Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering
Abstract
:1. Introduction
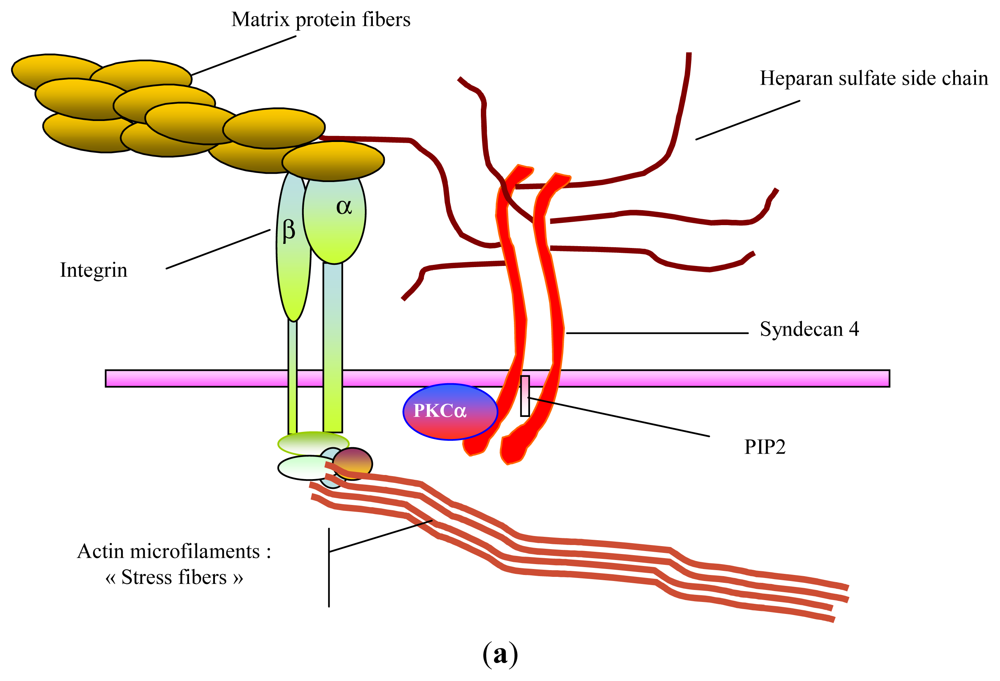
2. Glycosaminoglycans: Structural Features, Biological Properties and Limitations for Therapeutic Use
3. GAG-Like Polysaccharides from Marine Eukaryotes
3.1. GAGs
3.2. Alginate
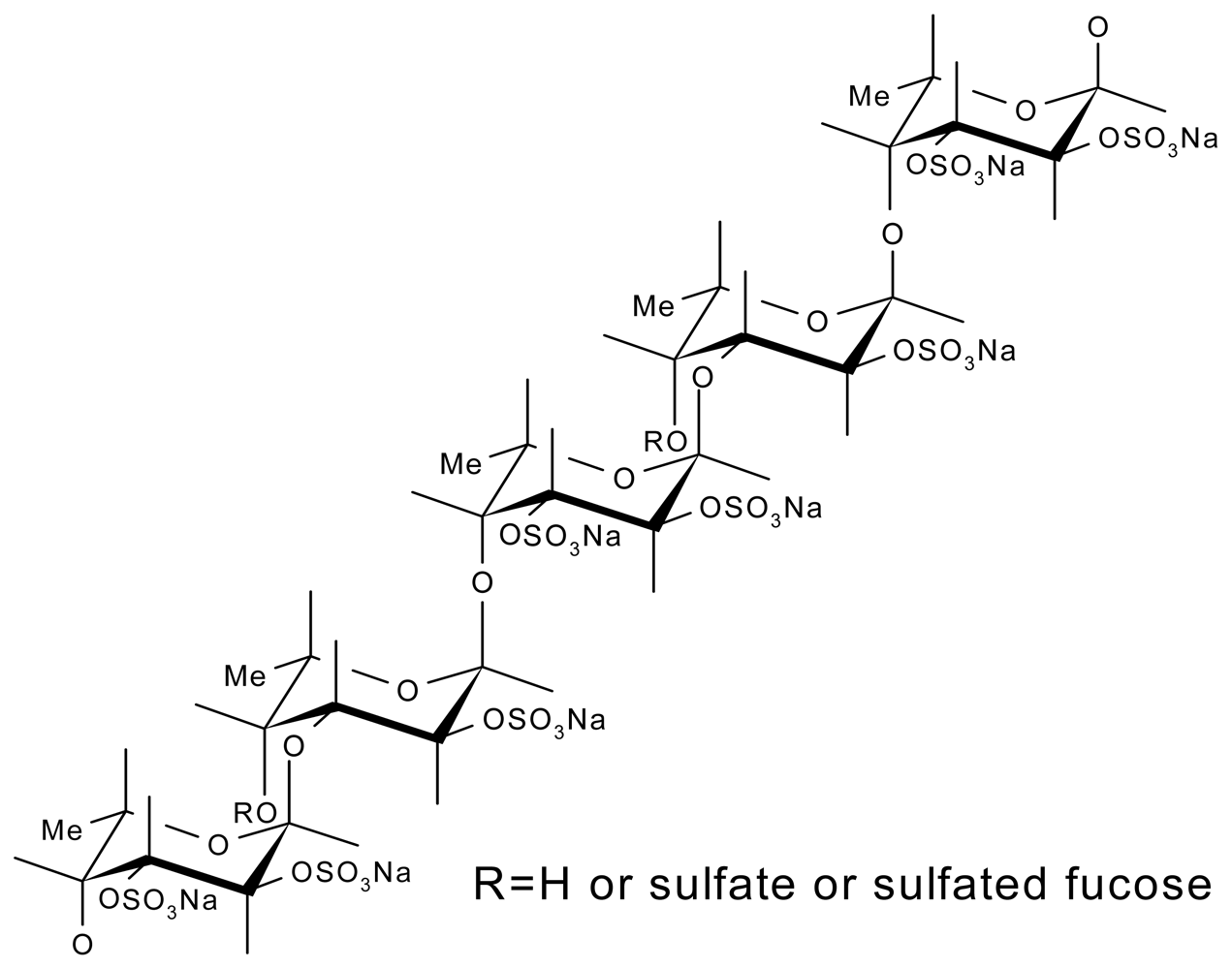
3.3. Fucoidans
3.3.1. From Marine Echinoderms
3.3.2. From Seaweeds
