Inhibitory Effects of Ecklonia cava Extract on High Glucose-Induced Hepatic Stellate Cell Activation
Abstract
:1. Introduction
2. Results
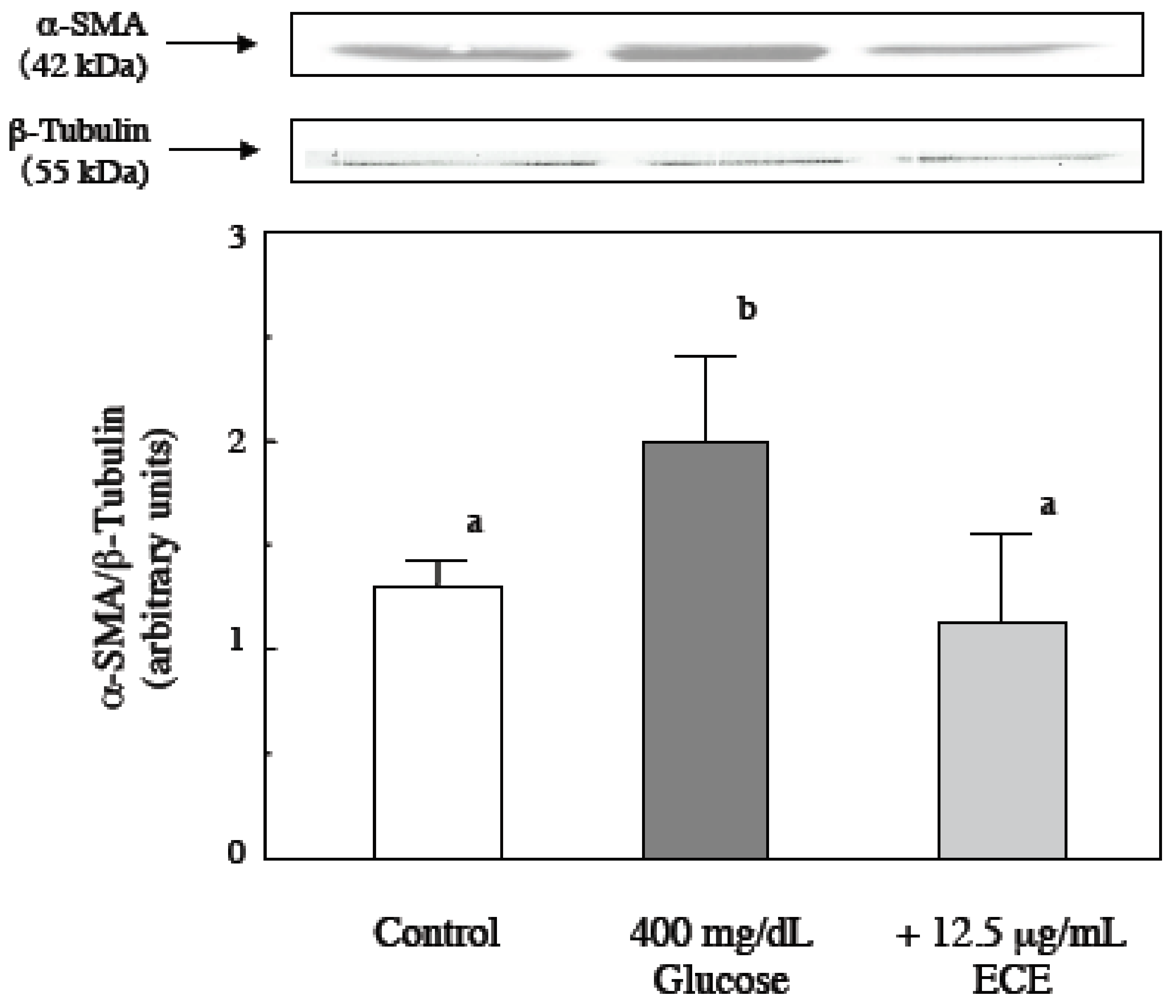
2.1. Changes in α-Smooth Muscle Actin Expression and Cell Proliferation after Hepatic Stellate Cell (HSC) Isolation
| Days after HSC isolation | Cell number (cells/field) |
|---|---|
| 3 | 40.2 ± 8.2 a |
| 5 | 56.3 ± 7.1 a |
| 7 | 77.5 ± 7.8 b |
| 9 | 119.9 ± 20.9 c |
| 11 | 151.8 ± 18.8 d |
| 13 | 166.5 ± 13.9 d |
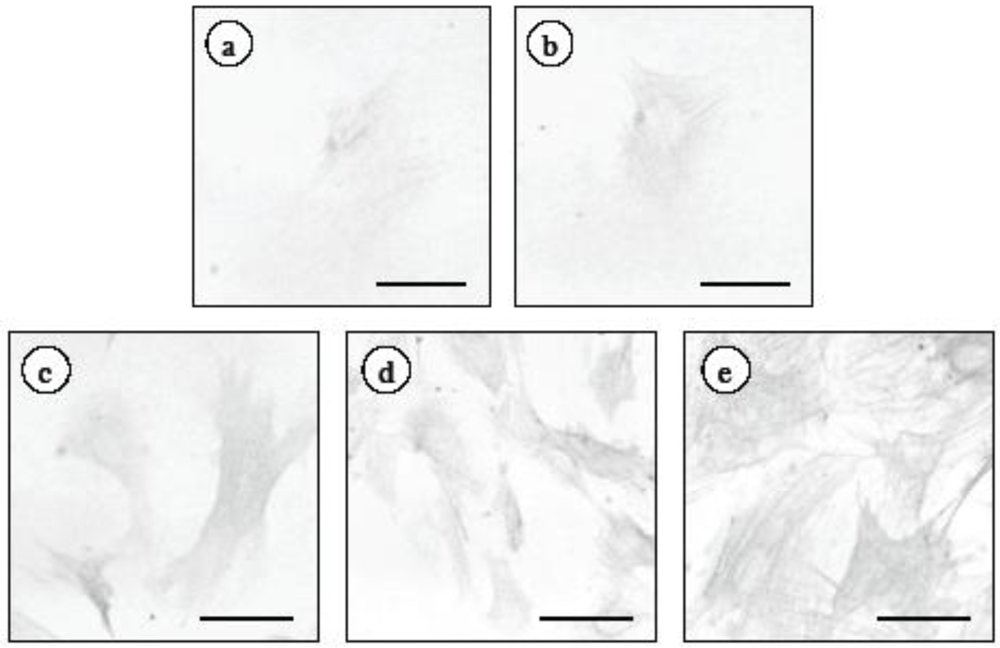
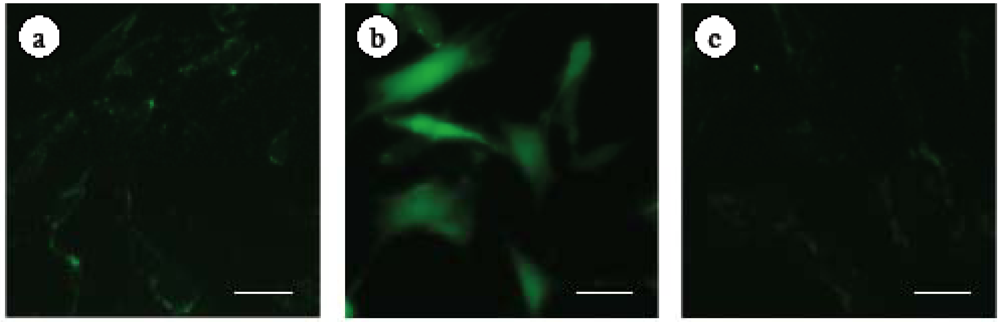
2.2. Effect of ECE on High Glucose-Induced HSC Activation
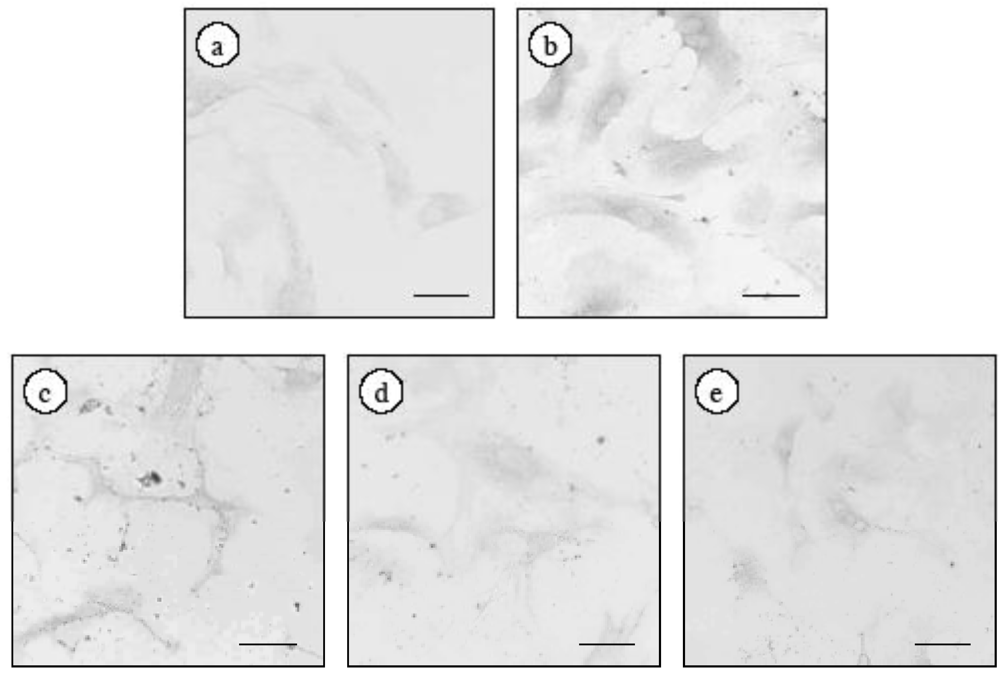
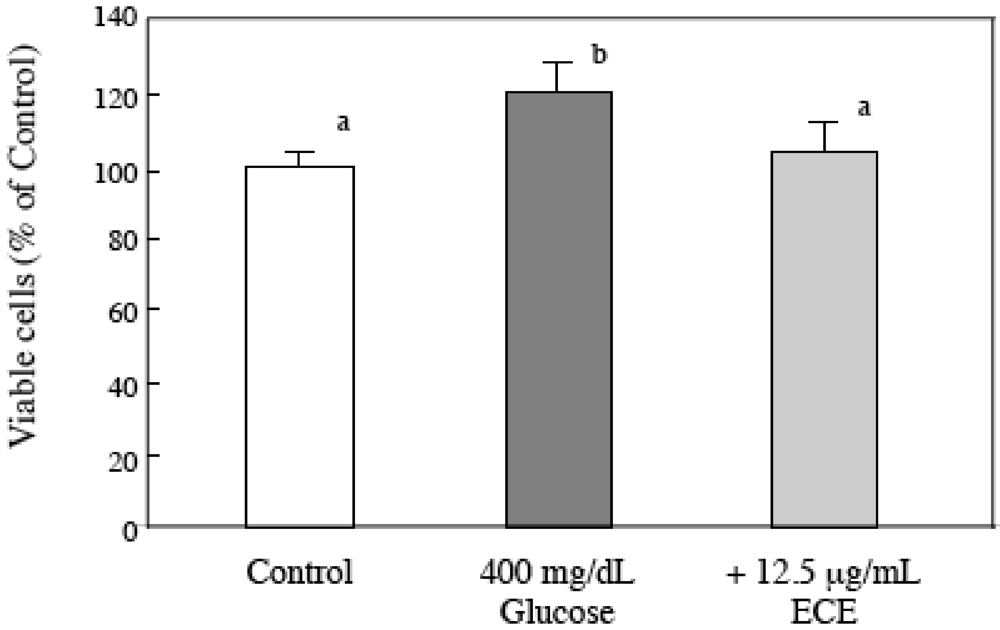
2.3. Effect of ECE on the Proliferation of High Glucose-Treated HSCs
2.4. Effect of ECE on Intracellular ROS Levels of High Glucose-Treated HSCs
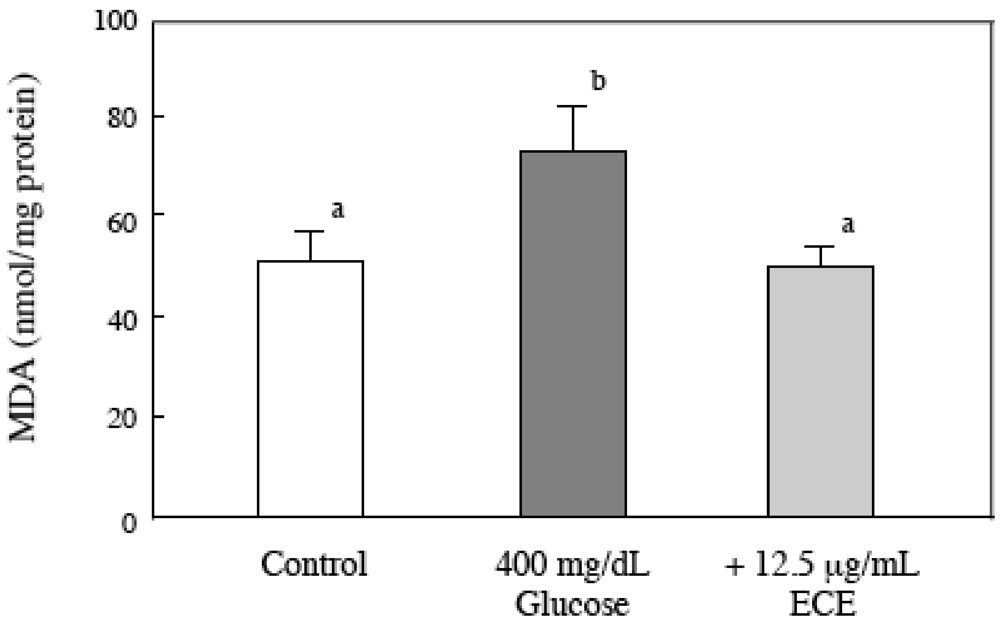
2.5. Effect of ECE on the Intracellular MDA Levels of Glucose-Treated HSCs
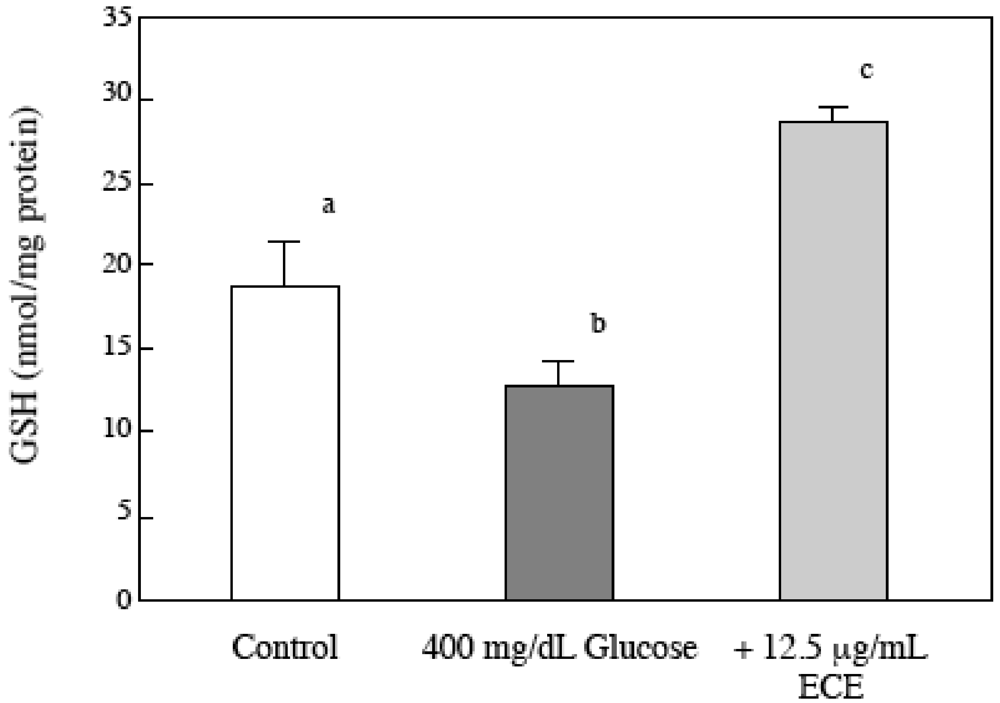
2.6. Effect of ECE on the Intracellular GSH Levels of High Glucose-Treated HSCs
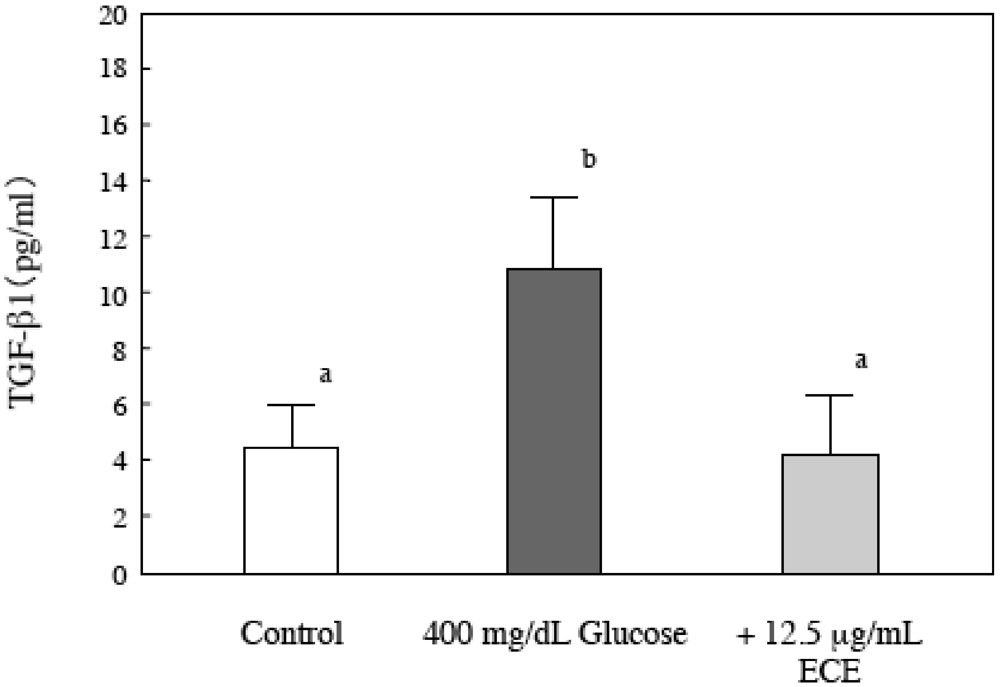
2.7. Effect of ECE on Bioactive TGF-β1 in High Glucose-Treated HSCs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ecklonia cava Extract
4.3. Animals
4.4. Isolation and Culturing of HSCs
4.5. Immunohistochemistry
4.6. Western Blot Analysis of α-SMA
4.7. Determination of Intracellular GSH Levels
4.8. MTT Assay
4.9. Intracellular ROS Formation
