Enteric Pathogen Survival Varies Substantially in Irrigation Water from Belgian Lettuce Producers
Abstract
:1. Introduction
2. Experimental Section
2.1. Strains and Growth Conditions
2.2. Irrigation Water Samples
2.3. Pathogen Inoculation of Irrigation Water Samples
2.4. Quantification of Pathogen Survival in the Irrigation Water Samples
| Water Treatment | Inoculation of the Water with | Storage Temperature of Inoculated Water | ||||
|---|---|---|---|---|---|---|
| Salmonella Thypimurium MB3885 | Salmonella Thompson RM1987N | E. coli O157:H7 MB3885 | E. coli O157:H7 NCTC12900 | Non Inoculated | ||
| untreated | x | x | x | 4 °C | ||
| filter sterilized | x | x | x | 4 °C | ||
| untreated | x | x | x | x | x | 20 °C |
| filter sterilized | x | x | x | 20 °C | ||
2.5. Quantification of the Resident Heterotrophic Bacteria in the Irrigation Water Samples
2.6. Plant Growth Conditions
2.7. Pathogen Inoculation of Lettuce Leaves
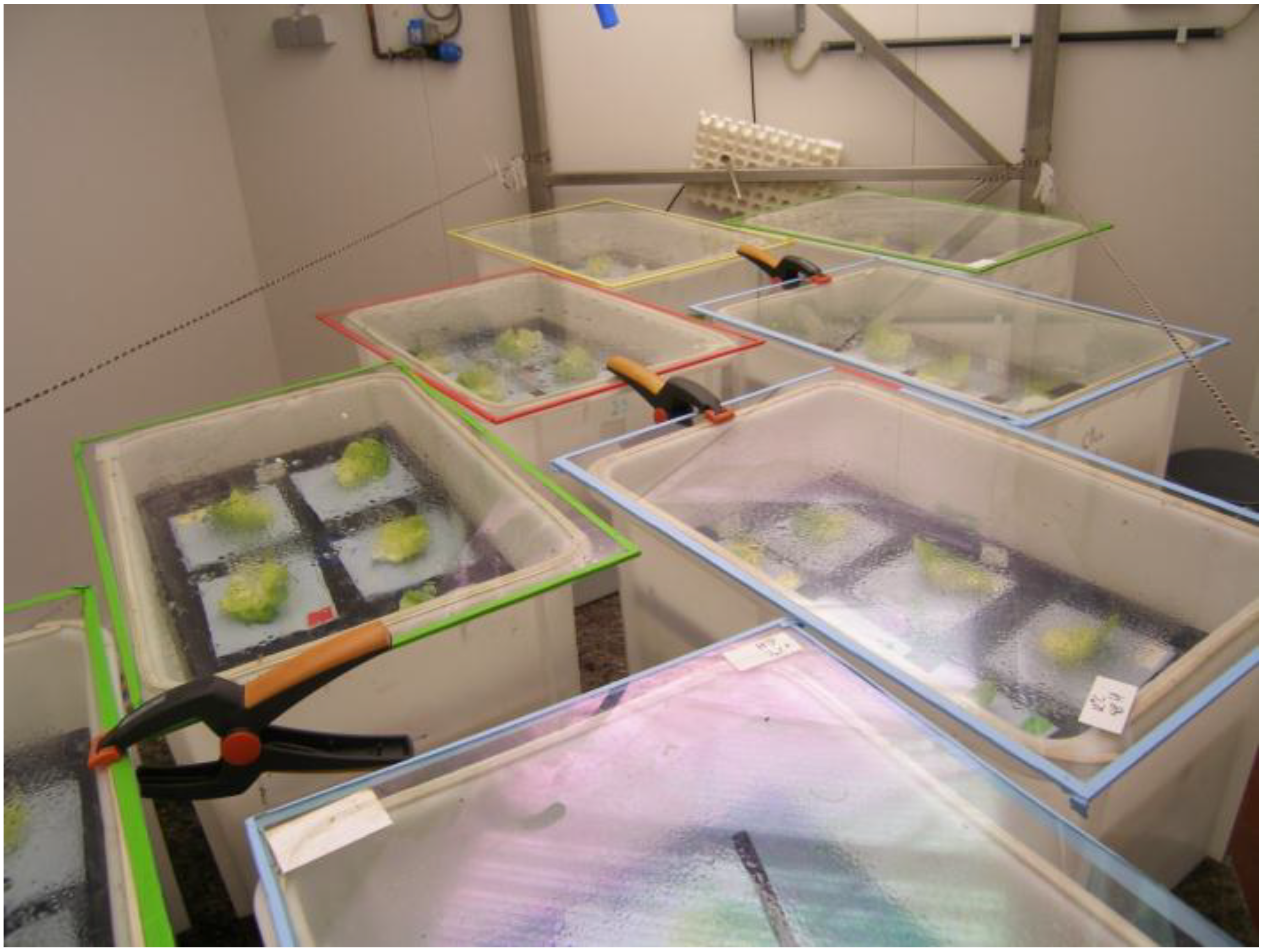
2.8. Quantification of Pathogen Survival on the Lettuce Leaves
2.9. Statistical Analysis
3. Results
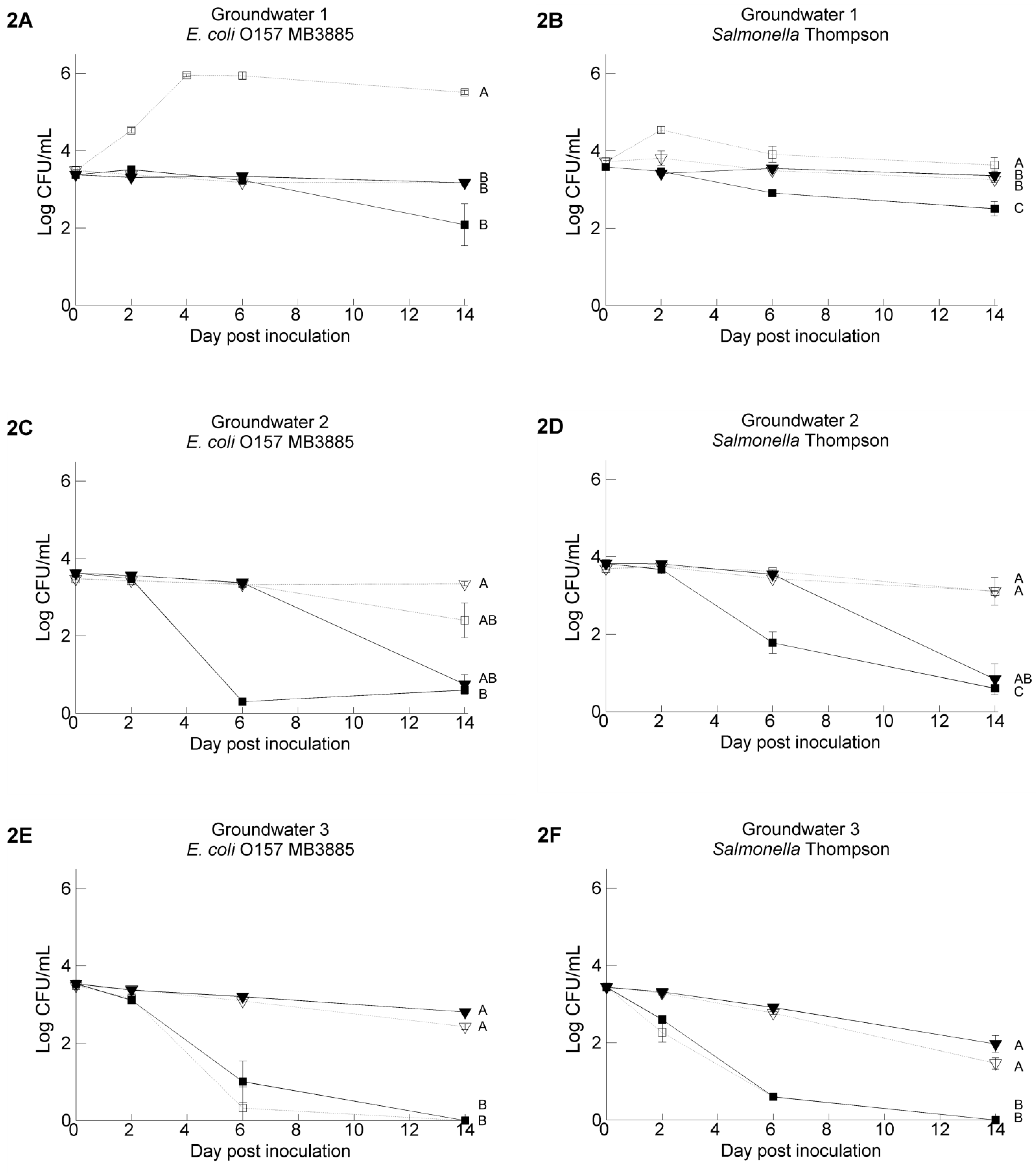
3.1. Pathogen’s Survival in Irrigation Water: Influence of Water Temperature and Resident Aquatic Microbiota
| Water Sample | pH-H2O | EC | SO4 | Cl | Fe | Mn | Mg | Ca | K | Na | Cu | Zn | NO2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µS/cm | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | µg/L | mg/L | mg/L | ||
| GW1 a | 7.45 | 710 | 0.383 | <0.22 | 78.64 | 51.2 | 0.52 | 0.15 | 15.60 | 94.30 | 19.00 | 29.40 | <0.01 | <0.01 | <0.12 |
| GW2 a | 6.99 | 1413.0 | 198.5 | <0.22 | 340.9 | 48.8 | 0.08 | 0.06 | 43.50 | 219.90 | 58.85 | 38.00 | 0.03 | 0.73 | <0.12 |