4. GAG-Like Polysaccharides from Marine Prokaryotes
4.1. Extracellular Polymeric Substances (EPS)-Producing Cyanobacteria
4.2. Spirulina (Arthrospira)
Other EPS-Producing Cyanobacteria
4.3. Bacterial Exopolysaccharides (EPS)
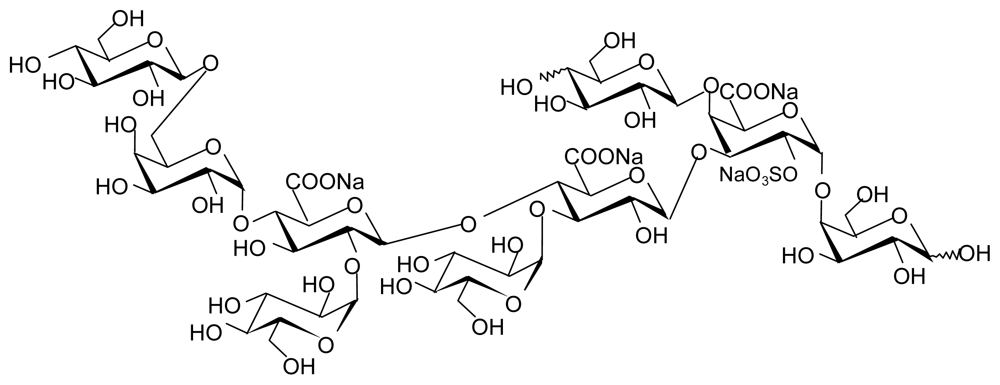
4.3.1. HE 800 EPS from Vibrio diabolicus
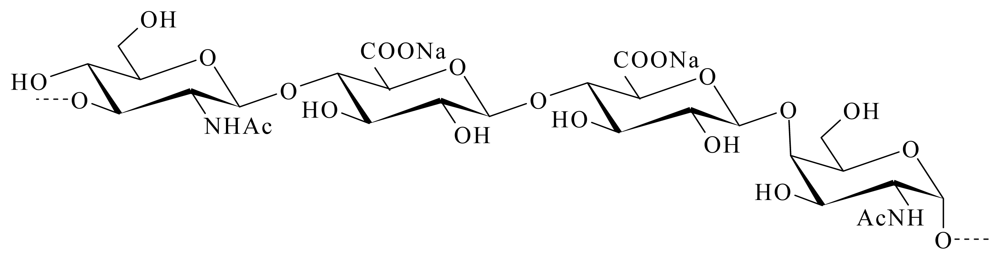
4.3.2. EPS GY 785 from Alteromonas infernus
5. Conclusions
- Samples Availability: Available from the authors.
