Examination of Marine-Based Cultivation of Three Demosponges for Acquiring Bioactive Marine Natural Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Environmental Conditions
2.2. Growth and Survival
2.3. Chemical Analysis
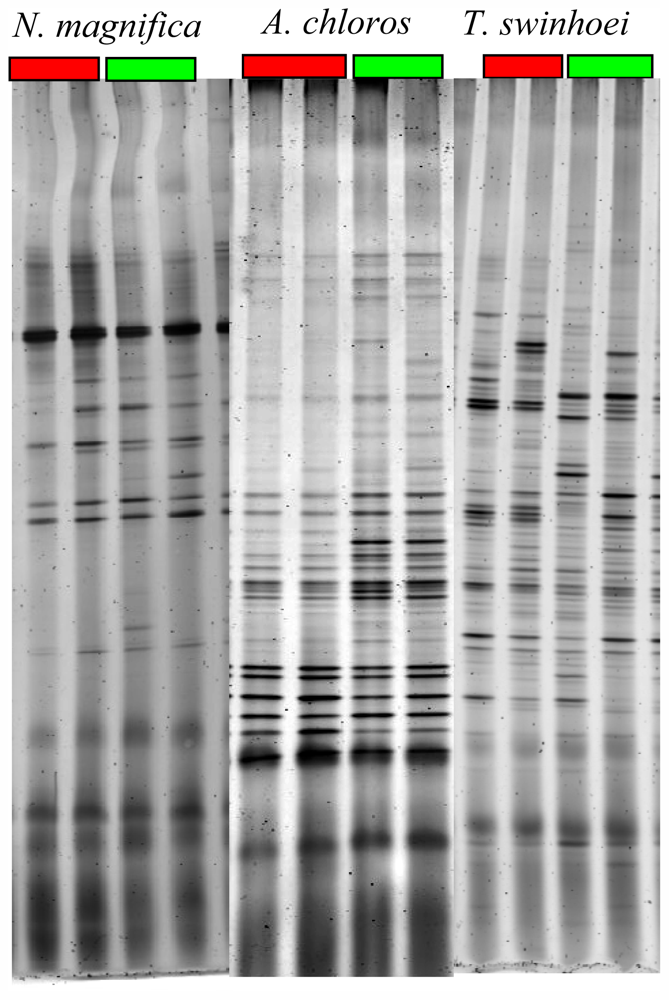
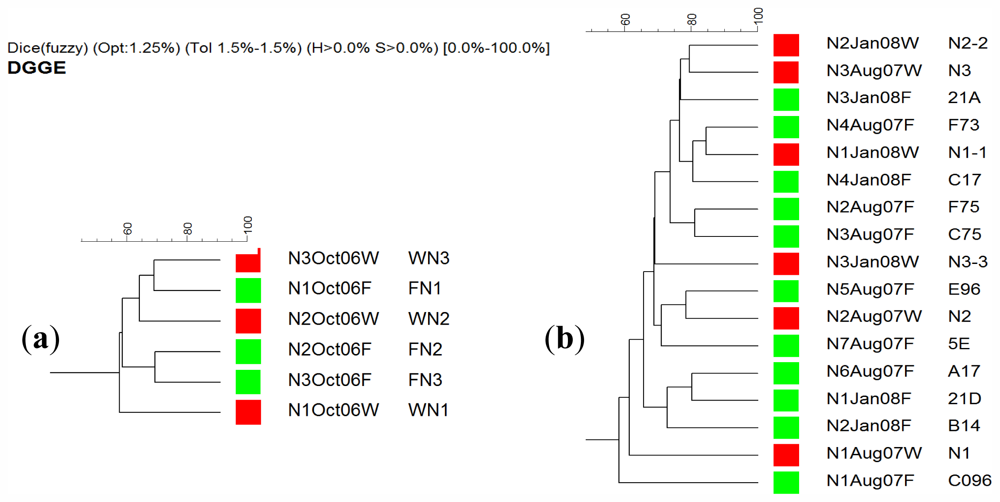
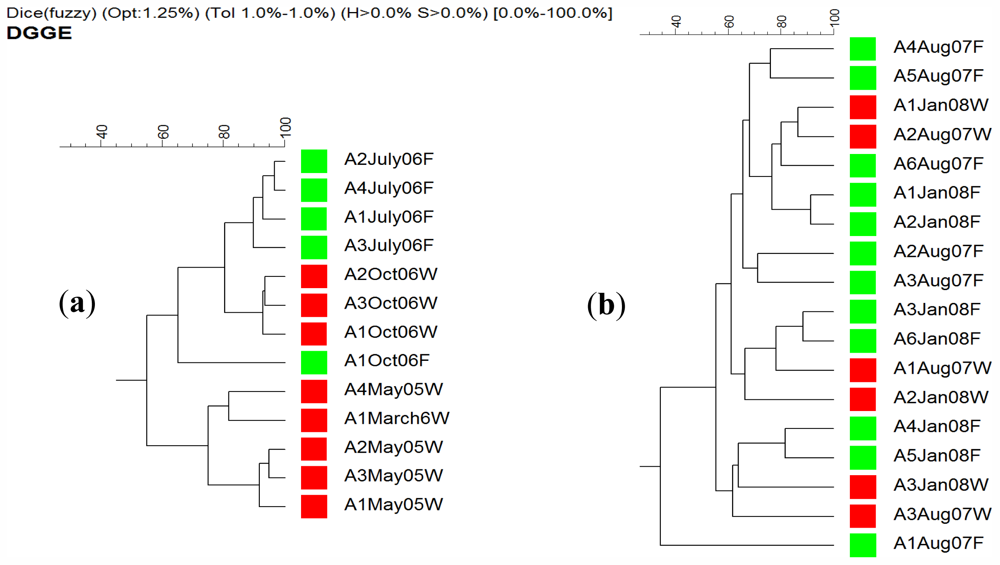
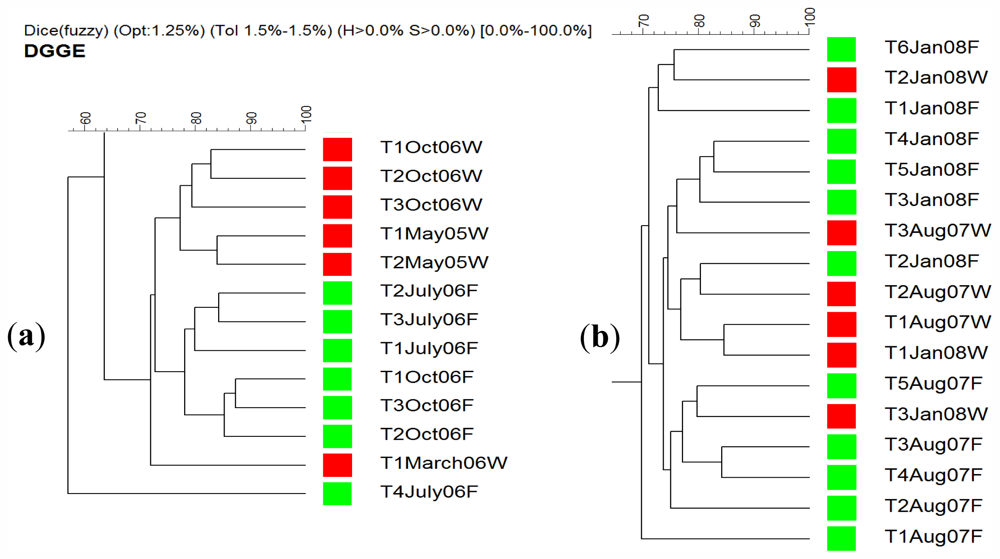
2.4. DGGE Analysis
3. Experimental Section
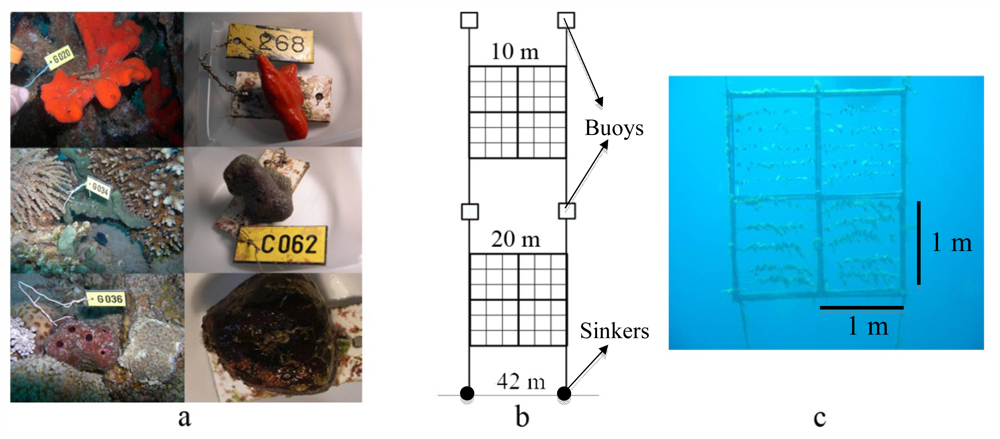
3.1. Study Area
3.2. Mariculture System Design
3.3. Analysis of Environmental Conditions
3.4. Sponge Collection and Preparation
3.5. Annual Specific Growth Rate (SGR) Measurements and Survival Monitoring
3.6. Chemical Analysis
3.7. Denaturing Gradient Gel Electrophoresis (DGGE)
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Hentschel, U.; Schmid, M.; Wagner, M.; Fieseler, L.; Gernert, C.; Hacker, J. Isolation and phylogenetic analysis of bacteria with antimicrobial activities from the Mediterranean sponges Aplysina aerophoba and Aplysina cavernicola. FEMS Microbiol. Ecol 2001, 35, 305–312. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.G.H.; Northcote, P.T.; Prinsep, M.P. Marine natural products. Nat. Prod. Rep 2009, 26, 170–244. [Google Scholar]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep 1998, 15, 113–158. [Google Scholar]
- Proksch, P.; Ebel, R.; Edrada, R.A.; Schupp, P.; Lin, W.H.; Sudarsono Wray, V.; Steube, K. Detection of pharmacologically active natural products using ecology. Selected examples from Indopacific marine invertebrates and sponge-derived fungi. Pure Appl. Chem 2003, 75, 343–352. [Google Scholar]
- Sipkema, D.; Franssen, M.C.; Osinga, R.; Tramper, J.; Wijffels, R.H. Marine sponges as pharmacy. Mar. Biotechnol 2005, 7, 142–162. [Google Scholar]
- Schmitz, F.J.; Bowden, B.F.; Toth, S.I. Antitumor and Cytotoxic Compounds from Marine Organisms. In Marine Biotechnology: Pharmaceutical and Bioactive Natural Products; Attaway, D.H., Zaborsky, O.R., Eds.; Plenum Press: New York, NY, USA, 1993; Volume 1, pp. 197–308. [Google Scholar]
- Carballo, J.; Yanez, B.; Zubia, E.; Ortega, M.; Vega, C. Culture of explants from the sponge Mycale cecilia to obtain bioactive mycalazal-type metabolites. Mar. Biotechnol 2010, 12, 516–525. [Google Scholar]
- Sipkema, D.; Osinga, R.; Schatton, W.; Mendola, D.; Tramper, J.; Wijffels, R.H. Large-scale production of pharmaceuticals by marine sponges: Sea, cell, or synthesis? Biotechnol. Bioeng 2005, 90, 201–222. [Google Scholar]
