Statistical Research on Marine Natural Products Based on Data Obtained between 1985 and 2008
Abstract
:1. Introduction
2. Data and Methods
3. Results and Analyses
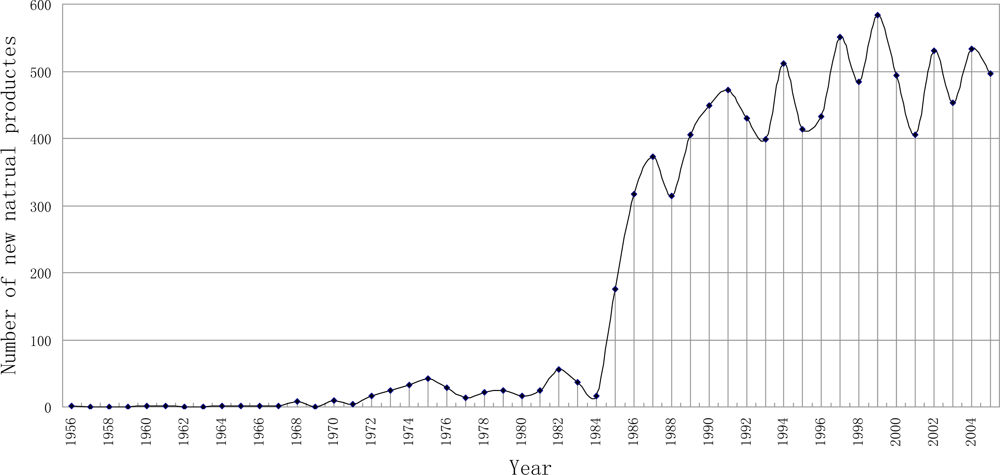
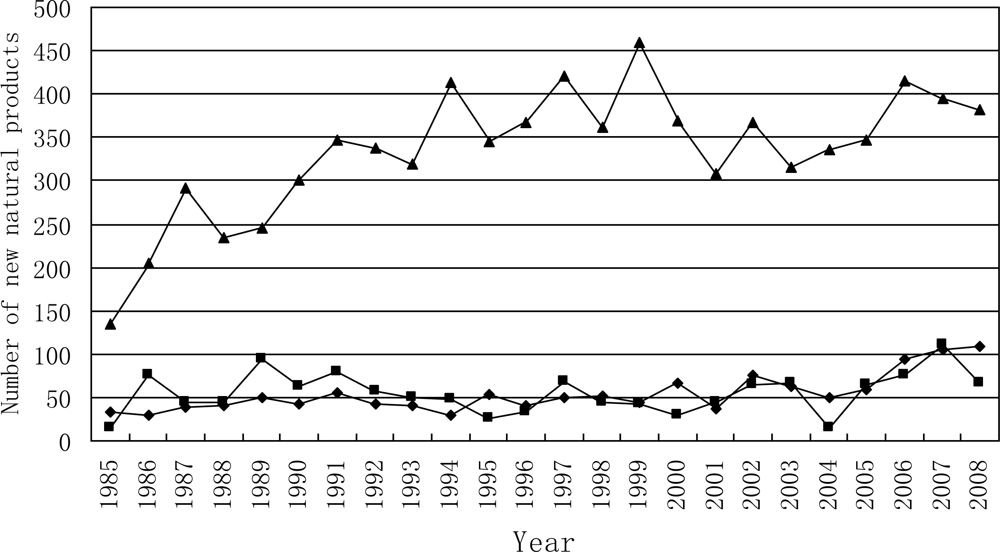