References
- Lindahl, U. Heparan sulfate—a polyanion with multiple messages. Pure Appl. Chem 1997, 69, 1897–1902. [Google Scholar]
- Shriver, Z; Raguram, S; Sasisekharan, R. Glycomics: A pathway to a class of new and improved therapeutics. Nat. Rev. Drug. Discov 2004, 3, 863–873. [Google Scholar]
- Berteau, O; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, biological properties of sulfated fucans and overview of enzymes active towards this class of polysaccharide. Glycobiology 2003, 6, 29R–40R. [Google Scholar]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar]
- Jackson, RL; Busch, SJ; Cardin, AD. Glycosaminoglycans—Molecular-properties, proteins interactions, and role in physiological processes. Physiol. Rev 1991, 71, 481–539. [Google Scholar]
- Iozzo, RV. Matrix proteoglycans: From molecular design to cellular function. Annu. Rev. Biochem 1998, 67, 609–652. [Google Scholar]
- Ernst, B; Magnani, JL. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug. Discov 2009, 8, 661–677. [Google Scholar]
- Gandhi, NS; Mancera, RL. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des 2008, 72, 455–482. [Google Scholar]
- Mulloy, B; Linhardt, RJ. Order out of complexity—Protein structures that interact with heparin. Curr. Opin. Struct. Biol 2001, 11, 623–628. [Google Scholar]
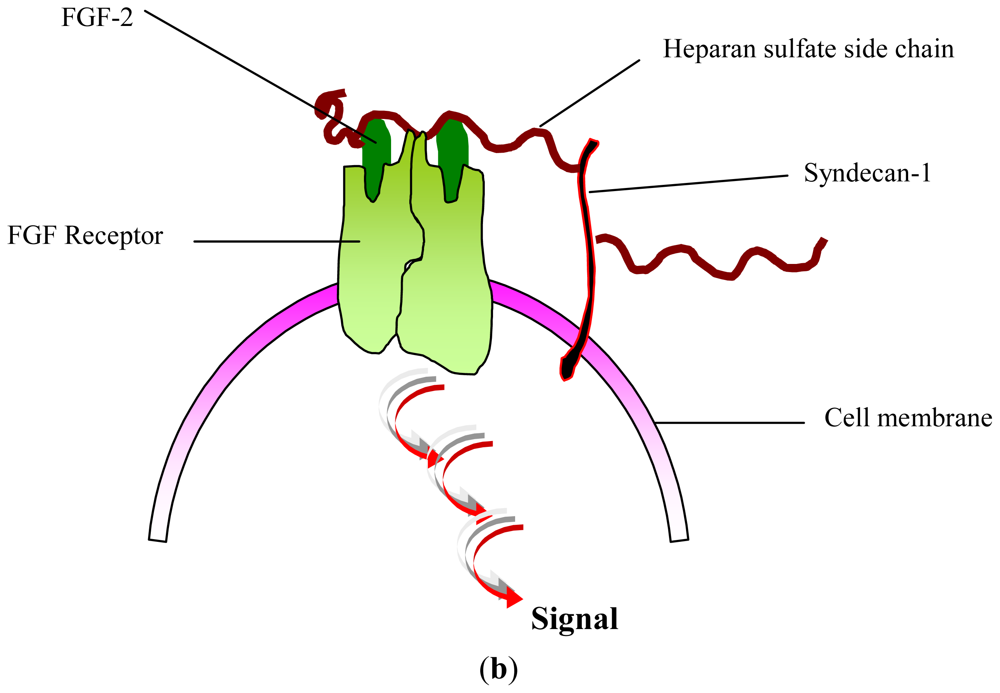
- Yayon, A; Klagsbrun, M; Esko, JD; Leder, P; Ornitz, DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 1991, 64, 841–848. [Google Scholar]
- Yu, W-H; Woessner, JF; McNeish, JD; Stamenkovic, I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev 2002, 16, 307–323. [Google Scholar]
- Stringer, SE. The role of heparan sulphate proteoglycans in angiogenesis. Biochem. Soc. Trans 2006, 34, 451–453. [Google Scholar]
- Penc, SF; Pomahac, B; Winkler, T; Dorschner, RA; Eriksson, E; Herndon, M; Gallo, RL. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J. Biol. Chem 1998, 273, 28116–28121. [Google Scholar]
- Feige, JJ; Baird, A. Crinopexy: Extracellular regulation of growth factor action. Kidney Int. Suppl 1995, 49, S15–S18. [Google Scholar]
- Chung, CY; Erickson, HP. Glycosaminoglycans modulate fibronectin matrix assembly and are essential for matrix incorporation of tenascin-C. J. Cell Sci 1997, 110, 1413–1419. [Google Scholar]
- Wu, WJ; Vrhovski, B; Weiss, AS. Glycosaminoglycans mediate the coacervation of human tropoelastin through dominant charge interactions involving lysine side chains. J. Biol. Chem 1999, 274, 21719–21724. [Google Scholar]
- Buczek-Thomas, JA; Chu, CL; Rich, CB; Stone, PJ; Foster, JA; Nugent, MA. Heparan sulfate depletion within pulmonary fibroblasts: Implications for elastogenesis and repair. J. Cell. Physiol 2002, 192, 294–303. [Google Scholar]
- Volpi, N. Inhibition of human leukocyte elastase activity by chondroitin sulfates. Chem. Biol. Interact 1997, 105, 157–167. [Google Scholar]
