Discovery of New Eunicellins from an Indonesian Octocoral Cladiella sp.
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Superoxide Anion Generation and Elastase Release by Human Neutrophils
Acknowledgments
- Samples Availability: Not available.
References and Notes
- Chang, Y-C; Huang, I-C; Chiang, MY; Hwang, T-L; Kung, T-H; Lin, C-S; Sheu, J-H; Sung, P-J. Briaviodiol A, a new cembranoid from a soft coral. Briareum violacea Chem Pharm Bull 2010, 58, 1666–1668. [Google Scholar]
- Chen, Y-H; Tai, C-Y; Kuo, Y-H; Kao, C-Y; Li, J-J; Hwang, T-L; Fang, L-S; Wang, W-H; Sheu, J-H; Sung, P-J. Cladieunicellins A–E, new eunicellins from an Indonesian soft coral Cladiella sp. Chem Pharm Bull 2011, 59, 353–358. [Google Scholar]
- Chen, Y-H; Tai, C-Y; Hwang, T-L; Weng, C-F; Li, J-J; Fang, L-S; Wang, W-H; Wu, Y-C; Sung, P-J. Cladielloides A and B: new eunicellin-type diterpenoids from an Indonesian octocoral Cladiella sp. Mar Drugs 2010, 8, 2936–2945. [Google Scholar]
- Tai, C-Y; Chen, Y-H; Hwang, T-L; Fang, L-S; Wang, W-H; Liu, M-C; Su, J-H; Wu, Y-C; Sung, P-J. Cladielloides C and D: novel eunicellin-based diterpenoids from an Indonesian octocoral Cladiella sp. Bull Chem Soc Jpn 2011, 84, 531–536. [Google Scholar]
- Sung, P-J; Chang, P-C; Fang, L-S; Sheu, J-H; Chen, W-C; Chen, Y-P; Lin, M-R. Survey of briarane-related diterpenoids-Part II. Heterocycles 2005, 65, 195–204, and references cited therein. [Google Scholar]
- Sung, P-J; Sheu, J-H; Wang, W-H; Fang, L-S; Chung, H-M; Pai, C-H; Su, Y-D; Tsai, W-T; Chen, B-Y; Lin, M-R; Li, G-Y. Survey of briarane-type diterpenoids–Part III. Heterocycles 2008, 75, 2627–2648, and references cited therein. [Google Scholar]
- Sung, P-J; Su, J-H; Wang, W-H; Sheu, J-H; Fang, L-S; Wu, Y-C; Chen, Y-H; Chung, H-M; Su, Y-D; Chang, Y-C. Survey of briarane-type diterpenoids–Part IV. Heterocycles 2011, 83, 1241–1258, and references cited therein. [Google Scholar]
- Bloor, SJ; Schmitz, FJ; Hossain, MB; van der Helm, D. Diterpenoids from the gorgonian. Solenopodium stechei J Org Chem 1992, 57, 1205–1216. [Google Scholar]
- Diphenylene indonium (DPI) and elastatinal were used as reference compounds in anti-inflammatory activity testing. DPI displayed an inhibitory effect on superoxide anion generation (IC50 = 0.8 μg/mL), and elastatinal exhibited an inhibitory effect on elastase release (IC50 = 31 μg/mL) by human neutrophils, respectively.