| GW3 a | 7.56 | 698.0 | 0.9 | <0.3 | 134.4 | 42.4 | 0.02 | <0.01 | 15.00 | 102.61 | 8.25 | 12.72 | <0.01 | 0.79 | <0.3 |
| PW1 b | 6.91 | 106 | 0.278 | <0.22 | 7.28 | 7.6 | 0.14 | 0.06 | 2.37 | 8.77 | 6.75 | 4.18 | 0.01 | <0.01 | <0.12 |
| PW2 b | 7.71 | 651.0 | 5.2 | <0.22 | 165.5 | 46.3 | 0.02 | 0.02 | 14.80 | 82.65 | 13.20 | 29.70 | <0.01 | 0.01 | <0.12 |
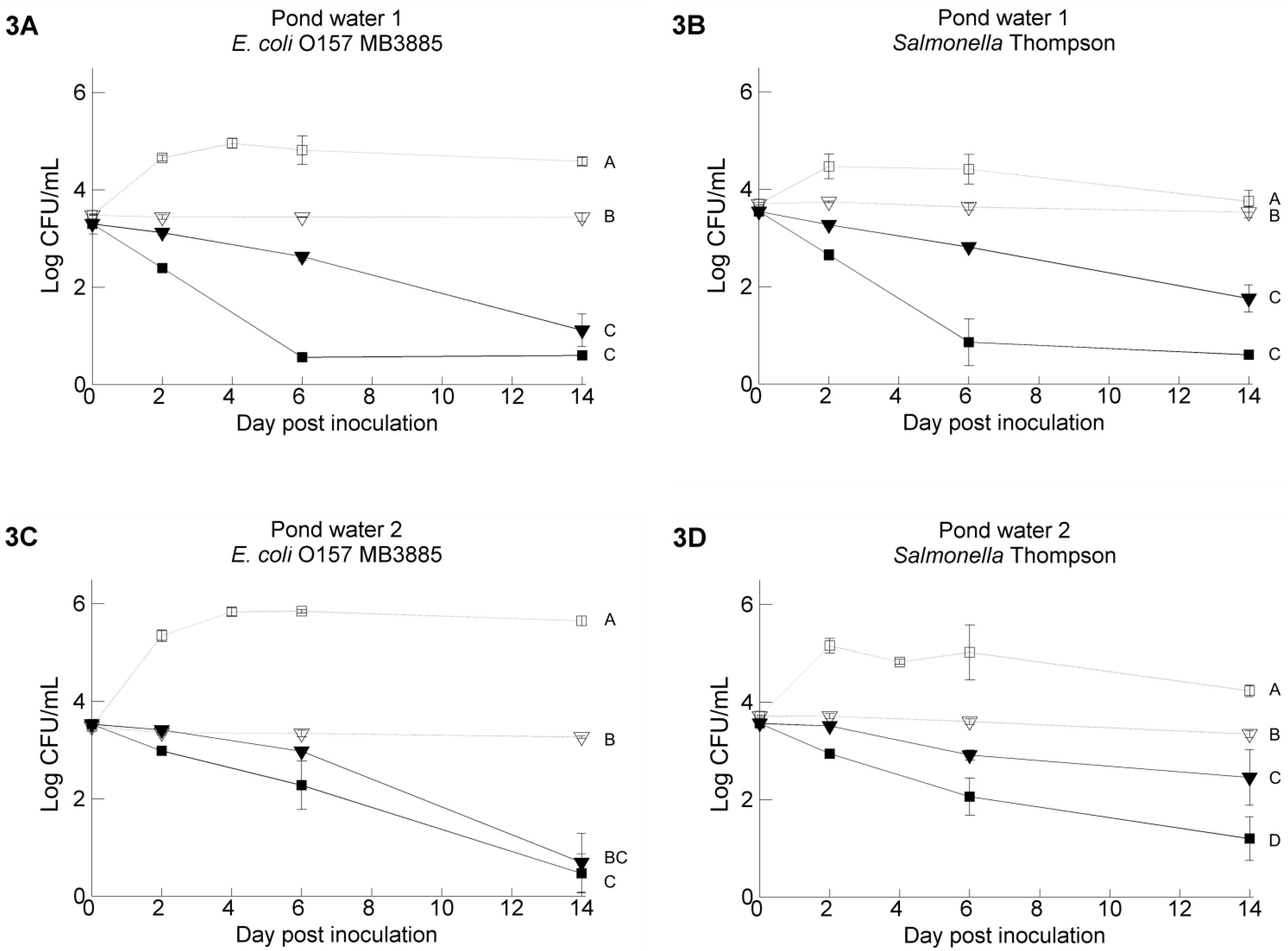
3.2. Pathogen’s Survival in Irrigation Water: Comparisons between Water Samples and Different Strains of Each Pathogen
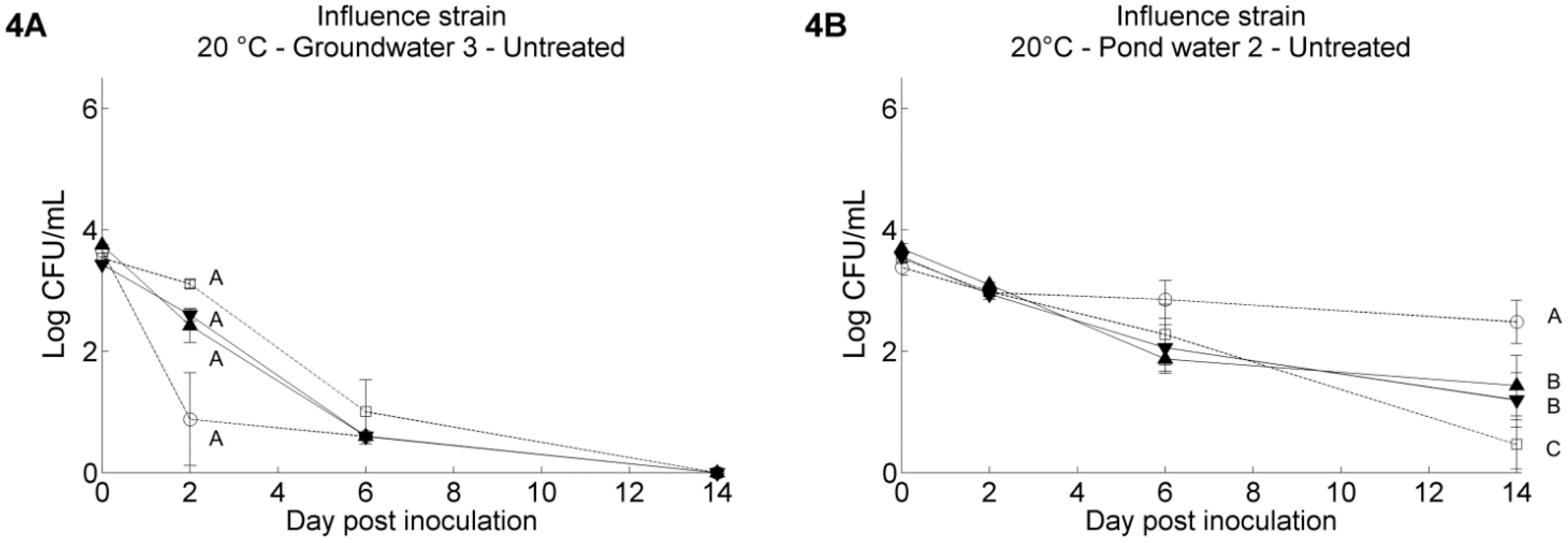
3.3. The Resident Background of Heterotrophic Microorganisms in the Irrigation Water Samples
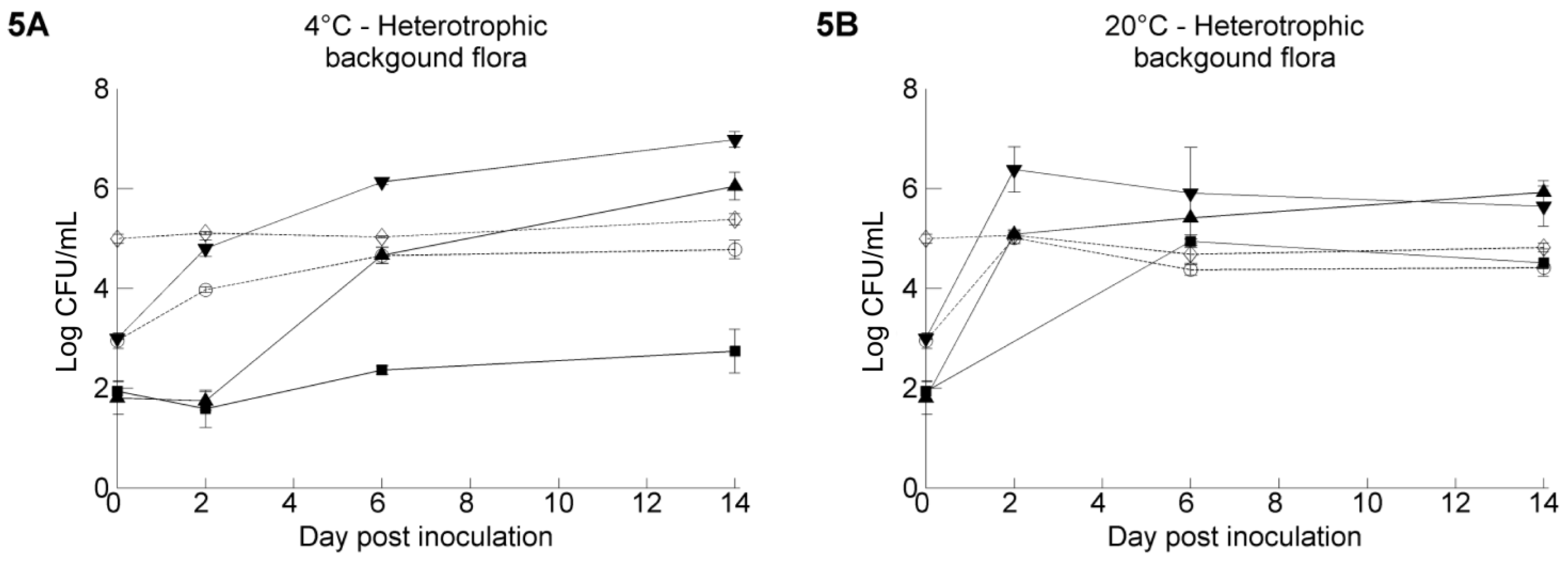
3.4. Pathogen Survival on Butterhead Lettuce Leaves: Influence of the Inoculum Carrier
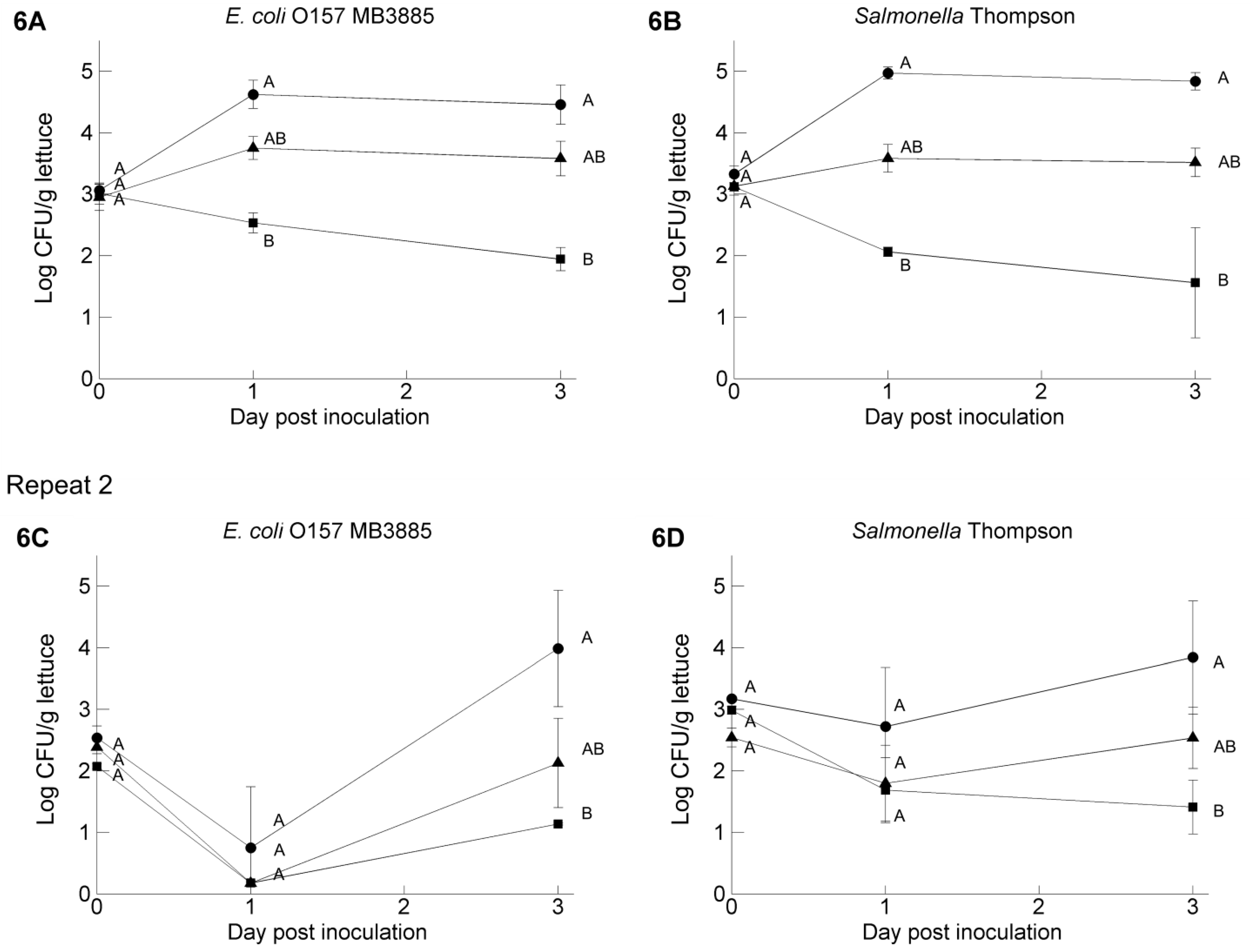
4. Discussion
| Water Sample | Initial Level Resident Heterotrophic Bacteria | Endpoint Pathogen Level | Tentative Interpretation | |||
|---|---|---|---|---|---|---|
| in presence of aquatic biota (in untreated water) | in absence of aquatic biota (in filter sterilized water) | combination of competitive survival and growth and nutrient availability | risk of pathogen transfer when using non-sterilized irrigation water | risk of pathogen transfer in case of pathogen contamination of sterilized water | ||
| GW1 | low | high | very high | -very low pathogen suppression (low bacterial background load) | high | very high |
| -nutrients for pathogen growth available | ||||||
| GW2 | medium | low | high | -important pathogen suppression by resident aquatic biota | low | high |