- Custodio, M.R.; Prokic, I.; Steffen, R.; Koziol, C.; Borojevic, R.; Brümmer, F.; Nickel, M.; Müller, W.E.G. Primmorphs generated from dissociated cells of the sponge Suberites domuncula: A model system for studies of cell proliferation and cell death. Mech. Ageing Dev 1998, 105, 45–59. [Google Scholar]
- Müller, W.E.G.; Wiens, M.; Batel, R.; Steffen, R.; Borojevic, R.; Custodio, M.R. Establishment of a primary cell culture from a sponge: primmorphs from Suberites domuncula. Mar. Ecol. Prog. Ser 1999, 178, 205–219. [Google Scholar]
- Zhang, X.; Cao, X.; Zhang, W.; Yu, X.; Jin, M. Primmorphs from archaeocytes-dominant cell population of the sponge Hymeniacidon perleve: Improved cell proliferation and spiculogenesis. Biotechnol. Bioeng 2003, 84, 583–590. [Google Scholar]
- Rinkevich, B.; Blisko, R.; Ilan, M. Further steps in the initiation of cell cultures from embryos and adult sponge colonies. Vitro Cell. Dev. Biol 1998, 34, 753–756. [Google Scholar]
- Sipkema, D.; Snijders, A.P.L.; Schroen, C.G.P.H.; Osinga, R.; Wijffels, R.H. The life and death of sponge cells. Biotechnol. Bioeng 2003, 85, 239–247. [Google Scholar]
- Ilan, M.; Contini, H.; Carmeli, S.; Rinkevich, B. Progress towards cell cultures from a marine sponge that produces bioactive compounds. J. Mar. Biotechnol 1996, 4, 145–149. [Google Scholar]
- Rinkevich, B. Cell cultures from marine invertebrates: Obstacles, new approaches and recent improvements. J. Biotechnol 1999, 70, 133–153. [Google Scholar]
- Osinga, R. Biotechnological aspects of marine sponges. J. Biotechnol 2003, 100, 91–92. [Google Scholar]
- Duckworth, A.R. Effect of wound size on the growth and regeneration of two temperate subtidal sponges. J. Exp. Mar. Biol. Ecol 2003, 287, 139–153. [Google Scholar]
- Belarbi, E.H.; Domínguez, M.R.; Cerón García, M.C.; Contreras Gómez, A.; García Camacho, F.; Molina Grima, E. Cultivation of explants of the marine sponge Crambe crambe in closed systems. Biomol. Eng 2003, 20, 333–337. [Google Scholar]
- Osinga, R.; de Beukelaer, P.B.; Meijer, E.M.; Tramper, J.; Wijffels, R.H. Growth of the sponge Pseudosuberites (aff.) andrewsi in a closed system. J. Biotechnol 1999, 70, 155–161. [Google Scholar]
- Belarbi, E.H.; Gomes, A.C.; Chisti, Y.; Garcia-Camacho, F.G.; Grima, E.M. Producing drugs from marine sponges. Biotechnol. Adv 2003, 21, 585–598. [Google Scholar]
- Duckworth, A.R.; Battershill, C.N. Developing farming structures for production of biologically active sponge metabolites. Aquaculture 2003, 217, 139–156. [Google Scholar]