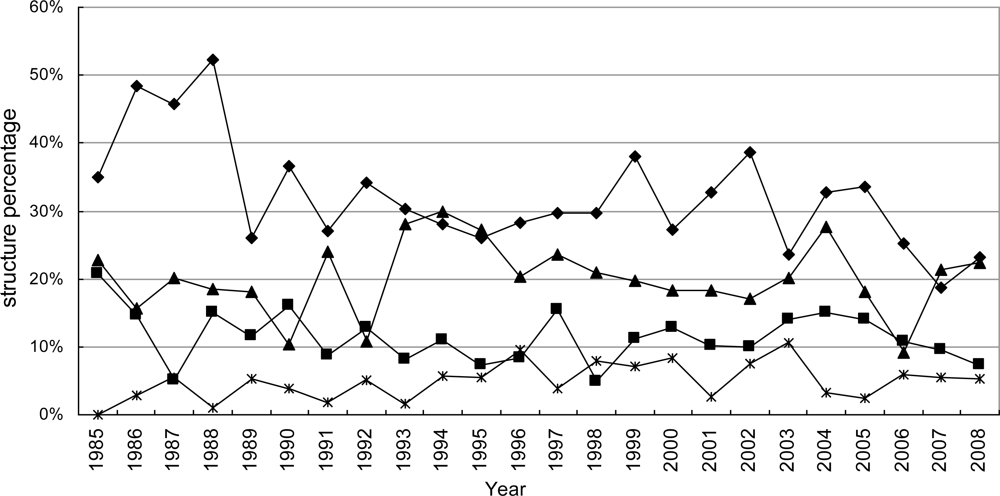
3.1. Temporal Distribution
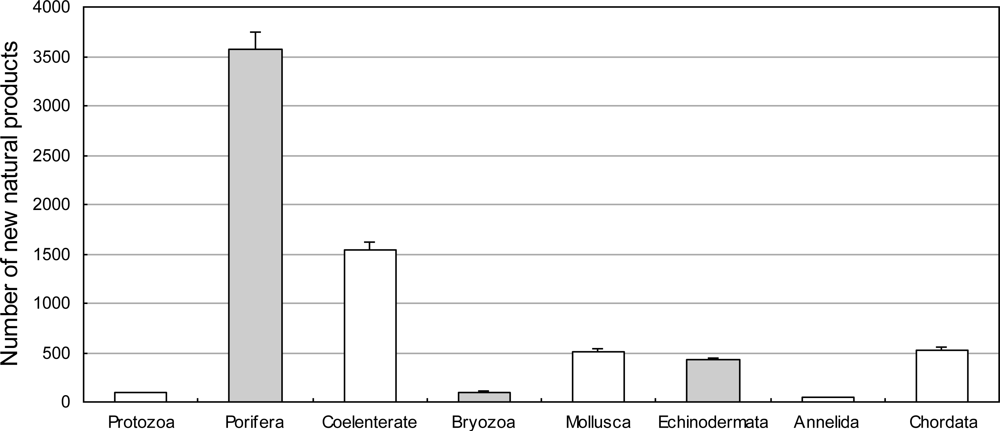
3.2. Species Distribution
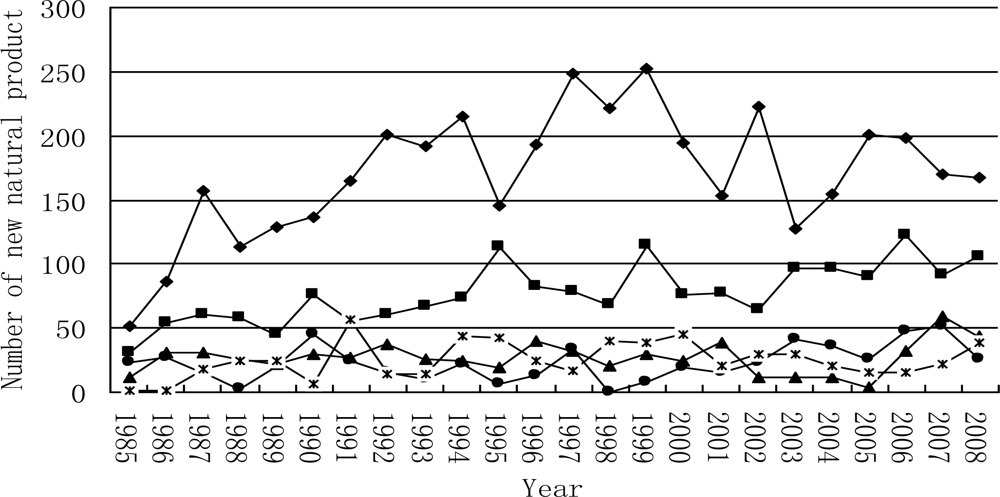
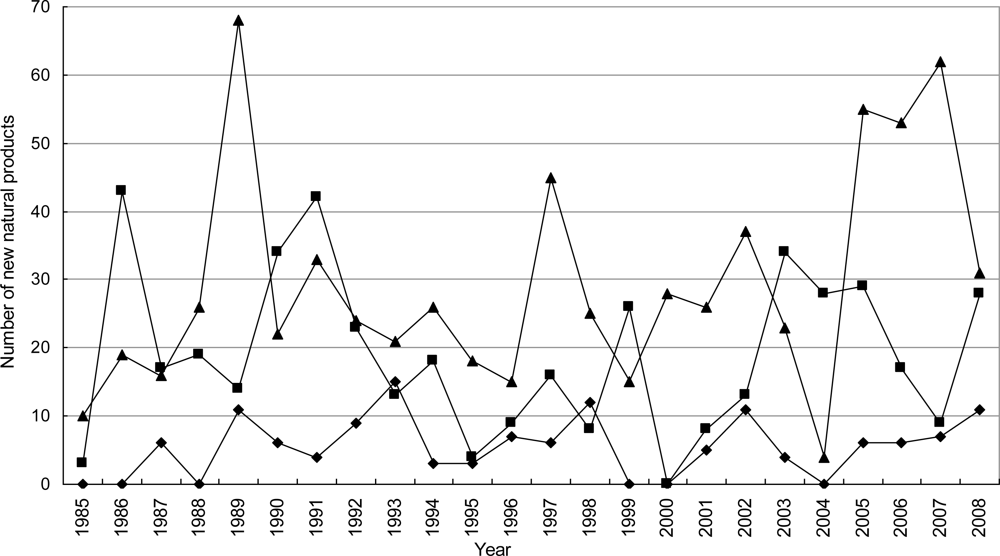
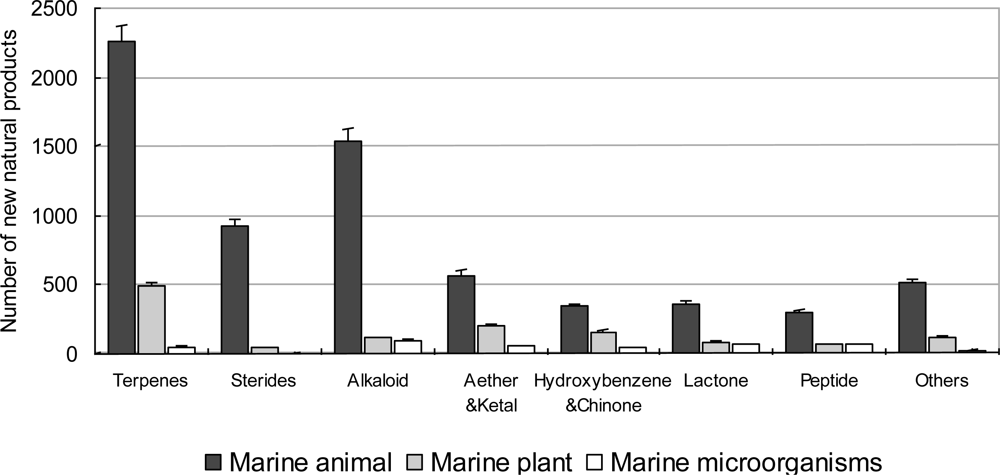
