Abstract
The structures, names, bioactivities, and references of 81 new secondary metabolites obtained from gorgonian corals belonging to the genus Junceella are described in this review. All compounds mentioned in this review were obtained from sea whip gorgonian corals Junceella fragilis and Junceella juncea, collected from the tropical and subtropical Indo-Pacific Ocean.
1. Introduction
This review describes 81 new natural products from gorgonian corals belonging to the genus Junceella (phylum Cnidaria, class Anthozoa, order Gorgonacea, family Ellisellidae) [1,2,3,4]. Extending from a previous review in 2004 [5], this review describes compounds reported from November 2003 to September 2011 and provides structures, names, bioactivities and references for all compounds in tabular form.
2. Natural Products from Gorgonian Corals Belonging to the Genus Junceella
2.1. Junceella fragilis
Two new chlorinated briarane-type diterpenoids (3,8-cyclized cembranoids), (−)-2-deacetyl-junceellin (1) and (−)-3-deacetyljunceellin (2) (Table 1), along with five known briaranes, junceellin, praelolide, and junceellolides A, B and D, were isolated from the gorgonian J. fragilis, collected at the Pass Reef of Madang, Papua New Guinea [6]. The absolute stereochemistry of (−)-3-deacetyljunceellin (2) was determined by the application of a new method using a combination of proton chemical shifts and molecular dynamic calculation.

Table 1.
The new natural products from Junceella fragilis-I.
| Structure | No. | Name | Biological Activity | Ref. |
|---|---|---|---|---|
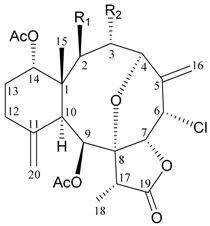 | 1 | (−)-2-Deacetyljunceellin (R1 = OH, R2 = OAc) | n.r. a | [6] |
| 2 | (−)-3-Deacetyljunceellin (R1 = OAc, R2 = OH) | n.r. | [6] |
a n.r. = not reported.
During the past 30 years, a series of interesting and bioactive natural products has been isolated from various marine invertebrates collected off the South China Sea [7,8]. Five new briaranes, junceellonoids A–E (3–7) (Table 2) [9,10], eight known briaranes, junceellins A and B, junceellolides A–D, umbraculolide A and praelolide, along with three known steroids, 24α-methylcholest-7,22-dien-3β,5α,6β-triol, cholestan-3-ol and cholesterol, were isolated from J. fragilis inhabiting the South China Sea [9,10,11,12]. Junceellonoids C (5) and D (6) exhibited cytotoxicity toward human breast carcinoma MDA-MB-231 and MCF-7 cells [10].

Table 2.
The new natural products from Junceella fragilis-II.
| Structure | No. | Name | Biological Activity | Ref. |
|---|---|---|---|---|
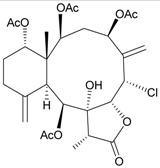 | 3 | Junceellonoid A | n.r. a | [9] |
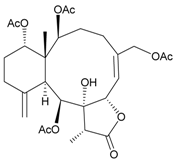 | 4 | Junceellonoid B | n.r. | [9] |
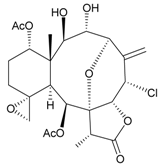 | 5 | Junceellonoid C | exhibited cytotoxicity toward MDA-MB-231 and MCF-7 cells at a concentration of 100 µM | [10] |
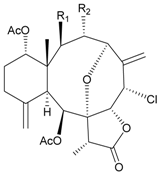 | 6 | Junceellonoid D (R1 = R2 = OH) | exhibited cytotoxicity toward MDA-MB-231 and MCF-7 cells at a concentration of 100 µM | [10] |
| 7 | Junceellonoid E (R1 = R2 = OAc) | n.r. | [10] |
a n.r. = not reported.
In continuing research on the new substances obtained from gorgonian corals distributed in the waters of Taiwan at the intersection of the Kuroshio current and the South China Sea surface current, the gorgonian J. fragilis was studied to examine the properties of its organic extract. Thirty-one new briaranes, 9-O-deacetylumbraculolide A (8) [13], junceellolides H–L (9–13) [14,15,16], fragilides A–J (14–23) [17,18,19,20,21,22,23,24] and frajunolides A–O (24–38) [25,26,27] (Table 3); 16 known briaranes, prarelolide [14,26,28], junceellin A [12,14,26,28], (1R,2R,5Z,7R,8S,9R,10R,12R,14R,17S)-2,14-diacetoxy-8,17-epoxide-9,12-dihydroxybriara-5,11(20)-dien-19-one [15], (−)-11β,20β-epoxy-4-deacetoxyjunceellolide D [16,25,26,29], junceellonoid D [22], juncins Y, Z and ZI [22,26], (+)-11β,20β-epoxyjunceellolide D [23,29], junceellolides A–E and K [25,26], and umbraculolide A [25,26]; and three known steroids, ergosterol peroxide [26], deoxycholic acid 3,12-diacetate, and deoxycholic acid 3,12-diacetate methyl ester [30], were isolated from J. fragilis collected off the waters of Taiwan. The structure, including the absolute configuration, of junceellolide J (11) was confirmed by single-crystal X-ray diffraction analysis and chemical conversion [16]. Fragilide A (14) was the first briarane derivative found to possess a 6-hydroxy group [17]. The geometry of the Δ3,5(16)-butadiene system in fragilide B (15) was found to be of an s-cis form [18]. The 13C NMR data for the known briaranes praelolide and junceellin were reassigned by 2D NMR experiments [14].

Table 3.