- Gogly, B; Dridi, M; Hornebeck, W; Bonnefoix, M; Godeau, G; Pellat, B. Effect of heparin on the production of matrix metalloproteinases and tissue inhibitors of metalloproteinases by human dermal fibroblasts. Cell Biol. Int 1999, 23, 203–209. [Google Scholar]
- Kawashima, H. Roles of sulfated Glycans in lymphocyte homing. Biol. Pharm. Bull 2006, 29, 2343–2349. [Google Scholar]
- Taylor, KR; Gallo, RL. Glycosaminoglycans and their proteoglycans: Host-associated molecular patterns for initiation and modulation of inflammation. FASEB J 2006, 20, 9–22. [Google Scholar]
- Juhlin, L. Hyaluronan in skin. J. Int. Med 1997, 242, 61–66. [Google Scholar]
- Croce, MA; Dyne, K; Boraldi, F; Quaglino, D; Cetta, G; Tiozzo, R; Ronchetti, IP. Hyaluronan affects protein and collagen synthesis by in vitro human skin fibroblasts. Tissue Cell 2001, 33, 326–331. [Google Scholar]
- Meyer, LJM; Russell, SB; Russell, JD; Trupin, JS; Egbert, BM; Shuster, S; Stern, R. Reduced hyaluronan in keloid tissue and cultured keloid fibroblasts. J. Invest. Dermatol 2000, 114, 953–959. [Google Scholar]
- Saravanan, R; Shanmugam, A. Isolation and Characterization of Low Molecular Weight Glycosaminoglycans from Marine Mollusc Amussium pleuronectus (Linne) using Chromatography. Appl. Biochem. Biotechnol 2010, 160, 791–799. [Google Scholar]
- Vilela-Silva, AC; Alves, AP; Valente, AP; Vacquier, VD; Mourao, PA. Structure of the sulfated alpha-L-fucan from the egg jelly coat of the sea urchin Strongylocentrotus franciscanus: Patterns of preferential 2-O- and 4-O-sulfation determine sperm cell recognition. Glycobiology 1999, 9, 927–933. [Google Scholar]
- Tapon-Bretaudiere, J; Chabut, D; Zierer, M; Matou, S; Helley, D; Bros, A; Mourao, PA; Fischer, AM. A fucosylated chondroitin sulfate from echinoderm modulates in vitro fibroblast growth factor 2-dependent angiogenesis. Mol. Cancer Res 2002, 1, 96–102. [Google Scholar]
- Nandini, CD; Itoh, N; Sugahara, K. Novel 70-kDa chondroitin sulfate/dermatan sulfate hybrid chains with a unique heterogenous sulfation pattern from shark skin, which exhibit neuritogenic activity and binding activities for growth factors and neurotrophic factors. J. Biol. Chem 2005, 280, 4058–4069. [Google Scholar]
- Gomez d’Ayala, G; Malinconico, M; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar]
- Yang, J-S; Xie, Y-J; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym 2011, 84, 33–39. [Google Scholar]
- Augst, AD; Kong, HJ; Mooney, DJ. Alginate Hydrogels as Biomaterials. Macromol. Biosci 2006, 6, 623–633. [Google Scholar]
- Chevolot, L; Mulloy, B; Ratiskol, J; Foucault, A; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res 2001, 330, 529–535. [Google Scholar]
- Bilan, MI; Grachev, AA; Ustuzhanina, NE; Shashkov, AS; Nifantiev, NE; Usov, AI. Structure of a fucoidan from the brown seaweed Fucus evanescens. Carbohydr. Res 2002, 337, 719–730. [Google Scholar]
- Nishino, T; Nagumo, T; Kiyohara, H; Yamada, H. Structural characterization of a new anticoagulant fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr. Res 1991, 211, 77–90. [Google Scholar]