3.2.1. Novel Compounds Isolated from Marine Invertebrates
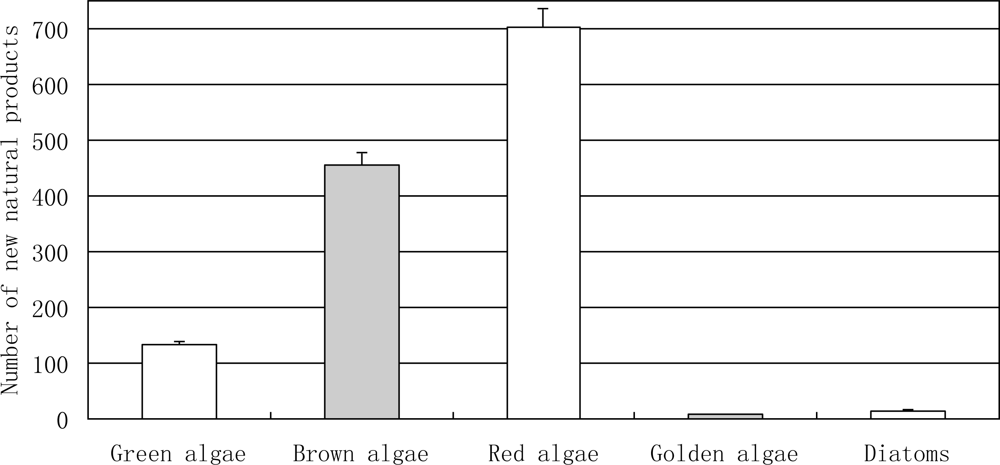
3.2.2. Novel Compounds Isolated from Marine Algae
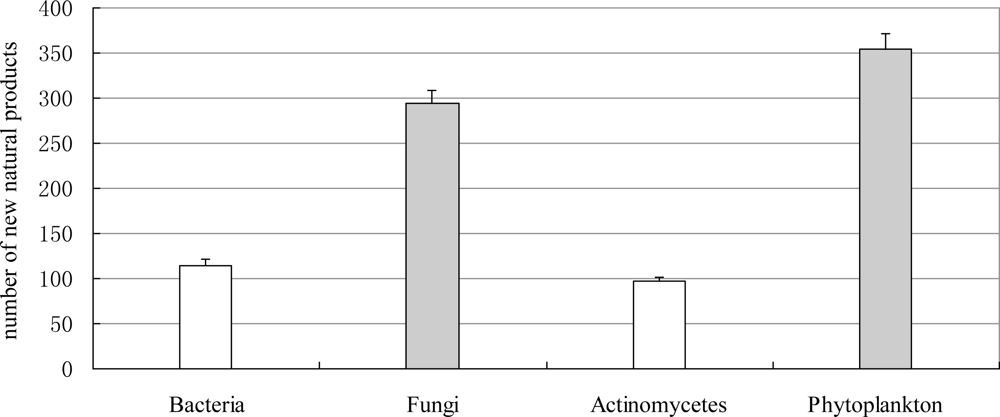
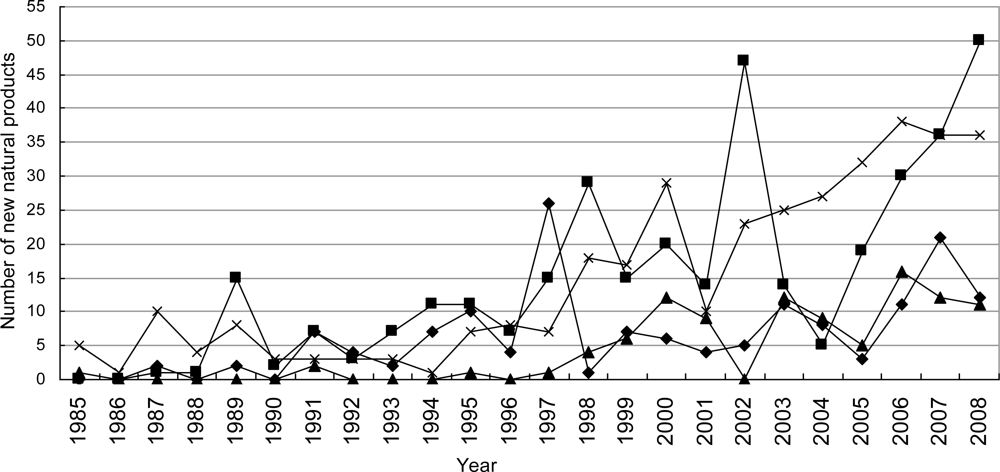