- Page, M.J.; Northcote, P.T.; Webb, V.L.; Mackey, S.; Handley, S.J. Aquaculture trials for the production of biologically active metabolites in the New Zealand sponge Mycale hentscheli (Demospongiae: Poecilosclerida). Aquaculture 2005, 250, 256–269. [Google Scholar]
- Milanese, M.; Sarà, M.; Manconi, R.; Ben Abdalla, A.; Pronzato, R. Commercial sponge fishing in Libya: historical records, present status and perspectives. Fish. Res 2008, 89, 90–96. [Google Scholar]
- Duckworth, A.R.; Battershill, C.N.; Bergquist, P.R. Influence of explant procedures and environmental factors on culture success of three sponges. Aquaculture 1997, 156, 251–267. [Google Scholar]
- Corriero, G.; Longo, C.; Mercurio, M.; Nonnis Marzano, C.; Lembo, G.; Spedicato, M.T. Rearing performance of Spongia officinalis on suspended ropes off the Southern Italian Coast (Central Mediterranean Sea). Aquaculture 2004, 238, 195–205. [Google Scholar]
- Hadas, E.; Shpigel, M.; Ilan, M. Sea ranching of the marine sponge Negombata magnifica (Demospongiae, Latrunculiidae) as a first step for latrunculin B mass production. Aquaculture 2005, 244, 159–169. [Google Scholar]
- Koopmans, M.; Wijffels, R.H. Seasonal growth rate of the sponge Haliclona oculata (Demospongiae: Haplosclerida). Mar. Biotechnol 2008, 10, 502–510. [Google Scholar]
- Duckworth, A.R. Farming sponges to supply bioactive metabolites and bath sponges: A review. Mar. Biotechnol 2009, 11, 669–679. [Google Scholar]
- de Caralt, S.; Sanchez-Fontenla, J.; Uriz, M.J.; Wijffels, R.H. In situ aquaculture methods for Dysidea avara (Demospongiae, Porifera) in the Northwestern Mediterranean. Mar. Drugs 2010, 8, 1731–1742. [Google Scholar]
- Wang, G. Diversity and biotechnological potential of the sponge-associated microbial consortia. J. Ind. Microbiol. Biotechnol 2006, 33, 545–551. [Google Scholar]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev 2007, 71, 295–347. [Google Scholar]
- Hentschel, U.; Hopke, J.; Horn, M.; Friedrich, A.B.; Wagner, M.; Hacker, J.; Moore, B.S. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl. Environ. Microbiol 2002, 68, 4431–4440. [Google Scholar]
- Hill, R.T. Microbes from marine sponges: A treasure trove of biodiversity for natural products discovery. In Microbial Diversity and Bioprospecting; Bull, A.T., Ed.; ASM Press: Washington, DC, USA, 2004; pp. 177–190. [Google Scholar]
- Berthold, R.J.; Borowitzka, M.A.; Mackay, M.A. The ultrastructure of Oscillatoria spongeliae, the blue-green algal endosymbiont of the sponge Dysidea herbacea. Phycologia 1982, 21, 327–335. [Google Scholar]
