The Acute Toxicity and Hematological Characterization of the Effects of Tentacle-Only Extract from the Jellyfish Cyanea capillata
Abstract
:1. Introduction
2. Results
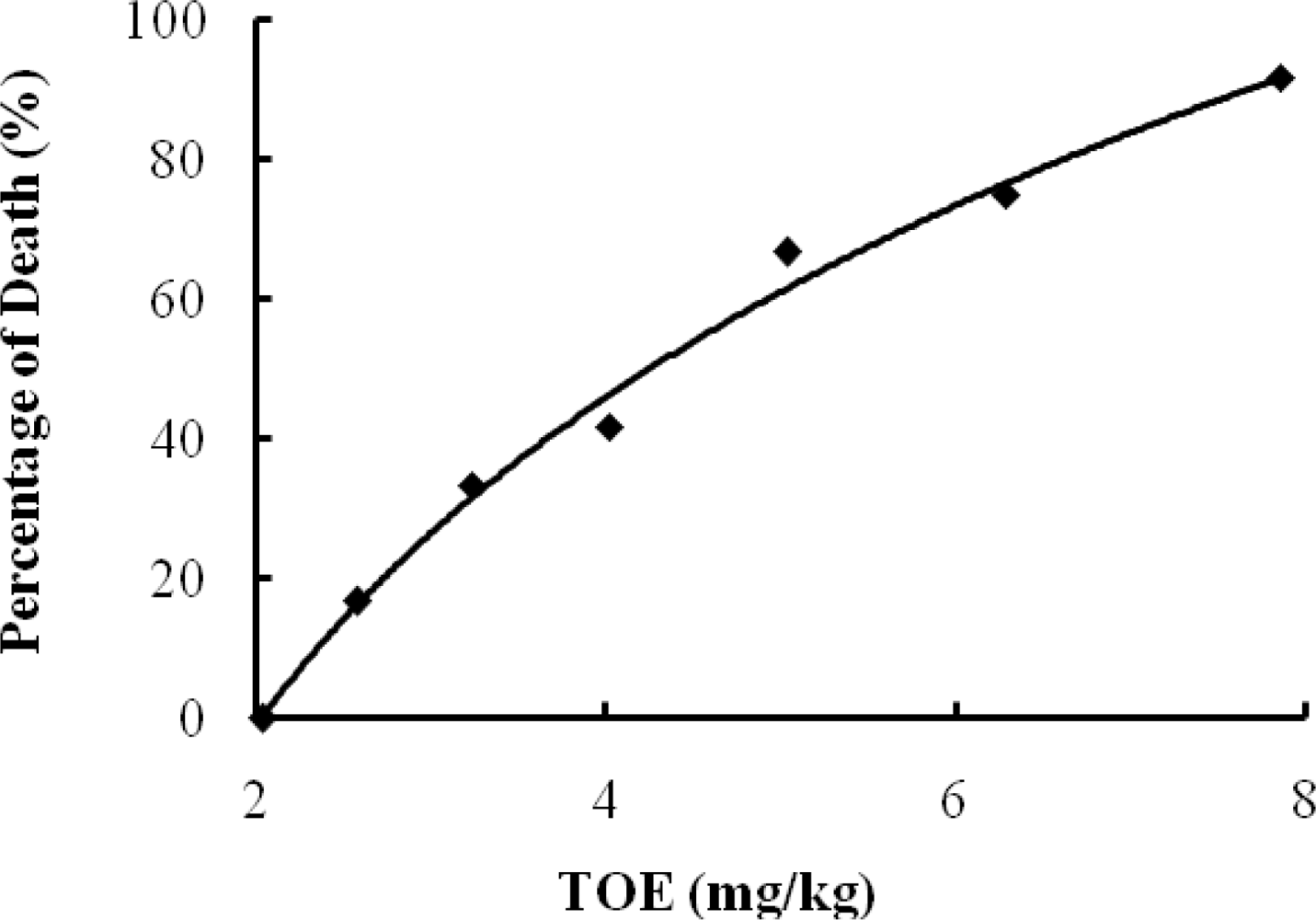
2.1. LD50 Determination and Toxic Symptoms
2.2. Effects of TOE on Arterial Blood Gas Indexes
2.3. Effects of TOE on Other Blood Indexes Determined by the Blood Gas Analyzer
2.4. Effects of TOE on Blood Biochemical Indexes
3. Discussion
4. Experimental Section
4.1. TOE Preparation from the Jellyfish C. capillata
4.2. LD50 Determination and Toxic Symptom
4.3. Hematological Indexes Determined by an Arterial Blood Gas Analyzer
4.4. Hematological Indexes Determined by an Automatic Biochemistry Analyzer
4.5. Data Analysis
5. Conclusions
Acknowledgments
- Sample Availability: Available upon request.