The new natural products from Junceella fragilis-III.
| Structure | No. | Name | Biological Activity | Ref. |
|---|---|---|---|---|
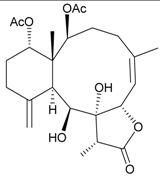 | 8 | 9-O-Deacetylumbraculolide A | n.r. a | [13] |
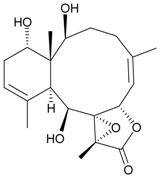 | 9 | Junceellolide H | not active in cytotoxicity testing with P-388D1, DLD-1, IMR-32, RPMI 7951 and CCRF-CEM tumor cells b | [14] |
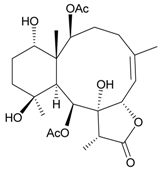 | 10 | Junceellolide I | n.r. | [15] |
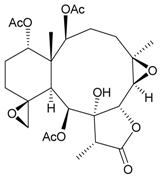 | 11 | Junceellolide J | not active in anti-inflammatory bioassay | [16] |
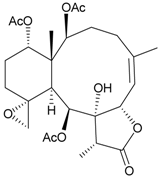 | 12 | Junceellolide K | weakly anti-inflammatory | [16] |
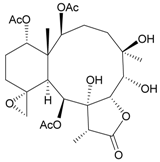 | 13 | Junceellolide L | not active in anti-inflammatory bioassay | [16] |
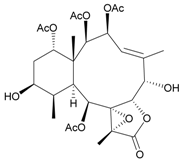 | 14 | Fragilide A | n.r. | [17] |
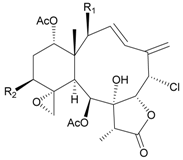 | 15 | Fragilide B (R1 = OC(O)CH2CH3, R2 = H) | weakly anti-inflammatory | [18] |
| 20 | Fragilide G (R1 = R2 = OAc) | not active in cytotoxicity testing with DLD-1 and CCRF-CEM cells | [22] | |
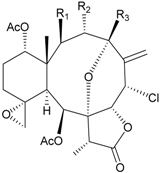 | 16 | Fragilide C (R1 = OCOCH2CH3, R2 = H, R3 = OH) | weakly anti-inflammatory | [19] |
| 23 | Fragilide J (R1 = OH, R2 = OAc, R3 = H) | weakly anti-inflammatory | [24] | |
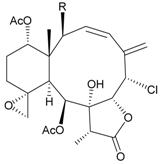 | 17 | Fragilide D (= Frajunolide G) (R = OC(O)CH2OC(O)CH2CH(CH3)2) | n.r. | [20,26] |
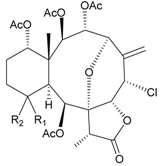 | 18 | Fragilide E (R1 = β-OH, R2 = α-CH2OAc) | weakly anti-inflammatory | [21] |
| 19 | Fragilide F (R1 = α-OH, R2 = β-CH2Cl) | not active in cytotoxicity testing with DLD-1 and CCRF-CEM cells | [22] | |
I 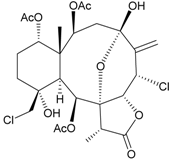 | 21 | Fragilide H | not active in cytotoxicity testing with P-388D1, DLD-1, HL-60 and CCRF-CEM cells b | [23] |
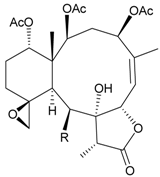 | 22 | Fragilide I (R = OC(O)CH2CH(CH3)2) | not active in cytotoxicity testing with P-388D1, DLD-1, HL-60 and CCRF-CEM cells | [23] |
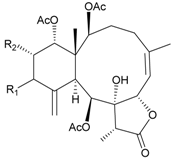 | 24 | Frajunolide A (R1 = α-OAc, R2 = H) | weakly anti-inflammatory | [25] |
| 25 | Frajunolide B (R1 = α-OAc, R2 = OAc) | weakly anti-inflammatory | [25] | |
| 28 | Frajunolide E (R1 = H, R2 = OAc) | frajunolides E, J and L were weakly anti-inflammatory | [26] | |
| 33 | Frajunolide J (R1 = α-OC(O)Et, R2 = H) | frajunolides E and J were not active in cytotoxicity testing with Hep2, Doay, WiDr and Hela cells b | [26] | |
| 35 | Frajunolide L (R1 = β-OAc, R2 = H) | [27] | ||
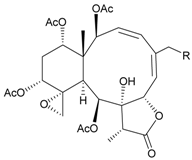 | 26 | Frajunolide C (R = Cl) | weakly anti-inflammatory | [25] |
| 27 | Frajunolide D (R = OAc) | not active in anti-inflammatory bioassay | [25] | |
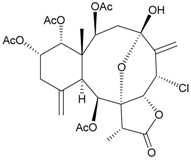 | 29 | Frajunolide F | weakly anti-inflammatory | [26] |
| not active in cytotoxicity testing with Hep2, Doay, WiDr and Hela cells | ||||
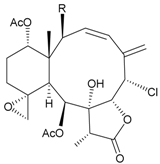 | 30 | Frajunolide G (= Fragilide D) (R = OC(O)CH2OC(O)CH2CH(CH3)2) | not active in anti-inflammatory bioassay | [20,26] |
| not active in cytotoxicity testing with Hep2, Doay, WiDr and Hela cells | ||||
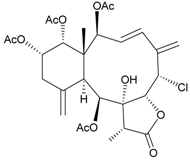 | 31 | Frajunolide H | not active in anti-inflammatory bioassay | [26] |
| not active in cytotoxicity testing with Hep2, Doay, WiDr and Hela cells | ||||
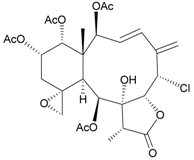 | 32 | Frajunolide I | weakly anti-inflammatory | [26] |
| not active in cytotoxicity testing with Hep2, Doay, WiDr and Hela cells | ||||
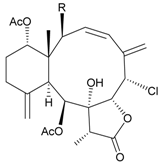 | 34 | Frajunolide K (R = OC(O)CH2OC(O)CH2CH(CH3)2) | not active in anti-inflammatory bioassay | [26] |
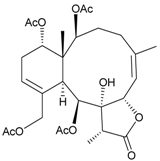 | 36 | Frajunolide M | weakly anti-inflammatory | [27] |
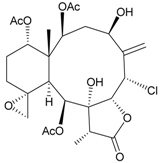 | 37 | Frajunolide N | modestly anti-inflammatory | [27] |
| not active in cytotoxicity testing with Hep2, Doay, WiDr and Hela cells | ||||
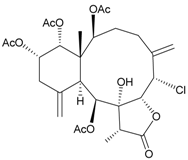 | 38 | Frajunolide O | weakly anti-inflammatory | [27] |
a n.r. = not reported; b P388D1 (mouse lymphoid neoplasm), DLD-1 (human colon adenocarcinoma), IMR-32 (human neuroblastoma), RPMI 7951 (human malignant melanoma), CCRF-CEM (human T-cell acute lymphoblastic leukemia), HL-60 (human promyelocytic leukemia), Hep2 (human liver carcinoma), Doay (medulloblastoma), WiDr (human colon adenocarcinoma), Hela (human cervical epitheloid carcinoma).