- Bayer, FM. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: anthozoa), with diagnoses of new taxa. Proc Biol Soc Wash 1981, 94, 902–947. [Google Scholar]
- Fabricius, K; Alderslade, P. Soft Corals and Sea Fans–A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed; Australian Institute of Marine Science: Queensland, Australia, 2001; Volume 49, pp. 84–85. [Google Scholar]
- Hwang, T-L; Li, G-L; Lan, Y-H; Chia, Y-C; Hsieh, P-W; Wu, Y-H; Wu, Y-C. Potent inhibitors of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb. Fissistigma oldhamii Free Radic Biol Med 2009, 46, 520–528. [Google Scholar]
- Hwang, T-L; Su, Y-C; Chang, H-L; Leu, Y-L; Chung, P-J; Kuo, L-M; Chang, Y-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J Lipid Res 2009, 50, 1395–1408. [Google Scholar]
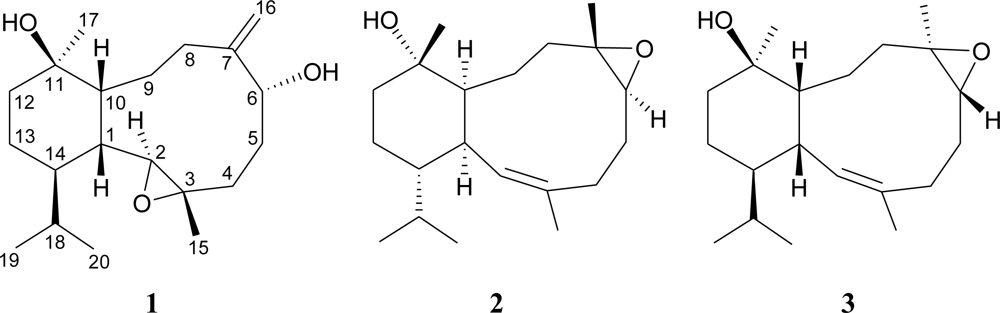
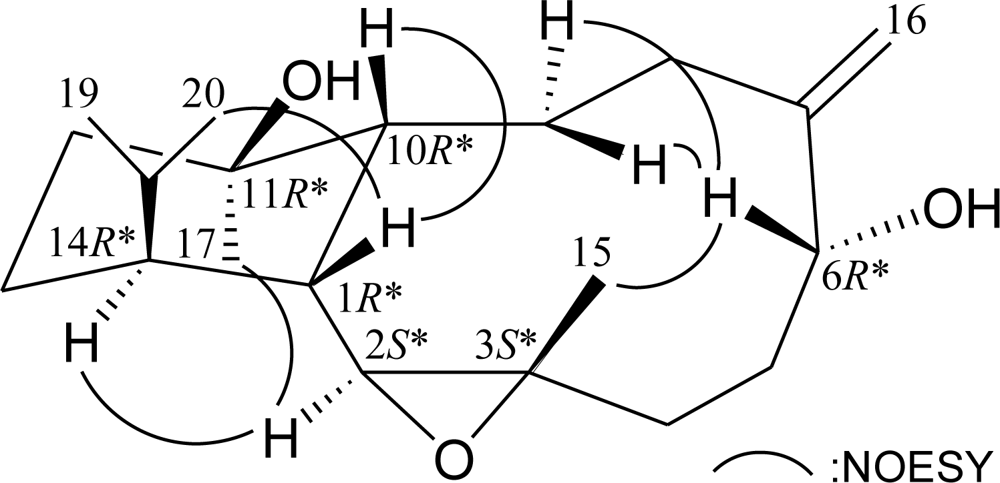

| C/H | 1Ha | 13Cb | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 2.12 m | 36.0 (d)d | H-2, H-10, H-14 | C-2, C-3, C-9, C-10, C-11, C-14, C-18 |
| 2 | 2.81 d (9.6)c | 64.8 (d) | H-1 | C-1, C-3, C-10, C-15 |
| 3 | 62.9 (s) | |||
| 4a | 1.27 m | 27.3 (t) | H-4b, H2-5 | C-3, C-5, C-15 |
| b | 1.81 m | H-4a, H2-5 | C-2, C-3, C-5, C-6, C-15 | |
| 5 | 2.01 m | 30.3 (t) | H2-4, H-6 | C-3, C-4, C-6, C-7 |
| 6 | 4.20 dd (10.4, 4.0) | 68.4 (d) | H2-5 | C-16 |
| 7 | 150.6 (s) | |||
| 8a | 2.16 m | 37.5 (t) | H-8b, H2-9 | n.o.e |
| b | 2.61 m | H-8a, H2-9, H2-16 | C-7, C-9, C-10, C-16 | |
| 9a | 1.44 m | 22.9 (t) | H2-8, H-9b, H-10 | n.o. |
| b | 1.96 m | H2-8, H-9a, H-10 | C-1, C-7, C-8 | |
| 10 | 1.68 dd (10.8, 6.0) | 46.1 (d) | H-1, H2-9 | C-1, C-2, C-8, C-9, C-11, C-12, C-17 |
| 11 | 72.9 (s) | |||
| 12 | 1.59 m | 37.2 (t) | H2-13 | C-10, C-11, C-13, C-14, C-17 |
| 13a | 1.55 m | 21.8 (t) | H2-12, H-13b, H-14 | C-11, C-12, C-14 |
| b | 1.71 m | H2-12, H-13a, H-14 | C-11, C-12, C-14 | |
| 14 | 1.32 m | 41.5 (d) | H-1, H2-13, H-18 | C-13 |
| 15 | 1.36 s | 21.8 (q) | C-2, C-3, C-4 | |
| 16 | 5.06 br s | 111.8 (t) | H-8b | C-6, C-7, C-8 |
