholar]
- Lemunier, M.; Francou, C.; Rousseaux, S.; Houot, S.; Dantigny, P.; Piveteau, P.; Guzzo, J. Long-term survival of pathogenic and sanitation indicator bacteria in experimental biowaste composts. Appl. Environ. Microbiol. 2005, 71, 5779–5786. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, F.A.; Groves, S.J.; Chambers, B.J. Pathogen survival during livestock manure storage and following land application. Bioresour. Technol. 2005, 96, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ravva, S.V.; Sarreal, C.Z.; Duffy, B.; Stanker, L.H. Survival of Escherichia coli O157:H7 in wastewater from dairy lagoons. J. Appl. Microbiol. 2006, 101, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Paluszak, Z.; Skowron, K.; Olszewska, H.; Skowron, K.J.; Bauza-Kaszewska, J.; Gryn, G. Sanitization efficacy of anaerobic digestion and aeration of slurry from the aspect of limiting emission of Salmonella into the environment. Ann. Agric. Environ. Med. 2012, 19, 427–430. [Google Scholar] [PubMed]
- Rumburg, B.; Neger, M.; Mount, G.H.; Yonge, D.; Filipy, J.; Swain, J.; Kincaid, R.; Johnson, K. Liquid and atmospheric ammonia concentrations from a dairy lagoon during an aeration experiment. Atmos. Environ. 2004, 38, 1523–1533. [Google Scholar] [CrossRef]
- Hammack, T.S.; Amaguana, R.M.; Andrews, W.H.; Lerner, I. Rappaport-Vassiliadis medium for recovery of Salmonella spp. from low microbial load foods: Collaborative study. J. AOAC Int. 2001, 84, 65–83. [Google Scholar] [PubMed]
- Weather Underground. Weather Forecast and Report. Available online: http://www.wunderground.com (accessed on 28 October 2014).
- Placha, I.; Venglovsky, J.; Makova, Z.; Martinez, J. The elimination of Salmonella typhimurium in sewage sludge by aerobic mesophilic stabilization and lime hydrated stabilization. Bioresour. Technol. 2008, 99, 4269–4274. [Google Scholar] [PubMed]
- Degnan, A.J. Examination of indigenous microbiota and survival of Escherichia coli 0157 and Salmonella in a paper milling environment. J. Appl. Microbiol. 2008, 104, 534–540. [Google Scholar] [PubMed]
- Hutchison, M.L.; Walters, L.D.; Moore, T.; Thomas, D.J.I.; Avery, S.M. Fate of pathogens present in livestock wastes spread onto fescue plots. Appl. Environ. Microbiol. 2005, 71, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Himathongkham, S.; Riemann, H. Destruction of Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes in chicken manure by drying and/or gassing with ammonia. FEMS Microbiol. Lett. 1999, 171, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Park, G.W.; Diez-Gonzalez, F. Utilization of carbonate and ammonia-based treatments to eliminate Escherichia coli O157:H7 and Salmonella Typhimurium DT104 from cattle manure. J. Appl. Microbiol. 2003, 94, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Ravva, S.V.; Korn, A. Extractable organic components and nutrients in wastewater from dairy lagoons influence the growth and survival of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Ravva, S.V.; Sarreal, C.Z.; Mandrell, R.E. Identification of protozoa in dairy lagoon wastewater that consume Escherichia coli O157:H7 preferentially. PLoS One 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Huws, S.A.; Morley, R.J.; Jones, M.V.; Brown, M.R.; Smith, A.W. Interactions of some common pathogenic bacteria with Acanthamoeba polyphaga. FEMS Microbiol. Lett. 2008, 282, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Gourabathini, P.; Brandl, M.T.; Redding, K.S.; Gunderson, J.H.; Berk, S.G. Interactions between food-borne pathogens and protozoa isolated from lettuce and spinach. Appl. Environ. Microbiol. 2008, 74, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, M.L.; Walters, L.D.; Moore, A.; Avery, S.M. Declines of zoonotic agents in liquid livestock wastes stored in batches on-farm. J. Appl. Microbiol. 2005, 99, 58–65. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Rankin, S.C.; Aceto, H.W.; Benson, C.E.; Toth, J.D.; Dou, Z. Survival of Salmonella enterica serovar Newport in manure and manure-amended soils. Appl. Environ. Microbiol. 2006, 72, 5777–5783. [Google Scholar] [CrossRef] [PubMed]
- Duffy, B.; Sarreal, C.; Ravva, S.; Stanker, L. Effect of molasses on regrowth of E.coli O157:H7 and Salmonella in compost teas. Compost Sci. Util. 2004, 12, 93–96. [Google Scholar] [CrossRef]
- Sidhu, J.; Gibbs, R.A.; Ho, G.E.; Unkovich, I. Selection of Salmonella Typhimurium as an indicator for pathogen regrowth potential in composted biosolids. Lett. Appl. Microbiol. 1999, 29, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Lung, A.J.; Lin, C.M.; Kim, J.M.; Marshall, M.R.; Nordstedt, R.; Thompson, N.P.; Wei, C.I. Destruction of Escherichia coli O157:H7 and Salmonella Enteritidis in cow manure composting. J. Food Protect. 2001, 64, 1309–1314. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravva, S.V.; Sarreal, C.Z. Survival of Salmonella enterica in Aerated and Nonaerated Wastewaters from Dairy Lagoons. Int. J. Environ. Res. Public Health 2014, 11, 11249-11260. https://doi.org/10.3390/ijerph111111249
Ravva SV, Sarreal CZ. Survival of Salmonella enterica in Aerated and Nonaerated Wastewaters from Dairy Lagoons. International Journal of Environmental Research and Public Health. 2014; 11(11):11249-11260. https://doi.org/10.3390/ijerph111111249
Chicago/Turabian StyleRavva, Subbarao V., and Chester Z. Sarreal. 2014. "Survival of Salmonella enterica in Aerated and Nonaerated Wastewaters from Dairy Lagoons" International Journal of Environmental Research and Public Health 11, no. 11: 11249-11260. https://doi.org/10.3390/ijerph111111249