- Hentschel, U.; Usher, K.M.; Taylor, M.W. Marine sponges as microbial fermenters. FEMS Microbiol. Ecol 2006, 55, 167–177. [Google Scholar]
- Weisz, J.B.; Lindquist, N.; Martens, C.S. Do associated microbial abundances impact marine demosponge pumping rates and tissue densities? Oecologia 2008, 155, 367–376. [Google Scholar]
- Mohamed, N.M.; Rao, V.; Hamann, M.T.; Kelly, M.; Hill, R.T. Monitoring bacterial diversity of the marine sponge Ircinia strobilina upon transfer into aquaculture. Appl. Environ. Microbiol 2008, 74, 4133–4143. [Google Scholar]
- Taylor, M.W.; Schupp, P.J.; de Nys, R.; Kjelleberg, S.; Steinberg, P.D. Biogeography of bacteria associated with the marine sponge Cymbastela concentrica. Environ. Microbiol 2005, 7, 419–433. [Google Scholar]
- Webster, N.S.; Taylor, M.W.; Behnam, F.; Lucker, S.; Rattei, T.; Whalan, S.; Horn, M.; Wagner, M. Deep sequencing reveals exceptional diversity and modes of transmission for bacterial sponge symbionts. Environ. Microbiol 2010, 12, 2070–2082. [Google Scholar]
- Lee, O.O.; Lau, S.C.K.; Qian, P.Y. Consistent bacterial community structure associated with the surface of the sponge Mycale adhaerens bowerbank. Microb. Ecol 2006, 52, 693–707. [Google Scholar]
- Webster, N.S.; Cobb, R.E.; Negri, A.P. Temperature thresholds for bacterial symbiosis with a sponge. ISME J 2008, 2, 830–842. [Google Scholar]
- Webster, N.S.; Webb, R.I.; Ridd, M.J.; Hill, R.T.; Negri, A.P. The effects of copper on the microbial community of a coral reef sponge. Mar. Biotechnol 2001, 3, 600–608. [Google Scholar]
- Webster, N.S.; Xavier, J.R.; Freckelton, M.; Motti, C.A.; Cobb, R. Shifts in microbial and chemical patterns within the marine sponge Aplysina aerophoba during a disease outbreak. Environ. Microbiol 2008, 10, 3366–3376. [Google Scholar]
- Webster, N.S.; Cobb, R.E.; Soo, R.; Anthony, S.L.; Battershill, C.N.; Whalan, S.; Evans-Illidge, E. Bacterial community dynamics in the marine sponge Rhopaloeides odorabile under in situ and ex situ cultivation. Mar. Biotechnol 2010, 13, 296–304. [Google Scholar]
- Mohamed, N.M.; Enticknap, J.J.; Lohr, J.E.; McIntosh, S.M.; Hill, R.T. Changes in bacterial communities of the marine sponge Mycale laxissima on transfer into aquaculture. Appl. Environ. Microbiol 2008, 74, 1209–1222. [Google Scholar]
- Isaacs, L.T.; Kan, J.; Nguyen, L.; Videau, P.; Anderson, M.A.; Wright, T.L.; Hill, R.T. Comparison of the bacterial communities of wild and captive sponge Clathria prolifera from the Chesapeake Bay. Mar. Biotechnol 2009, 11, 758–770. [Google Scholar]