| 17 | 1.24 s | 24.4 (q) | C-10, C-11, C-12 | |
| 18 | 1.81 m | 26.5 (d) | H-14, H3-19, H3-20 | C-14, C-19, C-20 |
| 19 | 0.88 d (6.4) | 20.6 (q) | H-18 | C-14, C-18, C-20 |
| 20 | 0.81 d (6.4) | 21.7 (q) | H-18 | C-14, C-18, C-19 |
| C/H | 2 | 3 | ||
|---|---|---|---|---|
| 1Ha | 13Cb | 1He | 13Ce | |
| 1 | 2.73 ddd (7.6, 6.0, 6.0)c | 36.6 (d)d | 2.73 ddd (7.5, 6, 6) | 44.8 (d) |
| 2 | 5.35 d (7.6) | 130.4 (d) | 5.34 br d (7.5) | 130.4 (d) |
| 3 | 134.3 (s) | 134.3 (s) | ||
| 4a | 2.47 ddd (13.2, 13.2, 2.8) | 28.0 (t) | 2.47 ddd (13.4, 13, 2.2) | 35.2 (t) |
| b | 1.95 m | 1.9 m | ||
| 5a | 2.13 m | 25.3 (t) | 2.1 m | 25.3 (t) |
| b | 1.37 m | 1.3 m | ||
| 6 | 3.10 dd (10.8, 3.6) | 65.9 (d) | 3.10 dd (11.2, 3.5) | 65.9 (d) |
| 7 | 61.2 (s) | 61.1 (s) | ||
| 8a | 2.14 m | 38.6 (t) | 2.3 m | 38.6 (t) |
| b | 0.93 m | |||
| 9a | 1.58 m | 22.5 (t) | 1.2–1.7 m | 22.2 (t) |
| b | 0.90 m | |||
| 10 | 1.95 m | 47.3 (d) | 1.95 ddd (6, 6, 6) | 47.3 (d) |
| 11 | 73.2 (s) | 73.2 (s) | ||
| 12a | 1.52 m | 35.2 (t) | 28.1 (t) | |
| b | 1.49 m | |||
| 13a | 1.56 m | 20.0 (t) | 20.0 (t) | |
| b | 1.45 m | |||
| 14 | 1.02 m | 44.7 (d) | 1.0 m | 36.6 (d) |
| 15 | 1.71 s | 24.9 (q) | 1.71 s | 24.9 (q) |
| 16 | 1.18 s | 18.2 (q) | 1.22 s | 18.2 (q) |
| 17 | 1.22 s | 26.7 (q) | 1.18 s | 26.8 (q) |
| 18 | 1.95 m | 26.6 (d) | 1.90 m | 26.6 (d) |
| 19 | 0.97 d (6.8) | 22.0 (q) | 0.78 d (7) | 22.0 (q) |
| 20 | 0.78 d (6.8) | 17.6 (q) | 0.98 d (7) | 17.7 (q) |
| C/H | 1Ha | 13Cb | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 2.74 ddd (8.0, 8.0, 4.0)c | 39.7 (d)d | H-2, H-10, H-14 | C-2, C-3, C-10, C-11, C-14 |
| 2 | 3.86 d (8.0) | 87.1 (d) | H-1 | C-3, C-4, C-14, C-15 |
| 3 | 74.1 (s) | |||
| 4 | 5.14 dd (4.4, 4.4) | 74.6 (d) | H2-5 | C-3, C-6, C-15, C-1′ |
| 5α | 2.97 ddd (16.0, 4.4, 2.8) | 37.2 (t) | H-4, H-5β, H-6 | C-3 |
| β | 1.75 ddd (16.0, 5.6, 3.6) | H-4, H-5α, H-6 | C-3, C-4, C-7 | |
| 6 | 4.21 br s | 72.6 (d) | H2-5, OH-6 | n.o.e |
| 7 | 147.6 (s) | |||