References
- Burnett, JW. Medical aspects of jellyfish envenomation, pathogenesis, case reporting and therapy. Hydrobiologia 2001, 451, 1–9. [Google Scholar]
- Tibballs, J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon 2006, 48, 830–859. [Google Scholar]
- Brinkman, DL; Burnell, JN. Biochemical and molecular characterization of cubozoan protein toxins. Toxicon 2009, 54, 1162–1173. [Google Scholar]
- Suput, D. In vivo effects of cnidarian toxins and venoms. Toxicon 2009, 54, 1190–1200. [Google Scholar]
- Nie, F; Xiao, L; Zhang, J; He, Q; Fan, JW; Li, Y; Zhang, LM. Comparison of haemolytic activities of venom separations from jellyfish Cyanea capillata and their influencing factors. Acad J Sec Mil Med Univ 2008, 29, 83–86. [Google Scholar]
- Xiao, L; Guo, YF; Zhang, J; He, Q; Nie, F; Li, Y; Ye, XF; Zhang, LM. Bioactive analysis of tentacle-only extract from jellyfish Cyanea capillata. Chin J Mar Drugs 2008, 27, 23–27. [Google Scholar]
- Xiao, L; He, Q; Guo, YF; Zhang, J; Nie, F; Li, Y; Ye, XF; Zhang, LM. Cyanea capillata tentacle-only extract as a potential alternative of nematocyst venom: Its cardiovascular toxicity and tolerance to isolation and purification procedures. Toxicon 2009, 53, 146–152. [Google Scholar]
- Xiao, L; Liu, GS; Wang, QQ; He, Q; Liu, SH; Li, Y; Zhang, J; Zhang, LM. The lethality of tentacle-only extract from jellyfish Cyanea capillata is primarily attributed to cardiotoxicity in anaesthetized SD rats. Toxicon 2010, 55, 838–845. [Google Scholar]
- Xiao, L; Zhang, J; Wang, Q; He, Q; Liu, S; Li, Y; Zhang, L. In vitro and in vivo haemolytic studies of tentacle-only extract from jellyfish Cyanea capillata. Toxicol in Vitro 2010, 24, 1203–1207. [Google Scholar]
- Ge, LJ; He, DM. The marking signal of ecosystem crisis. China Fish 2004, 9, 23–25. [Google Scholar]
- Edwards, L; Luo, E; Hall, R; Gonzalez, RR; Hessinger, DA. The effect of Portuguese Man-of-war (Physalia physalis) venom on calcium, sodium and potassium fluxes of cultured embryonic chick heart cells. Toxicon 2000, 38, 323–335. [Google Scholar]
- Edwards, L; Hessinger, DA. Portuguese Man-of-war (Physalia physalis) venom induces calcium influx into cells by permeabilizing plasma membranes. Toxicon 2000, 38, 1015–1028. [Google Scholar]
- Edwards, LP; Whitter, E; Hessinger, DA. Apparent membrane pore-formation by Portuguese Man-of-war (Physalia physalis) venom in intact cultured cells. Toxicon 2002, 40, 1299–1305. [Google Scholar]
- Houck, HE; Lipsky, MM; Marzella, L; Burnett, JW. Toxicity of sea nettle (Chrysaora quinquecirrha) fishing tentacle nematocyst venom in cultured rat hepatocytes. Toxicon 1996, 34, 771–778. [Google Scholar]
- Cao, CJ; Eldefrawi, ME; Eldefrawi, AT; Burnett, JW; Mioduszewski, RJ; Menking, DE; Valdes, JJ. Toxicity of sea nettle toxin to human hepatocytes and the protective effects of phosphorylating and alkylating agents. Toxicon 1998, 36, 269–281. [Google Scholar]