- Friedrich, A.B.; Fischer, I.; Proksch, P.; Hacker, H.; Hentschel, U. Temporal variation of the microbial community associated with the Mediterranean sponge Aplysina aerophoba. FEMS Microbiol. Ecol 2001, 38, 105–113. [Google Scholar]
- Thoms, C.; Horn, M.; Wagner, M.; Hentschel, U.; Proksch, P. Monitoring microbial diversity and natural product profiles of the sponge Aplysina cavernicola following transplantation. Mar. Biol 2003, 142, 685–692. [Google Scholar]
- Hoffmann, F.; Rapp, H.T.; Reitner, J. Monitoring microbial community composition by fluorescence in situ hybridization during cultivation of the marine cold-water sponge Geodia barretti. Mar. Biotechnol 2006, 8, 373–379. [Google Scholar]
- Ilan, M. Reproductive biology, taxonomy, and aspects of chemical ecology of Latrunculiidae (Porifera). Biol. Bull 1995, 188, 306–312. [Google Scholar]
- Kelman, D.; Kashman, Y.; Rosenberg, E.; Ilan, M.; Ifrach, I.; Loya, Y. Antimicrobial activity of the reef sponge Amphimedon viridis from the Red Sea: evidence for selective toxicity. Aquatic Microb. Ecol 2001, 24, 9–16. [Google Scholar]
- Burns, E.; Ifrach, I.; Carmeli, S.; Pawlik, J.R.; Ilan, M. Comparison of anti-predatory defenses of Red Sea and Caribbean sponges. I. Chemical defense. Mar. Ecol. Prog. Ser 2003, 252, 105–114. [Google Scholar]
- Oren, M. Aspects of Sponge Culture for the Production of Bioactive Materials. M.Sc. Thesis, Department of Zoology, Tel Aviv University, Tel Aviv, Israel, 2004. [Google Scholar]
- Carmely, S.; Rotem, M.; Kashman, Y. Swinholide A, a new marine macrolide. Complete assignment of the 1H and 13C NMR spectra by 2D NMR techniques. Magn. Res. Chem 1986, 24, 343–349. [Google Scholar]
- Kashman, Y.; Groweiss, A.; Shmueli, U. Latrunculin, a new 2-thiazolidinone macrolide from the marine sponge Latrunculia magnifica. Tetrahedron Lett 1980, 21, 3629–3632. [Google Scholar]
- Schmitz, F.J.; Hollenbeak, K.H.; Campbell, D.C. Marine natural products: Halitoxin, toxic complex of several marine sponges of the genus Haliclona. J. Org. Chem 1978, 43, 3916–3922. [Google Scholar]
- Berlinck, R.G.S.; Ogawa, C.A.; Almeida, A.M.P.; Sanchez, M.A.A.; Malpezzi, E.L.A.; Costa, L.V.; Hajdu, E.; de Freitas, J.C. Chemical and pharmacological characterization of halitoxin from Amphimedon viridis (Porifera) from the southeastern Brazilian coast. Comp. Biochem. Physiol. C 1996, 115, 155–163. [Google Scholar]
- Spector, I.; Braet, F.; Shochet, N.R.; Bubb, M.R. New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microsc. Res. Tech 1999, 47, 18–37. [Google Scholar]