3.2.3. Novel Compounds Isolated from Marine Microorganisms (including Phytoplankton)
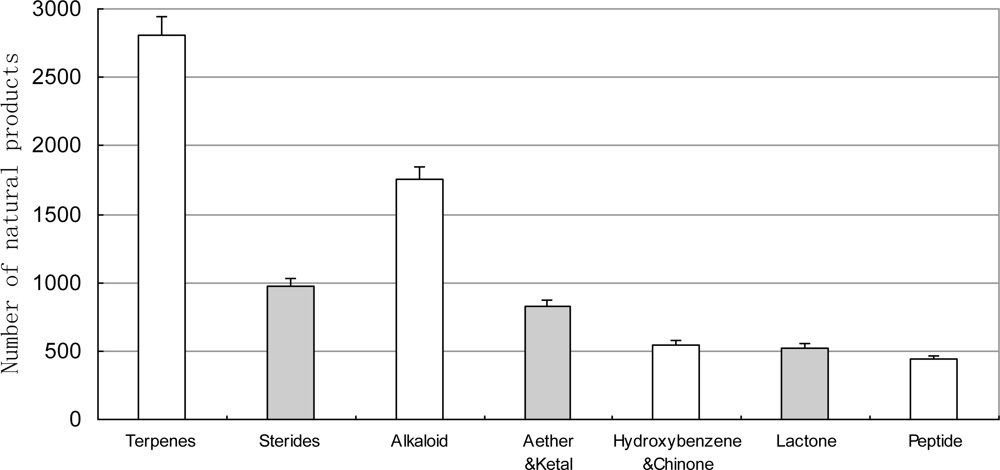
3.3. Chemical Compounds Isolated from Marine Organisms
4. Conclusions and Prospects
Acknowledgments
- Samples Availability: Available from the authors.