- Millet, J; Jouault, SC; Mauray, S; Theveniaux, J; Sternberg, C; Boisson Vidal, C; Fischer, AM. Antithrombotic and anticoagulant activities of a low molecular weight fucoidan by the subcutaneous route. J. Thromb. Haemost 1999, 81, 391–395. [Google Scholar]
- Colliec-Jouault, S; Millet, J; Helley, D; Sinquin, C; Fischer, AM. Effect of low-molecular-weight fucoidan on experimental arterial thrombosis in the rabbit and rat. J. Thromb. Haemost 2003, 1, 1114–1115. [Google Scholar]
- Colliec-Jouault, S; Durand, P; Fischer, A-M; Jozefonvicz, J; Letourneur, D; Millet, J. Low Molecular Weight Sulphated Polysaccharide to Obtain a Medicine with Antithrombotic Activity. US Patent 6,828,307, 7 December 2004. [Google Scholar]
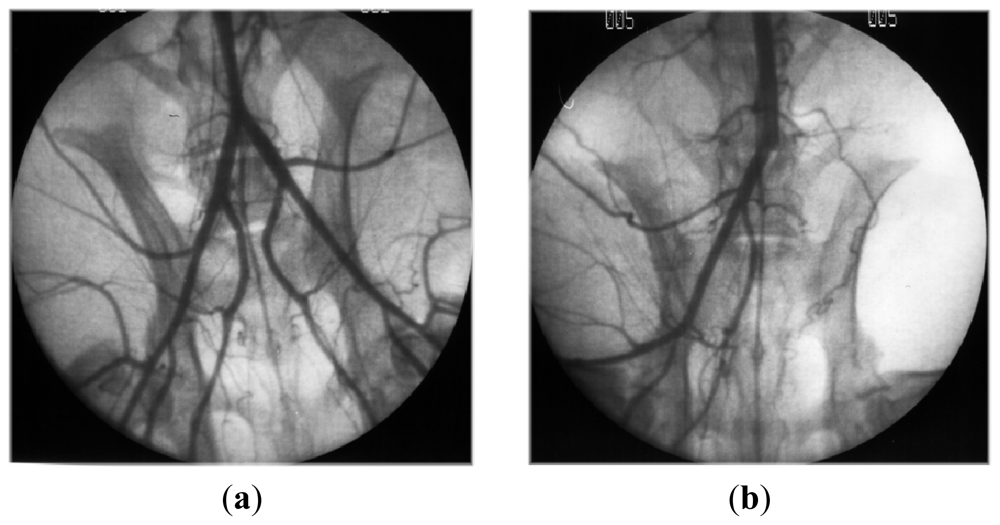
- Luyt, CE; Meddahi-Pelle, A; Ho-Tin-Noe, B; Colliec-Jouault, S; Guezennec, J; Louedec, L; Prats, HE; Jacob, MP; Osborne-Pellegrin, M; Letourneur, D; Michel, JB. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J. Pharmacol. Exp. Ther 2003, 305, 24–30. [Google Scholar]
- Durand, E; Helley, D; Zen, AAH; Dujols, C; Bruneval, P; Colliec-Jouault, S; Fischer, AM; Lafont, A. Effect of low molecular weight fucoidan and low molecular weight heparin in a rabbit model of arterial thrombosis. J. Vasc. Res 2008, 45, 529–537. [Google Scholar]
- Giraux, JL; Tapon-Bretaudiere, J; Matou, S; Fischer, AM. Fucoidan, as heparin, induces tissue factor pathway inhibitor release from cultured human endothelial cells. J. Thromb. Haemost 1998, 80, 692–695. [Google Scholar]
- Matou, S; Helley, D; Chabut, D; Bros, A; Fischer, A-M. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb. Res 2002, 106, 213–221. [Google Scholar]
- Chabut, D; Fischer, AM; Colliec-Jouault, S; Laurendeau, I; Matou, S; Le Bonniec, B; Helley, D. Low molecular weight fucoidan and heparin enhance the basic fibroblast growth factor-induced tube formation of endothelial cells through heparan sulfate-dependent alpha 6 overexpression. Mol. Pharmacol 2003, 64, 696–702. [Google Scholar]
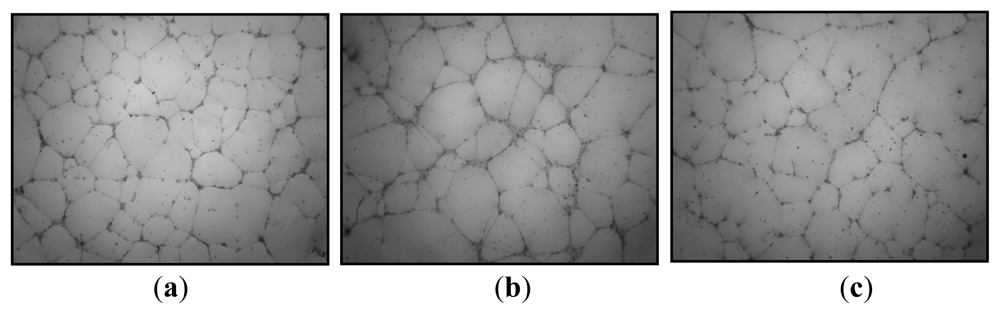
- Matou, S; Colliec-Jouault, S; Galy-Fauroux, I; Ratiskol, J; Sinquin, C; Guezennec, J; Fischer, A-M; Helley, D. Effect of an oversulfated exopolysaccharide on angiogenesis induced by fibroblast growth factor-2 or vascular endothelial growth factor in vitro. Biochem. Pharmacol 2005, 69, 751–759. [Google Scholar]
- Chabut, D; Fischer, AM; Helley, D; Colliec, S. Low molecular weight fucoidan promotes FGF-2-induced vascular tube formation by human endothelial cells, with decreased PAI-1 release and ICAM-1 downregulation. Thromb. Res 2004, 113, 93–95. [Google Scholar]
- Asahara, T; Krasinski, KL; Chen, DH; Sullivan, AB; Kearney, M; Silver, M; Li, T; Isner, JM. Circulating endothelial progenitor cells in peripheral blood incorporate into re-endothelialization after vascular injury. Circulation 1997, 96, 4064–4064. [Google Scholar]