- Nicolaou, K.C.; Patron, A.P.; Ajito, K.; Richter, P.K.; Khatuya, H.; Bertinato, P.; Miller, R.A.; Tomaszewski, M.J. Total synthesis of Swinholide A, Preswinholide A, and Hemiswinholide A. Chem. A Eur. J 1996, 2, 847–868. [Google Scholar]
- Webster, N.S. Sponge disease: A global threat? Environ. Microbiol 2007, 9, 1363–1375. [Google Scholar]
- Wulff, J.L. Disease prevalence and population density over time in three common Caribbean coral reef sponge species. J. Mar. Biol. Assoc. UK 2007, 87, 1715–1720. [Google Scholar]
- Duckworth, A.R.; Battershill, C.N.; Schiel, D.R. Effects of depth and water flow on growth, survival and bioactivity of two temperate sponges cultured in different seasons. Aquaculture 2004, 242, 237–250. [Google Scholar]
- Müller, W.E.G.; Wimmer, W.; Schatton, W.; Böhm, M.; Batel, R.; Filic, Z. Initiation of an aquaculture of sponges for the sustainable production of bioactive metabolites in open systems: Example, Geodia cydonium. Mar. Biotechnol 1999, 1, 569–579. [Google Scholar]
- Ferretti, C.; Vacca, S.; Ciucis, C.D.; Marengo, B.; Duckworth, A.R.; Manconi, R.; Pronzato, R.; Domenicotti, C. Growth dynamics and bioactivity variation of the Mediterranean demosponges Agelas oroides (Agelasida, Agelasidae) and Petrosia ficiformis (Haplosclerida, Petrosiidae). Mar. Ecol 2009, 30, 327–336. [Google Scholar]
- Novak, L. Tel Aviv University: Tel Aviv, Israel; Unpublished work; 2011. [Google Scholar]
- Webster, N.S.; Negri, A.P.; Munro, M.; Battershill, C.N. Diverse microbial communities inhabit Antarctic sponges. Environ. Microbiol 2004, 6, 288–300. [Google Scholar]
- Thiel, V.; Leininger, S.; Schmaljohann, R.; Brümmer, F.; Imhoff, J.F. Sponge-specific bacterial associations of the Mediterranean sponge Chondrilla nucula (Demospongiae, Tetractinomorpha). Microb. Ecol 2007, 54, 101–111. [Google Scholar]
- Thoms, C.; Hentschel, U.; Schmitt, S.; Schupp, P.J. Rapid tissue reduction and recovery in the sponge Aplysinella sp. Mar. Biol 2008, 156, 141–153. [Google Scholar]
- Bergman, O.; Haber, M.; Mayzel, B.; Anderson, M.A.; Shpigel, M.; Hill, R.T.; Ilan, M. Marine based cultivation of Diacarnus sponges and the bacterial community composition of wild and maricultured sponges and their larvae. Mar. Biotechnol 2011. [Google Scholar] [CrossRef]
- Barthel, D.; Theede, H. A new method for the culture of marine sponges and its application for experimental studies. Ophelia 1986, 25, 75–82. [Google Scholar]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S rRNA. Appl. Environ. Microbiol 1993, 59, 695–700. [Google Scholar]