In order to determine the stereochemistry of briaranes possessing an exocyclic 11,20-epoxy group, the 13C NMR data of the exocyclic 11,20-epoxy groups have been summarized; these appeared at δC 62–63 and 58–60 ppm, respectively, when the epoxy group existed in the 11S* form and led the cyclohexane rings to exhibit a twist boat conformation. If the epoxy group was in an 11R* configuration, the 13C NMR data for C-11 and C-20 appeared at δC 55–61 and 47–52 ppm, respectively, and the cyclohexane rings were in a chair conformation [16]. The 11,20-epoxybriaranes were only obtained from gorgonian corals belonging to the Ellisellidae family, and, thus compounds of this type could be a chemical marker for gorgonian corals belonging to the Ellisellidae family [31].
From the characteristics of the chemical shifts, it was shown that the briarane derivatives contained an exocyclic double bond between C-11/12. The proton chemical shifts were summed up for the olefin protons H2-20; these appear at δH 4.95–5.30 and 4.85–5.15 ppm, respectively, when the cyclohexane rings are in a twist boat conformation. Likewise, the 1H NMR data for H2-20 appear at δH 4.95–5.10 and 4.40–4.75, if the cyclohexane rings were found to exist in a chair conformation [22].
Symbiotic algae (zooxanthella) exist throughout the life cycle of J. fragilis, while J. juncea is a gorgonian coral free of zooxanthellae [32]. Two known chlorine-containing briaranes, junceellin and praelolide, were isolated in the same proportions from both J. fragilis and J. juncea, and this observation suggests that junceellin and praelolide could be chemical markers that enable one to infer that the briarane-type compounds are originally synthesized by the host corals [28] and are not produced by their zooxanthella.
In biological activity experiments, the new briaranes, junceellolide K (12) [16], fragilides B, C, E and J (15, 16, 18, 23) [18,19,21,24], frajunolides A–C (24–26), E (28), F (29), I (32), J (33), L–O (35–38) (Table 3), and the known compounds (−)-11β,20β-epoxy-4-deacetoxyjunceellolide D [16,25], junceellolide E [25] and umbraculolide A [25], displayed anti-inflammatory activity [33]. Juncin Z was found to exhibit cytotoxicity toward CCRF-CEM cells [22].
2.2. Junceella juncea
Five new steroidal glycosides, 4′-O-acetyl-3-O-[β-D-arabino-pyranosyl-oxy]-cholest-5-ene-3β,19-diol (39) [34] and junceellosides A–D (40–43) [35], and a new glycerol, 1,2-O-[2′-hydroxyoctadecyl]-glycerol (44) [34] (Table 4) along with various known metabolites, including four sterols, 24α-methylcholest-7,22-dien-3β,5α,6β-triol, 24α-methylcholest-3β,5α,6β-triol-25-monoacetate, 24α-methylcholest-3β,5α,6β-triol, and 24α-methylcholest-5,23-dien-3β-ol; six amines, 1-O-β-D-gluco-pyranosyl-(2S,3S,4R,8Z)-2-N-(2′-hydroxypalmitoyl)-octadecasphinga-8-ene, (2S,3R)-2-N-palmitoyl-octadecasphinga, (2S,3R,4E)-2-N-palmitoyloctadecasphinga-4-ene, thymine, uracil, and adenosine; and batyl alcohol, were isolated from the gorgonian coral J. juncea, collected off the South China Sea in 2004–2005 [34,35].

Table 4.
The new natural products from Junceella juncea-IV.
| Structure | No. | Name | Ref. |
|---|---|---|---|
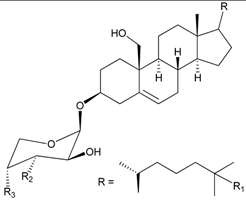 | 39 | 4′-O-Acetyl-3-O-[β-D-arabino-pyranosyl-oxy]-cholest-5-ene-3β,19-diol (R1 = H, R2 = OH, R3 = OAc) | [34] |
| 40 | Junceelloside A (R1 = R2 = OH, R3 = OAc) | [35] | |
| 41 | Junceelloside B (R1 = R3 = OH, R2 = OAc) | [35] | |
| 42 | Junceelloside C (R1 = OAc, R2 = R3 = OH) | [35] | |
| 43 | Junceelloside D (R1 = R2 = R3 = OH) | [35] | |
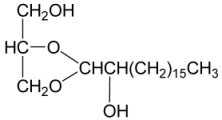 | 44 | 1,2-O-[2′-Hydroxyoctadecyl]-glycerol | [34] |
In addition, 14 new briarane derivatives, juncins O–Q (45–47) [36], R–ZI (48–57) [37], and ZII (58) [38] (Table 5), along with eight known briaranes, praelolide, junceellin, gemmacolides A–C and F, junceellolide D [34,38], and (+)-11β,20β-epoxyjunceellolide D [30,38], were also isolated from J. juncea.

Table 5.