- Zemani, F; Benisvy, D; Galy-Fauroux, I; Lokajczyk, A; Colliec-Jouault, S; Uzan, G; Fischer, AM; Boisson-Vidal, C. Low-molecular-weight fucoidan enhances the proangiogenic phenotype of endothelial progenitor cells. Biochem. Pharmacol 2005, 70, 1167–1175. [Google Scholar]
- Sweeney, EA; Priestley, GV; Nakamoto, B; Collins, RG; Beaudet, AL; Papayannopoulou, T. Mobilization of stem/progenitor cells by sulfated polysaccharides does not require selectin presence. Proc. Natl. Acad. Sci. USA 2000, 97, 6544–6549. [Google Scholar]
- Zemani, F; Silvestre, JS; Fauvel-Lafeve, F; Bruel, A; Vilar, J; Bieche, I; Laurendeau, I; Galy-Fauroux, I; Fischer, AM; Boisson-Vidal, C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler. Thromb. Vasc. Biol 2008, 28, 644–650. [Google Scholar]
- Senni, K; Gueniche, F; Foucault-Bertaud, A; Igondjo-Tchen, S; Fioretti, F; Colliec-Jouault, S; Durand, P; Guezennec, J; Godeau, G; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys 2006, 445, 56–64. [Google Scholar]
- Changotade, SI; Korb, G; Bassil, J; Barroukh, B; Willig, C; Colliec-Jouault, S; Durand, P; Godeau, G; Senni, K. Potential effects of a low-molecular-weight fucoidan extracted from brown algae on bone biomaterial osteoconductive properties. J. Biomed. Mater. Res. A 2008, 87, 666–675. [Google Scholar]
- De Philippis, R; Sili, C; Paperi, R; Vincenzini, M. Exopolysaccharide-producing cyanobacteria and their possible exploitation: A review. J. Appl. Phycol 2001, 13, 293–299. [Google Scholar]
- Matsui, MS; Muizzuddin, N; Arad, S; Marenus, K. Sulfated polysaccharides from red microalgae have antiinflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol 2003, 104, 13–22. [Google Scholar]
- Lee, JB; Hayashi, T; Hayashi, K; Sankawa, U. Structural Analysis of Calcium Spirulan (Ca-SP)-Derived Oligosaccharides Using Electrospray Ionization Mass Spectrometry. J. Nat. Prod 2000, 63, 136–138. [Google Scholar]
- de Morais, MG; Stillings, C; Dersch, R; Rudisile, M; Pranke, P; Costa, JAV; Wendorff, J. Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour. Technol 2010, 101, 2872–2876. [Google Scholar]
- Pereira, S; Micheletti, E; Zille, A; Santos, A; Moradas-Ferreira, P; Tamagnini, P; De Philippis, R. Using extracellular polymeric substances (EPS)-producing cyanobacteria for the bioremediation of heavy metals: Do cations compete for the EPS functional groups and also accumulate inside the cell? Microbiology 2011, 157, 451–458. [Google Scholar]
- Arrieta, JM; Arnaud-Haond, S; Duarte, CM. What lies underneath: Conserving the oceans’ genetic resources. Proc. Natl. Acad. Sci. USA 2010, 107, 18318–18324. [Google Scholar]
- Satpute, SK; Banat, IM; Dhakephalkar, PK; Banpurkar, AG; Chopade, BA. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv 2010, 28, 436–450. [Google Scholar]
- Deming, JW. Deep ocean environmental biotechnology. Curr. Opin. Biotechnol 1998, 9, 283–287. [Google Scholar]