- Winter, KL; Isbister, GK; Schneider, JJ; Konstantakopoulos, N; Seymour, JE; Hodgson, WC. An examination of the cardiovascular effects of an ‘Irukandji’ jellyfish, Alatina nr mordens. Toxicol Lett 2008, 179, 118–123. [Google Scholar]
- Ramasamy, S; Isbister, GK; Seymour, JE; Hodgson, WC. Pharmacologically distinct cardiovascular effects of box jellyfish (Chironex fleckeri) venom and a tentacle-only extract in rats. Toxicol Lett 2005, 155, 219–226. [Google Scholar]
- Ramasamy, S; Isbister, GK; Seymour, JE; Hodgson, WC. The in vivo cardiovascular effects of the Irukandji jellyfish (Carukia barnesi) nematocyst venom and a tentacle extract in rats. Toxicol Lett 2005, 155, 135–141. [Google Scholar]
- Bloom, DA; Burnett, JW; Alderslade, P. Partial purification of box jellyfish (Chironex fleckeri) nematocyst venom isolated at the beachside. Toxicon 1998, 36, 1075–1085. [Google Scholar]
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 1976, 72, 248–254. [Google Scholar]
- El-Sayed, YS; Khalil, RH; Saad, TT. Acute toxicity of ochratoxin-A in marine water-reared sea bass (Dicentrarchus labrax L.). Chemosphere 2009, 75, 878–882. [Google Scholar]
| TOE | Symptoms | Time to death | Autopsy | |
|---|---|---|---|---|
| Heart | Lung | |||
| <5 mg/kg | Little change in breathing. Progressive malaise, slowness, crouching and weakness | Mainly within 48 h | Enlarged, edema | Bright red, edema and congestion |
| 5 10 mg/kg | Early breathing deep and fast, gradually weakened, and finally arrested. Progressive malaise, slowness, crouching, muscle trembling and some double leg twitching | Mainly within 2 h | Enlarged, edema | Edema, some congestion |
| >10 mg/kg | Rapid emergence of breathing deep and fast. Progressive body trembling, double leg twitching, convulsion, opisthotonos and death | Mainly within 5 min, all within 15 min | Normal | Normal |
| Direct indexes | Indirect indexes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | PCO2 (mmHg) | PO2 (mmHg) | HCO3− (mmol/L) | HCO3std (mmol/L) | TCO2 (mmol/L) | BEecf (mmol/L) | BE(B) (mmol/L) | SO2c (%) | |
| 0 min | 7.37 ± 0.03 | 48 ± 4 | 78 ± 10 | 27.5 ± 3.1 | 25.9 ± 2.4 | 28.9 ± 3.2 | 2.1 ± 3.5 | 1.4 ± 3.0 | 95 ± 2 |
| 5 min | 7.32 ± 0.07 ** | 41 ± 7 * | 88 ± 12 | 20.8 ± 3.3 ** | 20.9 ± 2.7 ** | 22.0 ± 3.4 ** | −5.3 ± 3.8 ** | −5.1 ± 3.4 ** | 95 ± 2 |
| 10 min | 7.25 ± 0.05 ** | 41 ± 4 * | 93 ± 4 ** | 18.1 ± 3.2 ** | 17.9 ± 2.7 ** | 19.3 ± 3.3 ** | −9.2 ± 3.8 ** | −8.9 ± 3.5 ** | 96 ± 1 |
| 20 min | 7.27 ± 0.06 ** | 41 ± 8 * | 87 ± 8 ** | 18.6 ± 2.6 ** | 18.7 ± 2.0 ** | 19.9 ± 2.8 ** | −8.3 ± 2.8 ** | −7.9 ± 2.6 ** | 95 ± 2 |
| 60 min | 7.27 ± 0.05 ** | 36 ± 3 * | 89 ± 11 * | 16.5 ± 2.4 ** | 17.3 ± 2.3 ** | 17.5 ± 2.5 ** | −10.5 ± 3.1 ** | −9.7 ± 2.9 ** | 95 ± 2 |
| 180 min | 7.28 ± 0.02 ** | 34 ± 7 ** | 91 ± 11 * | 16.6 ± 3.2 ** | 17.6 ± 1.9 ** | 17.7 ± 3.5 ** | −10.1 ± 3.1 ** | −9.3 ± 2.5 ** | 94 ± 4 |