The new natural products from Junceella juncea-V.
| Structure | No. | Name | Biological Activity | Ref. |
|---|---|---|---|---|
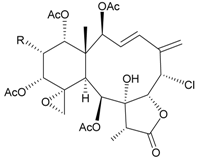 | 45 | Juncin O (R = OC(O)CH2CH(CH3)2) | juncins O–Q showed medium antifeedant activity (90.7, 69.0, 46.5%) toward the second-instar larvae of Spodoptera litura at a concentration of 500 µg/mL | [36,38] |
| juncins O–Q and ZII were not active in cytotoxicity testing with K562, A549, Hela and Hep2 cells a | ||||
| medium cytotoxicity (cell mortality: 8.7% in 24 h and 11.9% in 48 h) toward the second-instar larvae of S. litura at a concentration of 100 µg/mL | ||||
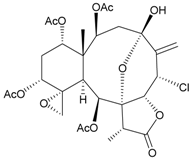 | 46 | Juncin P | medium cytotoxicity (cell mortality: 25.3% in 24 h and 29.7% in 48 h) toward the second-instar larvae of S. litura at a concentration of 100 µg/mL | [36,38] |
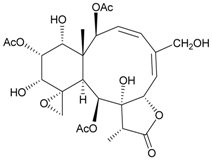 | 47 | Juncin Q | medium cytotoxicity (cell mortality: 31.3% in 24 h and 44.0% in 48 h) toward the second-instar larvae of S. litura at a concentration of 100 µg/mL | [36,38] |
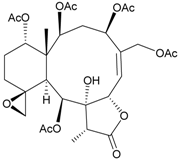 | 54 | Juncin X | [37] | |
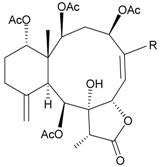 | 55 | Juncin Y (R = CH2OAc) | [37] | |
| 56 | Juncin Z (R = CO(O)CH3) | [37] | ||
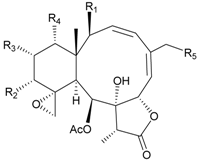 | 48 | Juncin R (R1 = R2 = R3 = OAc, R4 = OC(O)CH2CH(CH3)2, R5 = Cl) | juncins R–ZII (48–58) exhibited antifouling activity toward the barnacle Balanus amphitrite larvae (EC50 = 0.004, 0.3, 2.7, 1.6, 3.8, 21.1, 0.004, 0.1, 1.5, 0.5 and 0.004 µg/mL) | [37] |
| 49 | Juncin S (R1 = R3 = R4 = OAc, R2 = OC(O)CH2CH(CH3)2, R5 = Cl) | [37] | ||
| 50 | Juncin T (R1 = OC(O)CH2OC(O)(CH2)2CH(CH3)2, R2 = R3 = R4 = OAc, R5 = OH) | [37] | ||
| 51 | Juncin U (R1 = R2 = R4 = OAc, R3 = OC(O)CH2CH(CH3)2, R5 = OCH3) | [37] | ||
| 52 | Juncin V (R1 = R3 = OAc, R2 = R4 = OH, R5 = OCH3) | [37] | ||
| 53 | Juncin W (R1 = R3 = R5 = OAc, R2 = R4 = OH) | [37] | ||
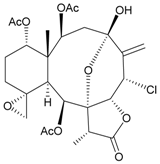 | 57 | Juncin ZI | [37] | |
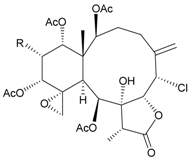 | 58 | Juncin ZII (R = OC(O)(CH2)2CH(CH3)2) | medium antifeedant activity (84.5%) toward the second-instar larvae of Spodoptera litura at a concentration of 500 µg/mL | [38] |
| medium cytotoxicity (cell mortality: 20.5% in 24 h and 43.2% in 48 h) toward the second-instar larvae of S. litura at a concentration of 100 µg/mL |
a K562 (human erythromyeloblastoid leukemia), A549 (human lung adenocarcinoma), Hela (human cervical epitheloid carcinoma), Hep2 (human liver carcinoma).
In biological activity testing, juncins R–ZII (48–58) showed potent antifouling activities against the larval settlement of barnacle Balanus amphitrite at a nontoxic concentration (Table 5), and the structure–activity relationships have been discussed [37,38]. The potency of these compounds to inhibit larval settlement was increased when the C-16 exocyclic oxymethylene was substituted by a methylene-bearing chlorine atom and decreased when the exocyclic oxymethylene C-16 was esterified or the acetoxymethylene C-16 was oxygenated to become an esterified group. The chain lengths of the ester moieties at C-1, C-12, C-13 and C-14 and the 11,20-epoxy group could also affect the antifouling activities [37,38].
The known briaranes, gemmacolides A, B, and junceellolide D, were also found to exhibit an antifouling activity as potent as that of juncins R–ZII [38], and these three compounds were not cytotoxic towards the K562, A549, Hela and Hep2 cells. In addition, all the known briaranes showed medium antifeedant activity toward the second-instar larvae of Spodoptera litura at a concentration of 500 µg/mL [38].
The gorgonian J. juncea collected off the Indian Ocean was proven to be a rich source of interesting natural products. The ethyl acetate extract of J. juncea exhibited anti-inflammatory activity at concentrations of 30–100 mg/kg body weight, while the oral median lethal dose (LD50) for the extract in albino mice was above 1000 mg/kg. The ethyl acetate extract of J. juncea also showed antibacterial activities toward Bacillus subtilis, B. pumilis and Escherichia coli [39]. Six new briaranes, juncins I–M (59–63) [40] and juncenolide B (64) [41], a new sphingolipid, (2R,3R,4E)-1,3-dihydroxy-2-[(nonadecanoyl) amino]-octadec-4-ene (65) [42] (Table 6), along with four known briaranes, gemmacolides A–C and juncin H [40], were obtained from the gorgonian coral J. juncea, collected from Tuticorin Coast of the Indian Ocean.

Table 6.