| -limited nutrients for pathogen growth available | ||||||
| -high Zn level | ||||||
| GW3 | low | low | low | -important pathogen suppression but not by the resident aquatic biota | low | low |
| -the pathogen does not survive in this water, although the bacterial background does | ||||||
| -high Zn level | ||||||
| PW1 | high | low | very high | -important pathogen suppression by resident aquatic biota | low | very high |
| -nutrients for pathogen growth available | ||||||
| PW2 | medium | medium | very high | -weaker pathogen suppression by resident aquatic biota | medium | very high |
| -nutrients for pathogen growth available | ||||||
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgements
Author Contributions
Conflicts of Interest
References
- Sivapalasingam, S.; Friedman, C.R.; Cohen, L.; Tauxe, R.V. Fresh produce: A growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 2004, 67, 2342–2353. [Google Scholar]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–200. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar]
- Nygård, K.; Lassen, J.; Vold, L.; Andersson, Y.; Fisher, I.; Löfdahl, S.; Threlfall, J.; Luzzi, I.; Peters, T.; Hampton, M.; et al. Outbreak of Salmonella Thompson infections linked to imported rucola lettuce. Foodborne Pathog. Dis. 2008, 5, 165–173. [Google Scholar]
- Söderström, A.; Österberg, P.; Lindqvist, A.; Jönsson, B.; Lindberg, A.; Blide Ulander, S.; Welinder-Olsson, C.; Löfdahl, S.; Kaijser, B.; de Jong, B.; et al. A large Escherichia coli O157 outbreak in Sweden associated with locally produced lettuce. Foodborne Pathog. Dis. 2008, 5, 339–349. [Google Scholar]