- Guezennec, J. Deep-sea hydrothermal vents: A new source of innovative bacterial exopolysaccharides of biotechnological interest? J. Ind. Microbiol. Biotechnol 2002, 29, 204–208. [Google Scholar]
- Nichols Mancuso, CA; Garon, S; Bowman, JP; Raguenes, G; Guezennec, J. Production of exopolysaccharides by Antarctic marine bacterial isolates. J. Appl. Microbiol 2004, 96, 1057–1066. [Google Scholar]
- Nichols Mancuso, CA; Guezennec, J; Bowman, JP. Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: A review. Mar. Biotechnol 2005, 7, 253–271. [Google Scholar]
- Arena, A; Gugliandolo, C; Stassi, G; Pavone, B; Iannello, D; Bisignano, G; Maugeri, TL. An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: Antiviral activity on immunocompetent cells. Immunol. Lett 2009, 123, 132–137. [Google Scholar]
- Guézennec, J; Moppert, X; Raguénès, G; Richert, L; Costa, B; Simon-Colin, C. Microbial mats in French Polynesia and their biotechnological applications. Process Biochem 2011, 46, 16–22. [Google Scholar]
- Pace, NR. Origin of life-facing up to the physical setting. Cell 1991, 65, 531–533. [Google Scholar]
- Desbruyeres, D; Chevaldonne, P; Alayse, AM; Jollivet, D; Lallier, FH; Jouin-Toulmond, C; Zal, F; Sarradin, PM; Cosson, R; Caprais, JC. Biology and ecology of the “Pompeii worm” (Alvinella pompejana), a normal dweller of an extreme deep-sea environment: A synthesis of current knowledge and recent developments. Deep Sea Res. Part II 1998, 45, 383–422. [Google Scholar]
- Vincent, P; Pignet, P; Talmont, F; Bozzi, L; Fournet, B; Guezennec, J. Production and characterization of an exopolysaccharide excreted by a deep-sea hydrothermal vent bacterium isolated from the polychaete annelid Alvinella pompejana. Appl. Environ. Microbiol 1994, 60, 4134–4141. [Google Scholar]
- Raguenes, G; Christen, R; Guezennec, J; Pignet, P; Barbier, G. Vibrio diabolicus sp. nov., a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent polychaete annelid, Alvinella pompejana. Int. J. Syst. Bacteriol 1997, 47, 989–995. [Google Scholar]
- Rougeaux, H; Kervarec, N; Pichon, R; Guezennec, J. Structure of the exopolysaccharide of Vibrio diabolicus isolated from a deep-sea hydrothermal vent. Carbohydr. Res 1999, 322, 40–45. [Google Scholar]
- Zanchetta, P; Lagarde, N; Guezennec, J. A new bone-healing material: A hyaluronic Acid-like bacterial exopolysaccharide. Calcif. Tissue Int 2003, 72, 74–79. [Google Scholar]
- Senni, K; Sinquin, C; Colliec-Jouault, S; Godeau, G; Guezennec, J. Use of a polysaccharide wich is excreted by the Vibrio diabolicus species for the engineering of non-mineralized connective-tissue. US Patent 0,317,860, 25 December 2008. [Google Scholar]
- Senni, K; Gueniche, F; Yousfi, M; Fioretti, F; Godeau, G; Colliec-Jouault, S; Ratiskol, J; Sinquin, C; Raguenes, G; Courtois, A; Guezennec, J. Sulfated depolymerized derivatives of exopolysaccharides (EPS) from mesophilic marine bacteria, method for preparing same, and use thereof in tissue regeneration. US Patent 0,131,472, 5 June 2008. [Google Scholar]