The new natural products from Junceella juncea-VI.
| Structure | No. | Name | Biological Activity | Ref. |
|---|---|---|---|---|
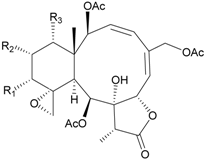 | 59 | Juncin I (R1 = R3 = OAc, R2 = OCOCH2CH(CH3)2) | n.r. a | [40] |
| 60 | Juncin J (R1 = R2 = OCOCH2CH(CH3)2, R3 = OAc) | n.r. | [40] | |
| 61 | Juncin K (R1 = R3 = OCOCH2CH(CH3)2, R2 = H) | n.r. | [40] | |
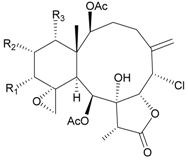 | 62 | Juncin L (R1 = R2 = OCOCH2CH(CH3)2, R3 = OAc) | n.r. | [40] |
| 63 | Juncin M (R1 = R3 = OCOCH2CH(CH3)2, R2 = H) | n.r. | [40] | |
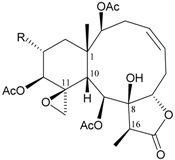 | 64 | Juncenolide B (R = OCOCH2CH(CH3)2) | n.r. | [41] |
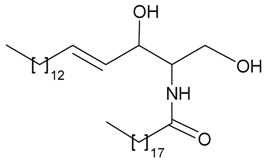 | 65 | (2R,3R,4E)-1,3-dihydroxy-2-[(nonadecanoyl) amino]-octadec-4-ene | n.r. | [42] |
a n.r. = not reported.
The molecular formula of juncenolide B was reported as C30H42O11 (M.W. = 578), but the structure presented in the article was found to possess the molecular formula C30H42O12 (M.W. = 594). The spectral data (such as from NOESY experiments) was not sufficient to support the structure presented in the article. We therefore suggested that the structure of this compound (juncenolide B) should be reexamined [41].
Sixteen new briaranes, juncenolides E–K (66–72) [43,44,45], juncin N (73) [46], and junceols A–H (74–81) [20,47] (Table 7), and two known briaranes, junceellolides B and C, were isolated from the gorgonian J. juncea, collected off the waters of Taiwan. Juncenolide G (68) is the first naturally-occurring briarane found to have an ether linkage between C-5/C-8 [44], and juncin N (73) is the first briarane derivative found to contain a carboxylic group [46].

Table 7.
The new natural products from Junceella juncea-VII.
| Structure | No. | Name | Biological Activity | Ref. |
|---|---|---|---|---|
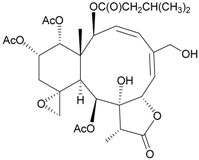 | 66 | Juncenolide E | n.r. a | [43] |
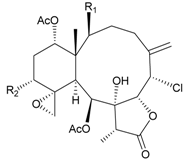 | 67 | Juncenolide F (R1 = OC(O)CH(CH3)2, R2 = OC(O)CH2CH(CH3)2) | n.r. | [44] |
| 69 | Juncenolide H (R1 = R2 = OAc) | modestly anti-inflammatory | [45] | |
| 70 | Juncenolide I (R1 = OC(O)CH(CH3)2, R2 = OAc) | weakly anti-inflammatory | [45] | |
| 71 | Juncenolide J (R1 = OAc, R2 = OC(O)CH2CH(CH3)2) | not active in anti-inflammatory bioassay | [45] | |
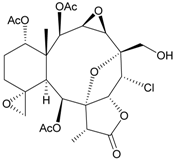 | 68 | Juncenolide G | n.r. | [44] |
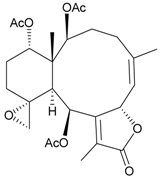 | 72 | Juncenolide K | weakly anti-inflammatory | [45] |
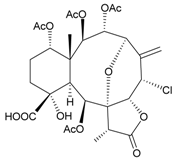 | 73 | Juncin N | not active in cytotoxicity testing with P-388D1, DLD-1, IMR-32, RPMI 7951 and CCRF-CEM cells b | [46] |
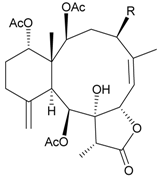 | 74 | Junceol A (R = OC(O)CH2CH(CH3)2) | significantly | [20] |
| anti-inflammatory | ||||
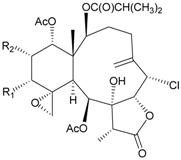 | 75 | Junceol B (R1 = OAc, R2 = OC(O)CH2CH(CH3)2) | significantly | [20] |
| anti-inflammatory | ||||
| 76 | Junceol C (R1 = R2 = OC(O)CH2CH(CH3)2) | significantly | [20] | |
| anti-inflammatory | ||||
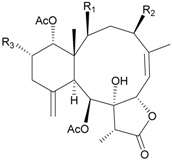 | 77 | Junceol D (R1 = OC(O)CH(CH3)2, R2 = OC(O)CH2CH(CH3)2, R3 = OAc) | not active in anti-inflammaory bioassay | [47] |
| exhibited cytotoxicity toward CCRF-CEM and DLD-1 (IC50 = 1.3, 10.0 µg/mL) cells | ||||
| 78 | Junceol E (R1 = OC(O)CH(CH3)2, R2 = OAc, R3 = H) | weakly anti-inflammatory | [47] | |
| not active in cytotoxicity testing with CCRF-CEM and DLD-1 (IC50 > 40 µg/mL) cells | ||||
| 79 | Junceol F (R1 = OC(O)CH(CH3)CH2CH3, R2 = OAc, R3 = H) | moderately anti-inflammatory | [47] | |
| exhibited cytotoxicity toward CCRF-CEM (IC50 = 4.9 µg/mL) cells | ||||
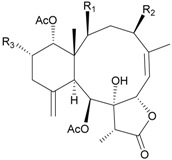 | 80 | Junceol G (R1 = OC(O)CH(CH3)CH2CH3, R2 = H, R3 = OAc) | weakly anti-inflammatory | [47] |
| exhibited cytotoxicity toward CCRF-CEM (IC50 = 4.4 µg/mL) | ||||
| 81 | Junceol H (R1 = OAc, R2 = H, R3 =OC(O)CH(CH3)2) | weakly anti-inflammatory | [47] | |
| exhibited cytotoxicity toward CCRF-CEM and DLD-1 (IC50 = 7.2, 17.0 µg/mL) cells |
a n.r. = not reported. b P388D1 (mouse lymphoid neoplasm), DLD-1 (human colon adenocarcinoma), IMR-32 (human neuroblastoma), RPMI 7951 (human malignant melanoma), CCRF-CEM (human T-cell acute lymphoblastic leukemia).