4.10. Evaluation of Intracellular Lipid Peroxidation
4.11. Measurement of Activated TGF-β1 Concentrations
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
References
- Poonawala, A.; Nair, S.P.; Thuluvath, P.J. Prevalence of obesity and diabetes inpatients with cryptogenic cirrhosis: A case-control study. Hepatology 2000, 32, 689–692. [Google Scholar]
- Tellez-Avila, F.I.; Sanchez-Avila, F.; Garcia-Saenz-de-Sicilia, M.; Chaves-Tapia, N.C.; Franco-Guzman, A.M.; Lopez-Arce, G.; Cerda-Contreras, E.; Uribe, M. Prevalence of metabolic syndrome, obesity and diabetes type 2 in cryptogenic cirrhosis. World J. Gastroenterol. 2008, 14, 4771–4775. [Google Scholar]
- Picardi, A.; D’Avola, D.; Gentolucci, U.V.; Galati, G.; Fiori, E.; Spataro, S.; Afeltra, A. Diabetes in chronic liver disease: Old concepts to new evidence. Diabetes Metab. Res. Rev. 2006, 22, 274–283. [Google Scholar]
- Iwamoto, K.; Kanno, K.; Hyogo, H.; Yamagishi, S.; Takeuchi, M.; Tazuma, S.; Chayama, K. Advanced glycation and products enhance the proliferation and activation of hepatic stellate cells. J. Gastroenterol. 2008, 43, 298–304. [Google Scholar]
- Ziyadeh, F.N.; Sharma, K.; Erickse, M.; Wolf, G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J. Clin. Invest. 1994, 93, 536–542. [Google Scholar]
- Sugimoto, R.; Enjoji, M.; Kohjima, M.; Turuta, S.; Fukushima, M.; Iwao, M.; Sonta, T.; Kotoh, K.; Inoguchi, T.; Nakamura, M. High glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinase. Liver Int. 2005, 25, 1018–1025. [Google Scholar]