- Pereira, J. The bacterial exopolysaccharides DRS HE800 and SDR GY785 have no effect on proangiogenic properties of human endothelial progenitors cells in vitro; Université Paris Descartes: Sorbonne Paris Cité, UMR-S765, Paris, France, Unpublished work; 2008. [Google Scholar]
- Raguenes, GH; Peres, A; Ruimy, R; Pignet, P; Christen, R; Loaec, M; Rougeaux, H; Barbier, G; Guezennec, JG. Alteromonas infernus sp. nov., a new polysaccharide-producing bacterium isolated from a deep-sea hydrothermal vent. J. Appl. Microbiol 1997, 82, 422–430. [Google Scholar]
- Roger, O; Kervarec, N; Ratiskol, J; Colliec-Jouault, S; Chevolot, L. Structural studies of the main exopolysaccharide produced by the deep-sea bacterium Alteromonas infernus. Carbohydr. Res 2004, 339, 2371–2380. [Google Scholar]
- Rederstorff, E; Fatimi, A; Sinquin, C; Ratiskol, J; Merceron, C; Vinatier, C; Weiss, P; Colliec-Jouault, S. Sterilization of Exopolysaccharides Produced by Deep-Sea Bacteria: Impact on Their Stability and Degradation. Mar. Drugs 2011, 9, 224–241. [Google Scholar]
- Rederstorff, E; Weiss, P; Sourice, S; Pilet, P; Xie, F; Sinquin, C; Colliec-Jouault, S; Guicheux, J; Laïb, S. An in vitro study of two GAG-like marine polysaccharides incorporated into injectable hydrogels for bone and cartilage tissue engineering. Acta Biomater 2011, 7, 2119–2130. [Google Scholar]
- Matou, S; Colliec-Jouault, S; Helley, D; Ratiskol, J; Sinquin, C; Boisset, C; Guezennec, J; Fischer, A-M. Use of low-molecular weight highly sulfated polysaccharide derivatives for modulating angiogenesis. US Patent 0,259,833, 8 November 2007. [Google Scholar]
- Velasco, CR; Colliec-Jouault, S; Redini, F; Heymann, D; Padrines, M. Proteoglycans on bone tumor development. Drug Discov. Today 2010, 15, 553–560. [Google Scholar]
- Ruiz Velasco, C; Baud’huin, M; Sinquin, C; Maillasson, M; Heymann, D; Colliec-Jouault, S; Padrines, M. Effects of a sulfated exopolysaccharide produced by Altermonas infernus on bone biology. Glycobiology 2011, 21, 781–795. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664-1681. https://doi.org/10.3390/md9091664
Senni K, Pereira J, Gueniche F, Delbarre-Ladrat C, Sinquin C, Ratiskol J, Godeau G, Fischer A-M, Helley D, Colliec-Jouault S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Marine Drugs. 2011; 9(9):1664-1681. https://doi.org/10.3390/md9091664
Chicago/Turabian StyleSenni, Karim, Jessica Pereira, Farida Gueniche, Christine Delbarre-Ladrat, Corinne Sinquin, Jacqueline Ratiskol, Gaston Godeau, Anne-Marie Fischer, Dominique Helley, and Sylvia Colliec-Jouault. 2011. "Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering" Marine Drugs 9, no. 9: 1664-1681. https://doi.org/10.3390/md9091664
APA StyleSenni, K., Pereira, J., Gueniche, F., Delbarre-Ladrat, C., Sinquin, C., Ratiskol, J., Godeau, G., Fischer, A.-M., Helley, D., & Colliec-Jouault, S. (2011). Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Marine Drugs, 9(9), 1664-1681. https://doi.org/10.3390/md9091664