3. Conclusions
The chemical class distribution of the natural products obtained from the organisms Junceella fragilis and Junceella juncea compiled in this review indicates that terpenoid derivatives, particularly briarane-type diterpenoids, are the major components of the natural products isolated. Of the 81 new metabolites, 74 compounds are briarane-type diterpenoids (91.4%). Of these briaranes, over 50% are chlorinated briaranes (38/74 = 51.4%), which are rarely found. Briarane-type compounds continue to attract attention owing to their structural novelty, complexity and interesting bioactivities, such as anti-inflammatory activity [48,49,50,51]. Terpenoid compounds are often present in large amounts in marine invertebrates, and as a major class represent the largest percentage of natural products isolated from marine organisms [52]. Over 500 naturally-occurring briarane derivatives have been isolated from various marine organisms [48,49,50,51]. However, owing to their structural complexity, it is difficult to obtain sufficient amounts of the bioactive metabolites, such as junceols B (75) and C (76), for further study of their potential medicinal usage. We have therefore begun to culture the potential useful gorgonian corals J. fragilis and J. juncea (Figure 1) in tanks using our highly developed aquaculture technology for extraction of natural products to establish a stable supply of bioactive materials, which also protects the natural population and habitats from over-exploitation.

Figure 1.
The cultured-type gorgonian corals Junceella fragilis (white) and Junceella juncea (red).
Acknowledgments
This work was supported by grants from the National Dong Hwa University; the National Museum of Marine Biology and Aquarium (Grant No. 100100101 and 100200311); the Division of Marine Biotechnology, Asia-Pacific Ocean Research Center, National Sun Yat-sen University (Grant No. 00C-0302-05); and the National Science Council, Taiwan, awarded to P.-J.S. (Grant No. NSC 100-2325-B-291-001, 99-2323-B-291-001, and 98-2320-B-291-001-MY3) and J.-H.S. (Grant No. NSC 98-2113-M-110-002-MY3).
References and Notes
- Bayer, F.M. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa) with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Bayer, F.M.; Grasshoff, M. The genus group taxa of the family Ellisellidae, with clarification of the genera established by J.E. Gray (Cnidaria: Octocorallia). Senckenberg. Biol. 1994, 74, 21–45. [Google Scholar]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans–A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed; Australian Institute of Marine Science: Queensland, Australia, 2001; pp. 62, 230-231. [Google Scholar]
- Chen, C.-C.; Chang, K.-H. Gorgonacea (Coelenterata: Anthozoa: Octocorallia) of Southern Taiwan. Bull. Inst. Zool. Acad. Sin. 1991, 30, 149–182. [Google Scholar]
- Sung, P.-J.; Gwo, H.-H.; Fan, T.-Y.; Li, J.-J.; Dong, J.; Han, C.-C.; Wu, S.-L.; Fang, L.-S. Natural product chemistry of gorgonian corals of the genus Junceella. Biochem. Syst. Ecol. 2004, 32, 185–196. [Google Scholar]
- Kubota, N.K.; Kobayashi, Y.; Iwamoto, H.; Fukazawa, Y.; Uchio, Y. Two new halogenated briarane diterpenes from the Papuan gorgonian coral Junceella fragilis. Bull. Chem. Soc. Jpn. 2006, 79, 634–636. [Google Scholar]
- Zhang, W.; Guo, Y.-W.; Gu, Y. Secondary metabolites from the South China Sea invertebrates: Chemistry and biological activity. Curr. Med. Chem. 2006, 13, 2041–2090. [Google Scholar]
- Shao, C.-L.; Fu, X.-M.; Wang, C.-Y.; Han, L.; Liu, X.; Fang, Y.-C.; Li, G.-Q.; Zeng, X.-Q.; Liu, G.-X.; Guan, H.-S. Investigation on the status of coral reef resources and medicinal research in China III. Status of folk medicinal usage and medicinal research. Zhongguo Haiyang Daxue Xuebao (Ziran Kexueban) 2009, 39, 691–698. [Google Scholar]
- Zhang, W.; Guo, Y.-W.; Mollo, E.; Cimino, G. Junceellonoids A and B, two new briarane diterpenoids from the Chinese gorgonian Junceella fragilis Ridley. Helv. Chim. Acta 2004, 87, 2341–2345. [Google Scholar]
- Qi, S.-H.; Zhang, S.; Wen, Y.-M.; Xiao, Z.-H.; Li, Q.-X. New briaranes from the South China Sea gorgonian Junceella fragilis. Helv. Chim. Acta 2005, 88, 2349–2354. [Google Scholar]
- Wen, Y.-M.; Qi, S.-H.; Zhang, S. Briarane diterpenes and steroids from the South China Sea gorgonian Junceella fragilis. Nat. Prod. Res. Dev. 2006, 18, 234–237. [Google Scholar]
- Lin, Y.; Long, K. Studies of the chemical constituents of the Chinese gorgonia (IV)-Junceellin, a new chlorine-containing diterpenoid from Junceella squamata. Zhongshan Daxue Xuebao (Ziran Kexueban) 1983, 22, 46–51. [Google Scholar]
- Sung, P.-J.; Fan, T.-Y. 9-O-Deacetylumbraculolide A, a new diterpenoid from the gorgonian Junceella fragilis. Heterocycles 2003, 60, 1199–1202. [Google Scholar]