- Svegliati-Baroni, G.; Saccomanno, S.; van Goor, H.; Jansen, P.; Benedetti, A.; Moshage, H. Involvement of reactive oxygen species and nitric oxide radicals in activation and proliferation of rat hepatic stellate cells. Liver 2001, 21, 1–12. [Google Scholar]
- Hui, A.Y.; Friedman, S.L. Molecular basis of hepatic fibrosis. Expert Rev. Mol. Med. 2003, 5, 1–23. [Google Scholar]
- Li, Y.; Qian, Z.-J.; Ryu, B.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Chemical constituents and its antioxidant properties in vitro: An edible marine alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar]
- Kang, H.S.; Chung, H.Y.; Kim, J.Y.; Son, B.W.; Jung, H.A.; Choi, J.S. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch. Pharm. Res. 2004, 27, 194–198. [Google Scholar]
- Kang, K.; Park, Y.; Hwang, H.J.; Kim, S.H.; Lee, J.G.; Shin, H.C. Antioxidative properties of brown algae polyphenolics and their perspectives as chemopreventive agents against vascular risk factors. Arch. Pharm. Res. 2003, 26, 286–293. [Google Scholar]
- Fukuyama, Y.; Kodama, M.; Miura, I.; Kinzyo, Z.; Mori, H.; Nakayama, Y.; Takahashi, H. Antiplasmin inhibitor. VI. Structure of Phlorofucofuroeckol-A, a novel phyrotannin with both Dibenzo-1,4-dioxin and Dibenzofuran elements, from Ecklonia-Kurome Okamura. Chem. Pharm. Bull. 1990, 38, 133–135. [Google Scholar]