- Gu, G.; Hu, J.; Cevallos-Cevallos, J.M.; Richardson, S.M.; Bartz, J.A.; van Bruggen, A.H.C. Internal colonization of Salmonella enterica serovar Typhimurium in tomato plants. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Danyluk, M.D.; Gu, G.; Vallad, G.E.; van Bruggen, A.H. Dispersal of Salmonella Typhimurium by rain splash onto tomato plants. J. Food Prot. 2012, 75, 472–479. [Google Scholar]
- Steele, M.; Odumeru, J. Irrigation water as source of foodborne pathogens on fruit and vegetables. J. Food Prot. 2004, 67, 2839–2849. [Google Scholar]
- Semenov, A.V.; van Overbeek, L.; van Bruggen, A.H. Percolation and survival of E. coli O157:H7 and Salmonella enterica serovar Typhimurium in soil amended with contaminated dairy manure or slurry. Appl. Environ. Microbiol. 2009, 75, 3206–3215. [Google Scholar]
- Gu, G.; Luo, Z.; Cevallos-Cevallos, J.M.; Adams, P.; Vellidis, G.; Wright, A.; van Bruggen, A.H. Factors affecting the occurrence of Escherichia coli O157 contamination in irrigation ponds on produce farms in the Suwannee River watershed. Can. J. Microbiol. 2012, 59, 175–182. [Google Scholar]
- Holvoet, K. Bacterial Safety of Lettuce in Primary Production and Fresh-Cut Processing Industry; Ghent University: Ghent, Belgium, 2014. [Google Scholar]
- Anibas, C.; Buis, K.; Verhoeven, R.; Meire, P.; Batelaan, O. A simple thermal mapping method for seasonal spatial patterns of groundwater-surface water interaction. J. Hydrol. 2011, 397, 93–104. [Google Scholar]
- Van Vliet, M.; Zwolsman, J. Impact of summer droughts on the water quality of the Meuse river. J. Hydrol. 2008, 353, 1–17. [Google Scholar]