- Sung, P.-J.; Fan, T.-Y.; Fang, L.-S.; Wu, S.-L.; Li, J.-J.; Chen, M.-C.; Cheng, Y.-M.; Wang, G.-H. Briarane derivatives from the gorgonian coral Junceella fragilis. Chem. Pharm. Bull. 2003, 51, 1429–1431. [Google Scholar]
- Sung, P.-J.; Lin, M.-R.; Fang, L.-S. Briarane diterpenoids from the Formosan gorgonian coral Junceella fragilis. Chem. Pharm. Bull. 2004, 52, 1504–1506. [Google Scholar]
- Sheu, J.-H.; Chen, Y.-P.; Hwang, T.-L.; Chiang, M.Y.; Fang, L.-S.; Sung, P.-J. Junceellolides J–L, 11,20-epoxybriaranes from the gorgonian coral Junceella fragilis. J. Nat. Prod. 2006, 69, 269–273. [Google Scholar]
- Sung, P.-J.; Lin, M.-R.; Chen, W.-C.; Fang, L.-S.; Lu, C.-K.; Sheu, J.-H. Fragilide A, a novel diterpenoid from Junceella fragilis. Bull. Chem. Soc. Jpn. 2004, 77, 1229–1230. [Google Scholar]
- Sung, P.-J.; Chen, Y.-P.; Su, Y.-M.; Hwang, T.-L.; Hu, W.-P.; Fan, T.-Y.; Wang, W.-H. Fragilide B: A novel briarane-type diterpenoid with a s-cis diene moiety. Bull. Chem. Soc. Jpn. 2007, 80, 1205–1207. [Google Scholar]
- Sung, P.-J.; Lin, M.-R.; Su, Y.-D.; Chiang, M.Y.; Hu, W.-P.; Su, J.-H.; Cheng, M.-C.; Hwang, T.-L.; Sheu, J.-H. New briaranes from the octocorals Briareum excavatum (Briareidae) and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 2596–2604. [Google Scholar]
- Sung, P.-J.; Pai, C.-H.; Su, Y.-D.; Hwang, T.-L.; Kuo, F.-W.; Fan, T.-Y.; Li, J.-J. New 8-hydroxybriarane diterpenoids from the gorgonians Junceella juncea and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 4224–4232. [Google Scholar]
- Sung, P.-J.; Li, G.-Y.; Chen, Y.-P.; Huang, I.-C.; Chen, B.-Y.; Wang, S.-H.; Huang, S.-K. Fragilide E, a novel chlorinated 20-acetoxybriarane from the gorgonian coral Junceella fragilis. Chem. Lett. 2009, 38, 454–455. [Google Scholar]
- Sung, P.-J.; Wang, S.-H.; Chiang, M.Y.; Su, Y.-D.; Chang, Y.-C.; Hu, W.-P.; Tai, C.-Y.; Liu, C.-Y. Discovery of new chlorinated briaranes from Junceella fragilis. Bull. Chem. Soc. Jpn. 2009, 82, 1426–1432. [Google Scholar]
- Sung, P.-J.; Wang, S.-H.; Chang, Y.-C.; Chen, Y.-H.; Lin, M.-R.; Huang, I.-C.; Chen, J.-J.; Li, J.-J.; Kung, T.-H.; Fang, L.-S.; Wang, W.-H.; Weng, C.-F. New briarane-related diterpenoids from the sea whip gorgonian coral Junceella fragilis (Ellisellidae). Bull. Chem. Soc. Jpn. 2010, 83, 1074–1078. [Google Scholar]
- Wang, S.-H.; Chang, Y.-C.; Chiang, M.Y.; Chen, Y.-H.; Hwang, T.-L.; Weng, C.-F.; Sung, P.-J. Chlorinated briarane diterpenoids from the sea whip gorgonian corals Junceella fragilis and Ellisella robusta (Ellisellidae). Chem. Pharm. Bull. 2010, 58, 928–933. [Google Scholar]
- Shen, Y.-C.; Chen, Y.-H.; Hwang, T.-L.; Guh, J.-H.; Khalil, A.T. Four new briarane diterpenoids from the gorgonian coral Junceella fragilis. Helv. Chim. Acta 2007, 90, 1391–1398. [Google Scholar]
- Liaw, C.-C.; Shen, Y.-C.; Lin, Y.-S.; Hwang, T.-L.; Kuo, Y.-H.; Khalil, A.T. Frajunolides E–K, briarane diterpenes from Junceella fragilis. J. Nat. Prod. 2008, 71, 1551–1556. [Google Scholar]
- Liaw, C.-C.; Kuo, Y.-H.; Lin, Y.-S.; Hwang, T.-L.; Shen, Y.-C. Frajunolides L–O, four new 8-hydroxybriarane diterpenoids from the gorgonian Junceella fragilis. Mar. Drugs 2011, 9, 1477–1486. [Google Scholar]
- Sung, P.-J.; Fan, T.-Y.; Chen, M.-C.; Fang, L.-S.; Lin, M.-R.; Chang, P.-C. Junceellin and praelolide, two briaranes from the gorgonian corals Junceella fragilis and Junceella juncea (Ellisellidae). Biochem. Syst. Ecol. 2004, 32, 111–113. [Google Scholar]
- The briaranes (−)-11β,20β-epoxy-4-deacetoxyjunceellolide D and (+)-11β,20β-epoxyjunceellolide D were originally named (−)-11α,20α-epoxy-4-deacetoxyjunceellolide D and (+)-11α,20α-epoxyjunceellolide D. The stereochemistry of the 11,20-epoxy groups in these two compounds was revised to a β-orientation, and these briaranes should be renamed (−)-11β,20β-epoxy-4-deacetoxyjunceellolide D and (+)-11β,20β-epoxyjunceellolide D, respectively.
- Sung, P.-J.; Fang, L.-S.; Chen, Y.-P.; Chen, W.-C.; Hu, W.-P.; Ho, C.-L.; Yu, S.-C. 5β-Steroids from the gorgonian coral Junceella fragilis (Ellisellidae). Biochem. Syst. Ecol. 2006, 34, 64–70. [Google Scholar]
- Su, Y.-M.; Fang, T.-Y.; Sung, P.-J. 11,20-Epoxybriaranes from the gorgonian coral Ellisella robusta (Ellisellidae). Nat. Prod. Res. 2007, 21, 1085–1090. [Google Scholar]
- Walker, T.A.; Bull, G.D. A newly discovered method of reproduction in gorgonian coral. Mar. Ecol. Prog. Ser. 1983, 12, 137–143. [Google Scholar]
- In the in vitro anti-inflammatory bioassay, the inhibitory effects on the generation of superoxide anions and the release of elastase by activated neutrophils were used as indicators. For significant activity of pure compounds, an inhibition rate ≥ 40% is required (inhibition rate ≤ 10%, not active, 20% ≥ inhibition rate ≥ 10%, weakly anti-inflammatory; 40% ≥ inhibition rate ≥ 20%, modestly anti-inflammatory).