- Fukuyama, Y.; Kodama, M.; Miura, I.; Kinzyo, Z.; Kido, M.; Mori, H.; Nakayama, Y.; Takahashi, H. Antiplasmin inhibitor. V. Structures of novel dimeric eckols isolated from the brown alga Ecklonia-Kurome Okamura. Chem. Pharm. Bull. 1989, 37, 349–353. [Google Scholar]
- Lee, J.H.; Kim, N.D.; Choi, J.S.; Kim, Y.J.; Moon, Y.H.; Lim, S.Y.; Park, S.Y. Inhibitory effects of the methanolic extract of anedible brown alga, Ecklonia stolonifera and its component, phlorglucinol on aflatoxin B1 mutagenicity in vitro (Ames test) and on benzo(a)pyrene or N-methyl N-nitrosourea clastogenicity in vivo (mouse micronucleus test). Nat. Prod. Sci. 1998, 4, 105–114. [Google Scholar]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactecidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar]
- Ahn, M.J.; Yoon, K.D.; Min, S.Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.G.; Huh, H.; Kim, J. Inhibition of HIV-1 reverse transcriptase and proteases by phlorotannins from brown alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from edible brown alga Ecklonia storonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Joen, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar]
- Garcia-Trevijano, E.R.; Iraburu, M.J.; Fontana, L.; Dominguez-Rosales, J.A.; Auster, A.; Covarrublas-Pinedo, A.; Rojkind, M. Transforming growth factor beta(1) induces the expression of alpha 1(I) procollagen mRNA by a hydrogen peroxide-C/EBP beta-dependent mechanism in rat hepatic stellate cells. Hepatology 1999, 29, 960–970. [Google Scholar]