References
- Faulkner, DJ. Marine natural products: metabolites of marine algae and herbivorous marine molluscs. Nat Prod Rep 1984, 1, 251–280. [Google Scholar]
- Faulkner, DJ. Marine natural products: metabolites of marine invertebrates. Nat Prod Rep 1984, 1, 551–598. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1986, 3, 1–33. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1987, 4, 539–576. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1988, 5, 613–663. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1990, 7, 269–309. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1991, 8, 97–147. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1992, 9, 323–364. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1993, 10, 497–539. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1994, 11, 355–394. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1995, 12, 223–269. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1996, 13, 75–125. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1997, 14, 259–302. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1998, 15, 113–158. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 1999, 16, 155–198. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 2000, 17, 7–55. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 2001, 18, 1R–49R. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 2002, 19, 1–48. [Google Scholar]
- Blunt, JW; Copp, BR; Munro, MHG; Northcotec, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2003, 20, 1–48. [Google Scholar]
- Blunt, JW; Copp, BR; Munro, MHG; Northcotec, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2004, 21, 1–49. [Google Scholar]
- Blunt, JW; Copp, BR; Munro, MHG; Northcotec, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2005, 22, 15–61. [Google Scholar]
- Blunt, JW; Copp, BR; Munro, MHG; Northcotec, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2006, 23, 26–78. [Google Scholar]
- Blunt, JW; Copp, BR; Hu, WP; Munro, MHG; Northcotec, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2007, 24, 31–86. [Google Scholar]
- Blunt, JW; Copp, BR; Hu, WP; Munro, MHG; Northcotec, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2008, 25, 35–94. [Google Scholar]
- Blunt, JW; Copp, BR; Hu, WP; Munro, MHG; Northcotec, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2009, 26, 170–244. [Google Scholar]
- Blunt, JW; Copp, BR; Munro, MHG; Northcotec, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2010, 27, 165–237. [Google Scholar]
- Bergmann, W; Feeney, RJ. Contributions to the study of marine products XXXII. The nucleosides of sponges. I. J Org Chem 1951, 16, 981–987. [Google Scholar]
- Weinheimer, J; Spraggins, RL. The occurrence of two new prostaglandin derivatives (15-epi-PGA2 and its acetate, methyl ester) in the Gorgonian Plexaura Homomalla Chemistry of Coelenterates. XV. Tetrahedron Lett 1969, 15, 5185–5188. [Google Scholar]
- Molinski, TF; Dalisay, DS; Lievens, SL; Saludes, JP. Drug development from marine natural products. Nat Rev Drug Discov 2009, 8, 69–85. [Google Scholar]
- Faulkner, DJ. Highlights of marine natural products chemistry 1972–1999). Nat Prod Rep 2000, 17, 1–6. [Google Scholar]
- Marine Organism Metabolite data center. Key Laboratory of Functional Molecules from Oceanic Microorganisms (Sun Yat-sen University). Available online: http:\\momdc.sysu.edu.cn (accessed on 28 January 2011).