- Qi, S.-H.; Zhang, S.; Xiao, Z.-H.; Huang, J.-S.; Wu, J.; Li, Q.-X. Study on the chemical constituents of the South China Sea gorgonian Junceella juncea. Chem. Pharm. Bull. 2004, 52, 1476–1478. [Google Scholar]
- Qi, S.; Zhang, S.; Huang, J.; Xiao, Z.; Wu, J.; Li, Q. Complete 1H and 13C NMR assignments of four new steroidal glycosides from a gorgonian coral Junceella juncea. Magn. Reson. Chem. 2005, 43, 266–268. [Google Scholar]
- Qi, S.-H.; Zhang, S.; Huang, H.; Xiao, Z.-H.; Huang, J.-S.; Li, Q.-X. New briaranes from the South China Sea gorgonian Junceella juncea. J. Nat. Prod. 2004, 67, 1907–1910. [Google Scholar]
- Qi, S.-H.; Zhang, S.; Qian, P.-Y.; Xiao, Z.-H.; Li, M.-Y. Ten new antifouling briarane diterpenoids from the South China Sea gorgonian Junceella juncea. Tetrahedron 2006, 62, 9123–9130. [Google Scholar]
- Qi, S.-H.; Zhang, S.; Qian, P.-Y.; Xu, H.-H. Antifeedant and antifouling briaranes from the South China Sea gorgonian Junceella juncea. Chem. Nat. Comp. 2009, 45, 49–54. [Google Scholar]
- Sastry, V.G.; Sastry, A.V.S.; Kumar, K.E.; Satyanarayana, S. Chemical examination and biological evaluation of some marine coelenterates of the Indian Ocean. Int. J. Chem. Sci. 2008, 6, 1291–1298. [Google Scholar]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G.; Venugopal, M.J.R.V.; Schmitz, F.J. Juncins I–M, five new briarane diterpenoids from the Indian Ocean gorgonian Junceella juncea Pallas. J. Nat. Prod. 2003, 66, 507–510. [Google Scholar]
- Krishna, N.; Muralidhar, P.; Kumar, M.M.K.; Rao, D.V.; Rao, C.H.B. Juncenolide B, a new briarane diterpene from the gorgonian Junceella juncea of the Indian Ocean. Asian J. Chem. 2003, 15, 344–348. [Google Scholar]
- Krishna, N.; Muralidhar, P.; Kumar, M.M.K.; Rao, D.V.; Rao, C.H.B. A new sphingolipid from the gorgonian Junceella juncea of the Indian Ocean. Nat. Prod. Res. 2004, 18, 551–555. [Google Scholar]
- Shen, Y.-C.; Lin, Y.-C.; Huang, Y.-L. Juncenolide E, a new briarane from Taiwanese gorgonian Junceella juncea. J. Chin. Chem. Soc. 2003, 50, 1267–1270. [Google Scholar]
- Lin, Y.-C.; Huang, Y.-L.; Khalil, A.T.; Chen, M.-H.; Shen, Y.-C. Juncenolides F and G, two new briarane diterpenoids from Taiwanese gorgonian Junceella juncea. Chem. Pharm. Bull. 2005, 53, 128–130. [Google Scholar]
- Wang, S.-S.; Chen, Y.-H.; Chang, J.-Y.; Hwang, T.-L.; Chen, C.-H.; Khalil, A.T.; Shen, Y.-C. Juncenolides H–K, new briarane diterpenoids from Junceella juncea. Helv. Chim. Acta 2009, 92, 2092–2100. [Google Scholar]
- Sung, P.-J.; Fan, T.-Y.; Fang, L.-S.; Sheu, J.-H.; Wu, S.-L.; Wang, G.-H.; Lin, M.-R. Juncin N, a new briarane-type diterpenoid from the gorgonian coral Junceella juncea. Heterocycles 2003, 61, 587–592. [Google Scholar]
- Sung, P.-J.; Pai, C.-H.; Hwang, T.-L.; Fan, T.-Y.; Su, J.-H.; Chen, J.-J.; Fang, L.-S.; Wang, W.-H.; Sheu, J.-H. Junceols D–H, new polyoxygenated briaranes from sea whip gorgonian coral Junceella juncea (Ellisellidae). Chem. Pharm. Bull. 2008, 56, 1276–1281. [Google Scholar]
- Sung, P.-J.; Sheu, J.-H.; Xu, J.-P. Survey of briarane-type diterpenoids of marine origin. Heterocycles 2002, 57, 535–579. [Google Scholar]
- Sung, P.-J.; Chang, P.-C.; Fang, L.-S.; Sheu, J.-H.; Chen, W.-C.; Chen, Y.-P.; Lin, M.-R. Survey of briarane-related diterpenoids–Part II. Heterocycles 2005, 65, 195–204. [Google Scholar]
- Sung, P.-J.; Sheu, J.-H.; Wang, W.-H.; Fang, L.-S.; Chung, H.-M.; Pai, C.-H.; Su, Y.-D.; Tsai, W.-T.; Chen, B.-Y.; Lin, M.-R.; Li, G.-Y. Survey of briarane-type diterpenoids—Part III. Heterocycles 2008, 75, 2627–2648. [Google Scholar]
- Sung, P.-J.; Su, J.-H.; Wang, W.-H.; Sheu, J.-H.; Fang, L.-S.; Wu, Y.-C.; Chen, Y.-H.; Chung, H.-M.; Su, Y.-D.; Chang, Y.-C. Survey of briarane-type diterpenoids—Part IV. Heterocycles 2011, 83, 1241–1258. [Google Scholar]
- Harper, M.K.; Bugni, T.S.; Copp, B.R.; James, R.D.; Lindsay, B.S.; Richardson, A.D.; Schnabel, P.C.; Tasdemir, D.; VanWagoner, R.M.; Verbitski, S.M.; Ireland, C.M. Introduction to the Chemical Ecology of Marine Natural Products. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Washington, DC, USA, 2001; pp. 3–69. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).