| 8 | 2.35 br d (2.4) | 40.0 (t) | H-9 | C-6, C-7, C-9, C-10, C-16 |
| 9 | 4.16 ddd (3.6, 3.2, 3.2) | 81.3 (d) | H2-8, H-10 | n.o. |
| 10 | 2.63 br s | 44.6 (d) | H-1, H-9 | C-11 |
| 11 | 132.1 (s) | |||
| 12 | 5.43 m | 122.2 (d) | H2-13, H3-17 | n.o. |
| 13α | 2.10 m | 22.8 (t) | H-12, H-13β, H-14 | n.o. |
| β | 1.98 m | H-12, H-13α, H-14 | n.o. | |
| 14 | 1.58 m | 39.0 (d) | H-1, H2-13, H-18 | n.o. |
| 15 | 1.37 s | 22.4 (q) | C-2, C-3, C-4 | |
| 16a | 5.21 s | 115.2 (t) | H-16b | C-6, C-8 |
| b | 5.58 s | H-16a | C-6, C-7, C-8 | |
| 17 | 1.68 d (0.8) | 22.0 (q) | H-12 | |
| 18 | 1.15 m | 28.8 (d) | H-14, H3-19, H3-20 | |
| 19 | 0.92 d (6.4) | 21.3 (q) | H-18 | C-14, C-18, C-20 |
| 20 | 0.83 d (6.4) | 20.5 (q) | H-18 | C-14, C-18, C-19 |
| OH-6 | 2.84 d (7.2) | H-6 | n.o. | |
| 1′ | 171.4 (s) | |||
| 2′ | 4.86 dd (6.8, 6.0) | 74.4 (d) | H2-3′ | C-1′, C-3′, C-4′, acetate carbonyl |
| 3′ | 1.91 m | 24.3 (t) | H-2′, H3-4′ | C-1′, C-2′, C-4′ |
| 4′ | 1.03 t (7.2) | 9.3 (q) | H2-3′ | C-2′, C-3′ |
| 2′-OAc | 171.1 (s) | |||
| 2.14 s | 20.6 (q) | Acetate carbonyl |
| C/H | 1Ha | 13Cb | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 2.51 m | 40.6 (d)d | H-2, H-10, H-14 | C-10 |
| 2 | 3.90 d (3.6)c | 88.1 (d) | H-1 | C-1, C-3, C-4, C-10 |
| 3 | 74.8 (s) | |||
| 4 | 5.21 dd (8.0, 4.0) | 73.8 (d) | H2-5 | C-5, C-6, C-1′ |
| 5α | 2.48 m | 34.2 (t) | H-4, H-5β, H-6 | C-6, C-7 |
| β | 1.97 m | H-4, H-5α, H-6 | n.o.e | |
| 6 | 4.66 dd (8.8, 3.2) | 83.8 (d) | H2-5 | C-4, C-7, C-16 |
| 7 | 144.2 (s) | |||
| 8α | 2.65 dd (14.0, 4.8) | 41.4 (t) | H-8β, H-9, H-16a | C-7, C-9, C-10, C-16 |
| β | 2.46 dd (14.0, 2.0) | H-8α, H-9 | C-6, C-7, C-16 | |
| 9 | 4.06 br s | 82.4 (d) | H2-8, H-10 | n.o. |
| 10 | 2.58 br s | 44.7 (d) | H-1, H-9 | C-8, C-9, C-11 |
| 11 | 131.1 (s) | |||
| 12 | 5.49 m | 123.1 (d) | H2-13, H3-17 | n.o. |
| 13α | 2.01 m | 22.9 (t) | H-12, H-13β, H-14 | n.o. |
| β | 1.80 m | H-12, H-13α, H-14 | n.o. | |
| 14 | 1.39 m | 39.8 (d) | H-1, H2-13, H-18 | C-1, C-2 |