- Proksch, P; Ebel, R; Edrada, RA; Schupp, P; Lin, WH; Sudarsono; Wray, V; Steube, K. Detection of pharmacologically active natural products using ecology. Selected examples from Indopacific marine invertebrates and sponge-derived fungi. Pure Appl Chem 2003, 75, 343–352. [Google Scholar]
- Proksch, P; Edrada, RA; Ebel, R. Drugs from the seas—current status and microbiological implications. Appl Microbiol Biotechnol 2002, 59, 125–134. [Google Scholar]
- Jha, RK; Xu, ZR. Biomedical Compounds from Marine organisms. Mar Drugs 2004, 2, 123–146. [Google Scholar]
- Ernst, RR; Bodcnhausen, G; Wokaun, A. Principles of Nuclear Magnetic Resonance in One and Two Dimensions; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Mendola, D. Aquacultural production of bryostatin 1 and ecteinascidin 743. In Drugs from the Sea; Fusetani, N, Ed.; Karger: Basel, Switzerland, 2000; pp. 120–133. [Google Scholar]
- Smit, AJ. Medicinal and pharmaceutical uses of seaweed natural products: A review. J Appl Phycol 2004, 16, 245–262. [Google Scholar]
- Tan, LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar]
- Gulder, TAM; Moore, BS. Chasing the treasures of the sea—bacterial marine natural products. Curr Opin Microbiol 2009, 12, 252–260. [Google Scholar]
- Piel, J. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc Natl Acad Sci USA 2002, 99, 14002–14007. [Google Scholar]
- Gulder, TAM; Moore, BS. Chasing the treasures of the sea—bacterial marine natural products. Curr Opin Microbiol 2009, 12, 252–260. [Google Scholar]
- Scherlach, K; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org Biomol Chem 2009, 7, 1753–1760. [Google Scholar]
- Chlipala, GE; Pham, HT; Nguyen, VH; Krunic, A; Shim, SH; Soejarto, DD; Orjala, J. Nhatrangins A and B, Aplysiatoxin-Related Metabolites from the Marine Cyanobacterium Lyngbya majuscula from Vietnam. J Nat Prod 2010, 73, 784–787. [Google Scholar]
- Lang, G; Mayhudin, NA; Mitova, MI; Sun, L; van der Sar, S; Blunt, JW; Cole, AL; Ellis, G; Laatsch, H; Munro, MH. Evolving Trends in the Dereplication of Natural Product Extracts: New Methodology for Rapid, Small-Scale Investigation of Natural Product Extracts. J Nat Prod 2008, 71, 1595–1599. [Google Scholar]
- Lacey, ME; Subramanian, R; Olson, DL; Webb, AG; Sweedler, JV. High-Resolution NMR Spectroscopy of Sample Volumes from 1 nL to 10 μL. Chem Rev 1999, 99, 3133–3152. [Google Scholar]
- Sabdono, A; Radjasa, OK. Microbial Symbionts in Marine Sponges: Marine natural product factory. J Coast Dev 2008, 11, 57–61. [Google Scholar]
- Piel, J. Metabolites from symbiotic bacteria. Nat Prod Rep 2004, 21, 519–538. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, G.-P.; Yuan, J.; Sun, L.; She, Z.-G.; Wu, J.-H.; Lan, X.-J.; Zhu, X.; Lin, Y.-C.; Chen, S.-P. Statistical Research on Marine Natural Products Based on Data Obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514-525. https://doi.org/10.3390/md9040514
Hu G-P, Yuan J, Sun L, She Z-G, Wu J-H, Lan X-J, Zhu X, Lin Y-C, Chen S-P. Statistical Research on Marine Natural Products Based on Data Obtained between 1985 and 2008. Marine Drugs. 2011; 9(4):514-525. https://doi.org/10.3390/md9040514
Chicago/Turabian StyleHu, Gu-Ping, Jie Yuan, Li Sun, Zhi-Gang She, Jue-Heng Wu, Xiu-Jian Lan, Xun Zhu, Yong-Cheng Lin, and Sheng-Ping Chen. 2011. "Statistical Research on Marine Natural Products Based on Data Obtained between 1985 and 2008" Marine Drugs 9, no. 4: 514-525. https://doi.org/10.3390/md9040514
APA StyleHu, G.-P., Yuan, J., Sun, L., She, Z.-G., Wu, J.-H., Lan, X.-J., Zhu, X., Lin, Y.-C., & Chen, S.-P. (2011). Statistical Research on Marine Natural Products Based on Data Obtained between 1985 and 2008. Marine Drugs, 9(4), 514-525. https://doi.org/10.3390/md9040514