- Gressner, A.M.; Weiskirchen, R.; Breitkopf, K.; Dooley, S. Roles of TGF-beta in hepatic fibrosis. Front. Biosci. 2002, 7, 793–807. [Google Scholar]
- Targher, G.; Marra, F.; Marchesini, G. Incresed risk of cardiovascular disease in non-alcoholic fatty liver disease: Causal effect or epiphenomenon? Diabetologia 2008, 51, 1947–1953. [Google Scholar]
- Nan, Y.-M.; Fu, N.; Wu, W.-J.; Liang, B.-L.; Wang, R.-Q.; Zhao, S.-X.; Zhao, J.-M.; Yu, J. Rosiglitazone prevents nutritional fibrosis and steatohepatitis in mice. Scand. J. Gastroenterol. 2009, 44, 358–365. [Google Scholar]
- Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar]
- Gressner, A.M. The cell biology of liver fibrogenesis—An imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998, 392, 447–452. [Google Scholar]
- Liu, X.; Hu, H.; Yin, J.Q. Therapeutic strategies against TGF-beta signaling pathway in hepatic fibrosis. Liver Int. 2006, 26, 8–22. [Google Scholar]
- Breitkopf, K.; Haas, S.; Wiercinska, E.; Singer, M.V.; Dooley, S. Anti-TGF-beta strategies for treatment of chronic liver disease, Alcohol. Clin. Exp. Res. 2005, 29, 121–131. [Google Scholar]
- Nakamura, T.; Ueno, T.; Sakamoto, M.; Sakata, R.; Torimura, T.; Hashimoto, O.; Ueno, H.; Sata, M. Suppression of transforming growth factor-beta results in upregulation of transcription of regeneration factors after chronic liver injury. J. Hepatol. 2004, 41, 974–982. [Google Scholar]