| Species | T. swinhoei | N. magnifica | A. chloros |
|---|---|---|---|
| Order and family | “Lithistida” Theonellidae | Poecilosclerida Podospongiidae | Haplosclerida Niphatidae |
| Main secondary metabolite | Swinholide A (Polyketides) [54] | Latrunculin B (Polyketide) [55] | Halitoxin (Pyridinium alkaloids) [56,57] |
| Bioactivity | Cytoskeleton affector: Antiactine Anticancer [59] | Anticancer [58] | Antimicrobial [57] |
| Sponge species | Variable measured | Maximal Growth rate * (%)(year−1) | Survival (%) | Duration of experiment | Reference |
|---|---|---|---|---|---|
| Mycale cecilia | Volume | 1260 | 95 | 60 days | [7] |
| Latrunculia wellingtonensis | Weight | 675 | 56 | 285 days | [21] |
| Geodia cydonium | Weight | 378 | - | 3–6 months | [63] |
| Agelas oroides | Volume | 336 | - | 15 months | [64] |
| Polymastia croceus | Weight | 260 | 59 | 285 days | [21] |
| Spongia officinalis | Wet weight | 120 | 75 | 3 years | [25] |
| Haliclona oculata | Volume | 4.4 | - | 1 year | [27] |
| Negombata magnifica | Wet weight | 324 | Between 40 and 75 | 177 days | [26] |
| Negombata magnifica | Wet weight | 308 | ** 54 | 592 days | Current study |
| Amphimedon chloros | Wet weight | 61 | 19 | 767 days | Current study |
| Theonella swinhoei | Wet weight | −19 | 11 | 767 days | Current study |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bergman, O.; Mayzel, B.; Anderson, M.A.; Shpigel, M.; Hill, R.T.; Ilan, M. Examination of Marine-Based Cultivation of Three Demosponges for Acquiring Bioactive Marine Natural Products. Mar. Drugs 2011, 9, 2201-2219. https://doi.org/10.3390/md9112201
Bergman O, Mayzel B, Anderson MA, Shpigel M, Hill RT, Ilan M. Examination of Marine-Based Cultivation of Three Demosponges for Acquiring Bioactive Marine Natural Products. Marine Drugs. 2011; 9(11):2201-2219. https://doi.org/10.3390/md9112201
Chicago/Turabian StyleBergman, Oded, Boaz Mayzel, Matthew A. Anderson, Muki Shpigel, Russell T. Hill, and Micha Ilan. 2011. "Examination of Marine-Based Cultivation of Three Demosponges for Acquiring Bioactive Marine Natural Products" Marine Drugs 9, no. 11: 2201-2219. https://doi.org/10.3390/md9112201
APA StyleBergman, O., Mayzel, B., Anderson, M. A., Shpigel, M., Hill, R. T., & Ilan, M. (2011). Examination of Marine-Based Cultivation of Three Demosponges for Acquiring Bioactive Marine Natural Products. Marine Drugs, 9(11), 2201-2219. https://doi.org/10.3390/md9112201