- Brandl, M.T.; Miller, W.G.; Bates, A.H.; Mandrell, R.E. Production of autoinducer 2 in Salmonella enterica serovar Thompson contributes to its fitness in chickens but not on cilantro leaf surfaces. Appl. Environ. Microbiol. 2005, 71, 2653–2662. [Google Scholar]
- Verstraete, K.; de Reu, K.; van Weyenberg, S.; Piérard, D.; de Zutter, L.; Herman, L.; Robyn, J.; Heyndrickx, M. Genetic characteristics of Shiga toxin-producing E. coli O157, O26, O103, O111 and O145 isolates from humans, food, and cattle in Belgium. Epidemiol. Infect. 2013, 141, 1–13. [Google Scholar]
- Dibb-Fuller, M.P.; Best, A.; Stagg, D.A.; Cooley, W.A.; Woodward, M.J. An in vitro model for studying the interaction of Escherichia coli O157:H7 and other enteropathogens with bovine primary cell cultures. J. Med. Microbiol. 2001, 50, 759–769. [Google Scholar]
- Skandamis, P.N.; Nychas, G.J.E. Development and evaluation of a model predicting the survival of Escherichia coli O157:H7 NCTC12900 in homemade eggplant salad at various temperatures, pHs, and oregano essential oil concentrations. Appl. Environ. Microbiol. 2000, 66, 1646–1653. [Google Scholar]
- Vande Walle, K.; Atef Yekta, M.; Verdonck, F.; de Zutter, L.; Cox, E. Rectal inoculation of sheep with E. coli O157:H7 results in persistent infection in the absence of a protective immune response. Vet. Microbiol. 2011, 147, 376–382. [Google Scholar]
- Woodward, M.J.; Best, A.; Sprigings, K.A.; Pearson, G.R.; Skuse, A.M.; Wales, A.; Hayes, C.M.; Roe, J.M.; Low, J.C.; la Ragione, R.M. Non-toxigenic Escherichia coli O157:H7 strain NCTC12900 causes attaching-effacing lesions and eae-dependent persistence in weaned sheep. Int. J. Med. Microbiol. 2003, 293, 299–308. [Google Scholar]