| 15 | 1.33 s | 22.8 (q) | C-2, C-3, C-4 | |
| 16a | 5.26 s | 117.7 (t) | H-8α, H-16b | C-6, C-8 |
| b | 5.47 s | H-16a | C-6, C-7, C-8 | |
| 17 | 1.69 d (1.2) | 22.8 (q) | H-12 | C-10, C-11, C-12 |
| 18 | 1.80 m | 27.8 (d) | H-14, H3-19, H3-20 | C-14, C-19, C-20 |
| 19 | 0.94 d (6.8) | 21.7 (q) | H-18 | C-14, C-18, C-20 |
| 20 | 0.77 d (6.8) | 17.5 (q) | H-18 | C-14, C-18, C-19 |
| 1′ | 170.2 (s) | |||
| 2′ | 4.87 dd (6.8, 6.0) | 74.3 (d) | H2-3′ | C-1′, C-3′, C-4′, acetate carbonyl |
| 3′ | 1.91 m | 24.5 (t) | H-2′, H3-4′ | C-1′, C-2′, C-4′ |
| 4′ | 1.02 t (7.2) | 9.3 (q) | H2-3′ | C-2′, C-3′ |
| 2′-OAc | 171.6 (s) | |||
| 2.14 s | 20.6 (q) | Acetate carbonyl |
| Superoxide anion | Elastase release | |
|---|---|---|
| Compounds | Inh% | Inh% |
| 1 | 6.46 ± 1.28 ** | 12.91 ± 3.56 * |
| 2 | 45.82 ± 2.49 *** | 40.45 ± 5.80 ** |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.-H.; Tai, C.-Y.; Su, Y.-D.; Chang, Y.-C.; Lu, M.-C.; Weng, C.-F.; Su, J.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Discovery of New Eunicellins from an Indonesian Octocoral Cladiella sp. Mar. Drugs 2011, 9, 934-943. https://doi.org/10.3390/md9060934
Chen Y-H, Tai C-Y, Su Y-D, Chang Y-C, Lu M-C, Weng C-F, Su J-H, Hwang T-L, Wu Y-C, Sung P-J. Discovery of New Eunicellins from an Indonesian Octocoral Cladiella sp. Marine Drugs. 2011; 9(6):934-943. https://doi.org/10.3390/md9060934
Chicago/Turabian StyleChen, Yung-Husan, Chia-Ying Tai, Yin-Di Su, Yu-Chia Chang, Mei-Chin Lu, Ching-Feng Weng, Jui-Hsin Su, Tsong-Long Hwang, Yang-Chang Wu, and Ping-Jyun Sung. 2011. "Discovery of New Eunicellins from an Indonesian Octocoral Cladiella sp." Marine Drugs 9, no. 6: 934-943. https://doi.org/10.3390/md9060934
APA StyleChen, Y.-H., Tai, C.-Y., Su, Y.-D., Chang, Y.-C., Lu, M.-C., Weng, C.-F., Su, J.-H., Hwang, T.-L., Wu, Y.-C., & Sung, P.-J. (2011). Discovery of New Eunicellins from an Indonesian Octocoral Cladiella sp. Marine Drugs, 9(6), 934-943. https://doi.org/10.3390/md9060934