| Na+ (mmol/L) | K+ (mmol/L) | Ca2+ (mmol/L) | Ca2+ (7.4) (mmol/L) | Glu (mmol/L) | Lac (mmol/L) | Hct (%) | THbc (g/dL) | |
|---|---|---|---|---|---|---|---|---|
| 0 min | 143 ± 2 | 3.6 ± 0.2 | 1.18 ± 0.04 | 1.17 ± 0.05 | 8.1 ± 1.3 | 2.7 ± 0.7 | 47 ± 4 | 14.8 ± 1.1 |
| 5 min | 129 ± 4 ** | 11.7 ± 1.9 ** | 0.82 ± 0.12 ** | 0.79 ± 0.12 ** | 9.3 ± 2.2 | 5.1 ± 0.7 ** | 51 ± 5 | 15.8 ± 1.5 |
| 10 min | 133 ± 4 ** | 9.7 ± 2.0 ** | 0.78 ± 0.16 ** | 0.73 ± 0.15 ** | 10.0 ± 2.8 | 5.9 ± 0.1 ** | 53 ± 4 * | 16.3 ± 1.2 * |
| 20 min | 134 ± 3 ** | 6.8 ± 1.1 ** | 1.07 ± 0.17 | 1.05 ± 0.14 * | 11.0 ± 2.3 * | 5.3 ± 0.7 ** | 52 ± 5 | 16.0 ± 1.4 |
| 60 min | 135 ± 4 ** | 5.3 ± 1.5 ** | 1.25 ± 0.09 | 1.18 ± 0.10 | 10.9 ± 1.7 ** | 4.7 ± 0.7 ** | 49 ± 3 | 15.1 ± 0.7 |
| 180 min | 133 ± 3 ** | 6.5 ± 0.8 ** | 1.26 ± 0.05 ** | 1.20 ± 0.05 | 9.5 ± 2.4 | 4.3 ± 1.0 ** | 46 ± 4 | 14.4 ± 1.3 |
| LDH (U/L) | CK (U/L) | CK-MB (U/L) | ALT (U/L) | AST (U/L) | sCr (μmol/L) | BUN (mmol/L) | AMY (U/L) | |
|---|---|---|---|---|---|---|---|---|
| 0 min | 695 ±187 | 649 ±393 | 1067 ±227 | 46 ±8 | 114 ±55 | 22 ±4 | 5.1 ±1.0 | 1946 ±254 |
| 5 min | 692 ±295 | 987 ±588 | 1211 ±303 | 59 ±13 | 157 ±33 | 25 ±11 | 6.3 ±1.3 | 2021 ±581 |
| 10 min | 718 ±358 | 1113 ±539 | 1574 ±456 * | 56 ±20 | 154 ±53 | 29 ±9 | 6.0 ±1.7 | 2099 ±494 |
| 20 min | 760 ±496 | 1192 ±697 | 1826 ±616 * | 68 ±19 * | 185 ±80 | 28 ±4 * | 6.7 ±1.5 | 2343 ±393 |
| 60 min | 3171 ±4422 | 2085 ±2782 | 1889 ±607 * | 166 ±235 | 362 ±372 | 36 ±9 ** | 5.1 ±1.6 | 2070 ±342 |
| 180 min | 5816 ±5335 * | 2231 ±1212 * | 2772 ±885 ** | 354 ±257 * | 785 ±201 ** | 57 ±19 ** | 6.8 ±1.8 | 1913 ±664 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiao, L.; Liu, S.; He, Q.; Wang, Q.; Ye, X.; Liu, G.; Nie, F.; Zhao, J.; Zhang, L. The Acute Toxicity and Hematological Characterization of the Effects of Tentacle-Only Extract from the Jellyfish Cyanea capillata. Mar. Drugs 2011, 9, 526-534. https://doi.org/10.3390/md9040526
Xiao L, Liu S, He Q, Wang Q, Ye X, Liu G, Nie F, Zhao J, Zhang L. The Acute Toxicity and Hematological Characterization of the Effects of Tentacle-Only Extract from the Jellyfish Cyanea capillata. Marine Drugs. 2011; 9(4):526-534. https://doi.org/10.3390/md9040526
Chicago/Turabian StyleXiao, Liang, Sihua Liu, Qian He, Qianqian Wang, Xuting Ye, Guoyan Liu, Fei Nie, Jie Zhao, and Liming Zhang. 2011. "The Acute Toxicity and Hematological Characterization of the Effects of Tentacle-Only Extract from the Jellyfish Cyanea capillata" Marine Drugs 9, no. 4: 526-534. https://doi.org/10.3390/md9040526
APA StyleXiao, L., Liu, S., He, Q., Wang, Q., Ye, X., Liu, G., Nie, F., Zhao, J., & Zhang, L. (2011). The Acute Toxicity and Hematological Characterization of the Effects of Tentacle-Only Extract from the Jellyfish Cyanea capillata. Marine Drugs, 9(4), 526-534. https://doi.org/10.3390/md9040526