- Semenov, A.M.; Kuprianov, A.A.; van Bruggen, A.H. Transfer of enteric pathogens to successive habitats as part of microbial cycles. Microb. Ecol. 2010, 60, 239–249. [Google Scholar]
- Franz, E.; Visser, A.A.; van Diepeningen, A.D.; Klerks, M.M.; Termorshuizen, A.J.; van Bruggen, A.H.C. Quantification of contamination of lettuce by GFP-expressing E. coli O157:H7 and Salmonella enterica serovar Typhimurium. Food Microbiol. 2007, 24, 106–112. [Google Scholar]
- Franz, E.; Klerks, M.A.; de Vos, O.J.; Termorshuizen, A.J.; van Bruggen, A.H.C. Prevalence of Shiga toxin-producing Escherichia coli stx(1), stx(2), eaeA, and rfbE genes and survival of E. coli O157:H7 in manure from organic and low-input conventional dairy farms. Appl. Environ. Microbiol. 2007, 73, 2180–2190. [Google Scholar]
- Lapidot, A.; Yaron, S. Transfer of Salmonella enterica serovar Typhimurium from contaminated irrigation water to parsley is dependent on curli and cellulose, the biofilm matrix components. J. Food Prot. 2009, 72, 618–623. [Google Scholar]
- Semenov, A.V.; van Overbeek, L.; Termorshuizen, A.J.; van Bruggen, A.H. Influence of aerobic and anaerobic conditions on survival of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in Luria-Bertani broth, farm-yard manure and slurry. J. Environ. Manag. 2011, 92, 780–787. [Google Scholar]
- Erickson, M.C.; Liao, J.; Payton, A.S.; Riley, D.G.; Webb, C.C.; Davey, L.E.; Kimbrel, S.; Ma, L.; Zhang, G.; Flitcroft, I. Preharvest internalization of Escherichia coli O157:H7 into lettuce leaves, as affected by insect and physical damage. J. Food Prot. 2010, 73, 1809–1816. [Google Scholar]