- Nakamura, T.; Sakata, R.; Ueno, T.; Sata, M.; Ueno, H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology 2000, 32, 247–255. [Google Scholar]
- Inagaki, Y.; Truter, S.; Ramirez, F. Transforming growth factor-beta stimulates alpha 2(I) collagen gene expression through a cis-acting element that contains a Sp1-binding site. J. Biol. Chem. 1994, 269, 14828–14834. [Google Scholar]
- Rahman, I.; MacNee, W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000, 16, 534–554. [Google Scholar]
- Kang, K.A.; Zhang, R.; Chae, S.; Lee, S.J.; Kim, J.; Kim, J.; Jeong, J.; Lee, J.; Shin, T.; Lee, N.H.; Hyun, J.W. Phloroglusinol (1,3,5-trihydroxybenzene) protects against ionizing radiation-induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 215–226. [Google Scholar]
- Regan, M.A.; Glombitza, K.W. Handbook of Physiological Methods; Cambridge University Press: Cambridge, UK, 1986; pp. 129–241. [Google Scholar]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Hoe, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; Heu, M.S.; Jeon, Y.J. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–76. [Google Scholar]
- Kojima-Yuasa, A.; Ohkita, T.; Ichikawa, H.; Takami, N.; Nakatani, T.; Kennedy, D.O.; Nishiguchi, S.; Matsui-Yuasa, I. Involvement of intracellular glutathione in zinc deficiency-induced activation of hepatic stellate cells. Chem. Biol. Interact. 2003, 146, 89–99. [Google Scholar]