- Brandl, M.T.; Amundson, R. Leaf age as a risk factor in contamination of lettuce with Escherichia coli O157:H7 and Salmonella enterica. Appl. Environ. Microbiol. 2008, 74, 2298–2306. [Google Scholar]
- Rice, E.W.; Johnson, C.H.; Wild, D.K.; Reasoner, D.J. Survival of Escherichia coli O157:H7 in drinking water associated with a waterborne disease outbreak of hemorrhagic colitis. Lett. Appl. Microbiol. 1992, 15, 38–40. [Google Scholar]
- Wang, G.; Doyle, M.P. Survival of enterohemorrhagic Escherichia coli O157:H7 in water. J. Food Prot. 1998, 61, 662–667. [Google Scholar]
- Vital, M.; Hammes, F.; Egli, T. Escherichia coli O157 can grow in natural freshwater at low carbon concentrations. Environ. Microbiol. 2008, 10, 2387–2396. [Google Scholar]
- Vital, M.; Stucki, D.; Egli, T.; Hammes, F. Evaluating the growth potential of pathogenic bacteria in water. Appl. Environ. Microbiol. 2010, 76, 6477–6484. [Google Scholar]
- Semenov, A.V.; Franz, E.; van Bruggen, A.H. COLIWAVE a simulation model for survival of E. coli O157:H7 in dairy manure and manure-amended soil. Ecol. Model. 2010, 221, 599–609. [Google Scholar]
- Ravva, S.V.; Sarreal, C.Z.; Mandrell, R.E. Identification of protozoa in dairy lagoon wastewater that consume E. coli O157:H7 preferentially. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Ravva, S.; Sarreal, C.; Mandrell, R. Intra and Inter-Strain Differences in Fitness of E. coli O157:H7 to Protozoan Predation and Survival in Soil; Michael Davidson, J.F.P., Ryser, E.T., Sofos, J.N., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2013; p. 141.
- Preston, S.; Coad, N.; Townend, J.; Killham, K.; Paton, G.I. Biosensing the acute toxicity of metal interactions: Are they additive, synergistic, or antagonistic? Environ. Toxicol. Chem. 2000, 19, 775–780. [Google Scholar]
- Avery, L.M.; Williams, A.P.; Killham, K.; Jones, D.L. Survival of E. coli O157:H7 in waters from lakes, rivers, puddles and animal-drinking troughs. Sci. Total Environ. 2008, 389, 378–385. [Google Scholar]
- Semenov, A.V.; van Bruggen, A.H.C.; van Overbeek, L.; Termorshuizen, A.J.; Semenov, A.M. Influence of temperature fluctuations on E. coli O157:H7 and Salmonella enterica serovar Typhimurium in cow manure. FEMS Microbiol. Ecol. 2007, 60, 419–428. [Google Scholar]
- Barker-Reid, F.; Harapas, D.; Engleitner, S.; Kreidl, S.; Holmes, R.; Faggian, R. Persistence of E. coli on injured iceberg lettuce in the field, overhead irrigated with contaminated water. J. Food Prot. 2009, 72, 458–464. [Google Scholar]
- Solomon, E.B.; Pang, H.J.; Matthews, K.R. Persistence of E. coli O157:H7 on lettuce plants following spray irrigation with contaminated water. J. Food Prot. 2003, 66, 2198–2202. [Google Scholar]
- Theofel, C.G.; Harris, L.J. Impact of preinoculation culture conditions on the behavior of E. coli O157:H7 inoculated onto romaine lettuce (Lactuca sativa) plants and cut leaf surfaces. J. Food Prot. 2009, 72, 1553–1559. [Google Scholar]
- Choi, S.; Bang, J.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Survival and colonization of E. coli O157:H7 on spinach leaves as affected by inoculum level and carrier, temperature and relative humidity. J. Appl. Microbiol. 2011, 111, 1465–1472. [Google Scholar]
- Brandl, M.T.; Mandrell, R.E. Fitness of Salmonella enterica serovar Thompson in the cilantro phyllosphere. Appl. Environ. Microbiol. 2002, 68, 3614–3621. [Google Scholar]
- Van der Linden, I.; Cottyn, B.; Uyttendaele, M.; Vlaemynck, G.; Heyndrickx, M.; Maes, M. Survival of enteric pathogens during butterhead lettuce growth: Crop stage, leaf age, and irrigation. Foodborne Pathog. Dis. 2013, 10, 485–491. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Der Linden, I.; Cottyn, B.; Uyttendaele, M.; Berkvens, N.; Vlaemynck, G.; Heyndrickx, M.; Maes, M. Enteric Pathogen Survival Varies Substantially in Irrigation Water from Belgian Lettuce Producers. Int. J. Environ. Res. Public Health 2014, 11, 10105-10124. https://doi.org/10.3390/ijerph111010105
Van Der Linden I, Cottyn B, Uyttendaele M, Berkvens N, Vlaemynck G, Heyndrickx M, Maes M. Enteric Pathogen Survival Varies Substantially in Irrigation Water from Belgian Lettuce Producers. International Journal of Environmental Research and Public Health. 2014; 11(10):10105-10124. https://doi.org/10.3390/ijerph111010105
Chicago/Turabian StyleVan Der Linden, Inge, Bart Cottyn, Mieke Uyttendaele, Nick Berkvens, Geertrui Vlaemynck, Marc Heyndrickx, and Martine Maes. 2014. "Enteric Pathogen Survival Varies Substantially in Irrigation Water from Belgian Lettuce Producers" International Journal of Environmental Research and Public Health 11, no. 10: 10105-10124. https://doi.org/10.3390/ijerph111010105
APA StyleVan Der Linden, I., Cottyn, B., Uyttendaele, M., Berkvens, N., Vlaemynck, G., Heyndrickx, M., & Maes, M. (2014). Enteric Pathogen Survival Varies Substantially in Irrigation Water from Belgian Lettuce Producers. International Journal of Environmental Research and Public Health, 11(10), 10105-10124. https://doi.org/10.3390/ijerph111010105