- Sack, R.; Willi, A.; Hunziker, P.E. Determination of total glutathione in cell lysates by high performance liquid chromatography with O-phthalaldehyde precolumn derivatization in the presence of tris(1-carboxyethyl)-phosphate. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 2947–2962. [Google Scholar]
- Royall, J.A.; Ischiropoulos, H. Evaluation of 2',7'-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 1993, 302, 348–355. [Google Scholar]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipidperoxide in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yokogawa, K.; Matsui-Yuasa, I.; Tamura, A.; Terada, M.; Kojima-Yuasa, A. Inhibitory Effects of Ecklonia cava Extract on High Glucose-Induced Hepatic Stellate Cell Activation. Mar. Drugs 2011, 9, 2793-2808. https://doi.org/10.3390/md9122793
Yokogawa K, Matsui-Yuasa I, Tamura A, Terada M, Kojima-Yuasa A. Inhibitory Effects of Ecklonia cava Extract on High Glucose-Induced Hepatic Stellate Cell Activation. Marine Drugs. 2011; 9(12):2793-2808. https://doi.org/10.3390/md9122793
Chicago/Turabian StyleYokogawa, Kumiko, Isao Matsui-Yuasa, Akiko Tamura, Masaki Terada, and Akiko Kojima-Yuasa. 2011. "Inhibitory Effects of Ecklonia cava Extract on High Glucose-Induced Hepatic Stellate Cell Activation" Marine Drugs 9, no. 12: 2793-2808. https://doi.org/10.3390/md9122793
APA StyleYokogawa, K., Matsui-Yuasa, I., Tamura, A., Terada, M., & Kojima-Yuasa, A. (2011). Inhibitory Effects of Ecklonia cava Extract on High Glucose-Induced Hepatic Stellate Cell Activation. Marine Drugs, 9(12), 2793-2808. https://doi.org/10.3390/md9122793





