Enhanced Productivity of a Lutein-Enriched Novel Acidophile Microalga Grown on Urea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Coccomyxa acidophila enhanced growth on urea
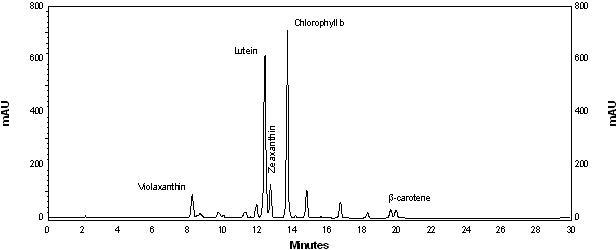
2.2. Carotenoid accumulation and xanthophylls cycle activity in urea grown Coccomyxa acidophila cells
3. Conclusions
4. Experimental Section
4.1. Microorganism and cultivation conditions
4.2. Dry weight measurements
4.3. Measurements of fluorescence
4.4. Oxygen evolution
4.5. Analytical determinations
4.6. Statistics
4.7. Cell counting
Acknowledgements
- Samples Availability: Available from the authors.
References
- Cuaresma, C; Garbayo, I; Vega, JM; Vílchez, C. Growth and photosynthetic utilization of inorganic carbon of the microalga Chlamydomonas acidophila isolated from Tinto river. Enzyme Microb Technol 2006, 40, 158–162. [Google Scholar]
- Bartlett, HE; Eperjesi, F. Effect of lutein and antioxidant dietary supplementation on contrast sensitivity in age-related macular disease: a randomized controlled trial. Eur J Clin Nutr 2007, 61, 1121–1127. [Google Scholar]
- Thurnham, DI. Macular zeaxanthins and lutein a review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nut Res Rev 2007, 20, 163–179. [Google Scholar]
- Schiraldi, C; De Rosa, M. The production of biocatalysts and biomolecules from extremophiles. Trends Biotechnol 2002, 20, 515–521. [Google Scholar]
- Pulz, O; Gross, W. Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 2004, 65, 635–648. [Google Scholar]
- Spolaore, P; Joannis-Cassan, C; Duran, E; Isambert, A. Commercial application of microalgae. J Biosci Bioeng 2006, 101, 87–96. [Google Scholar]
- Li, Y; Horsman, M; Wang, B; Wu, N; Lan, CQ. Effect of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 2008, 81, 629–636. [Google Scholar]
- Packer, M. Algal capture of carbon dioxide; biomass generation as a tool for greenhouse mitigation with reference to New Zealand energy strategy and policy. Energ Policy 2009, 37, 3428–3437. [Google Scholar]
- Becker, EW. Microalgae: Biotechnology and Microbiology; Cambridge University Press: New York, NY, USA, 2004; pp. 18–24. [Google Scholar]
- Mobley, HLT; Hausinger, RP. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev 1989, 53, 85–108. [Google Scholar]
- Sachs, G; Kraut, JA; Wen, Y; Feng, J; Scott, DR. Urea Transport in Bacteria: Acid Acclimation by Gastric Helicobacter spp. J Membr Biol 2006, 212, 71–82. [Google Scholar]
- Zawada, RJX; Kwan, P; Olszewski, KL; Llinas, M; Huang, S-G. Quantitative determination of urea concentrations in cell culture medium. Biochem Cell Biol 2009, 87, 541–544. [Google Scholar]
- Rangel-Yagui, CdO; Danesi, EDG; Carvalho, JCM; Sato, S. Chlorophyll production from Spirulina platensis: cultivation with urea addition by fed-batch process. Bioresour Technol 2004, 92, 133–141. [Google Scholar]
- Sánchez-Luna, LD; Converti, A; Tonini, GC; Sato, S; Carvalho, JCM. Continuous and pulse feedings of urea as a nitrogen source in fed-batch cultivation of Spirulina platensis. Aquacult Eng 2004, 31, 237–245. [Google Scholar]
- Soletto, D; Binaghi, L; Lodi, A; Carvalho, JCM; Converti, A. Batch and fed-batch cultivations of Spirulina platensis using ammonium sulphate and urea as nitrogen sources. Aquaculture 2005, 243, 217–224. [Google Scholar]
- Hsieh, CH; Wu, WT. Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 2009, 100, 3921–3026. [Google Scholar]
- Tittel, J; Bissinger, V; Gaedke, U; Kamjunke, N. Inorganic carbon limitation and mixotrophic growth in Chlamydomonas from an acidic mining lake. Protist 2005, 156, 63–75. [Google Scholar]
- Rocha, JMS; Garcia, JEC; Henriques, MHF. Growth aspects of the marine microalga Nannochloropsis gaditana. Biomol Eng 2003, 20, 237–242. [Google Scholar]
- Ellner, PD; Steers, E. Urea as a carbon source for Chlorella and Scenedesmus. Arch Biochem Biophys 1955, 59, 534–535. [Google Scholar]
- Cochlan, WP; Harrison, PJ. Inhibition of nitrate uptake by ammonium and urea in the eucaryotic picoflagellate Micromonas pusilla (Butcher) Manton et Parke. J Exp Mar Biol Ecol 1991, 153, 143–152. [Google Scholar]
- Smith, FW; Thompson, JF. Regulation of Nitrate Reductase in Chlorella vulgaris. Plant Physiol 1971, 48, 224–227. [Google Scholar]
- Mérigout, P; Lelandais, M; Bitton, F; Renou, J-P; Briand, X; Meyer, C; Daniel-Vedele, F. Physiological and Transcriptomic Aspects of Urea Uptake and Assimilation in Arabidopsis Plants. Plant Physiol 2008, 147, 1225–1238. [Google Scholar]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol Plant 2000, 108, 111–117. [Google Scholar]
- Richmond, A. Biological Principles of Mass Cultivation. In Handbook of Microalgal Culture; Blackwell Press: Boca Raton, FL, USA, 2004; pp. 125–177. [Google Scholar]
- Fernández-Sevilla, JM; Acién-Fernández, F; Molina-Grima, E. Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol 2010, 86, 27–40. [Google Scholar]
- Sánchez, JF; Fernández-Sevilla, JM; Acién, FG; Cerón, MC; Pérez-Parra, J; Molina-Grima, E. Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Appl Microbiol Biotechnol 2008, 79, 719–729. [Google Scholar]
- Del Campo, JA; Rodríguez, H; Moreno, J; Vargas, MA; Rivas, J; Guerrero, MG. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J Biotechnol 2001, 85, 289–295. [Google Scholar]
- Wei, D; Chen, F; Chen, G; Zhang, XW; Liu, LJ; Zhang, H. Enhanced production of lutein in heterotrophic Chlorella protothecoides by oxidative stress. Sci China Ser C Life Sci 2008, 51, 1088–1093. [Google Scholar]
- Silverman, MP; Lundgren, DG. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. J Bacteriol 1959, 77, 642–647. [Google Scholar]
- Schreiber, U; Schliwa, U; Bilger, W. Continuous recording of photochemical and non photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 1986, 10, 51–62. [Google Scholar]
- Harmut, A; Lichtenthaler, K. Chlorophylls and carotenoids: pigments of photosynthetic membranes. Method Enzymol 1987. [Google Scholar]
- Cawse, PA. The determination of nitrate in soil solution by ultraviolet spectrophotometry. Analyst 1967, 92, 311–315. [Google Scholar]
- Wilcox, AA; Carroll, WE; Sterling, RE; Davis, HA; Ware, AG. Use of Berthelot reaction in automatic analysis of serum urea nitrogen. Clin Chem 1966, 12, 151–154. [Google Scholar]
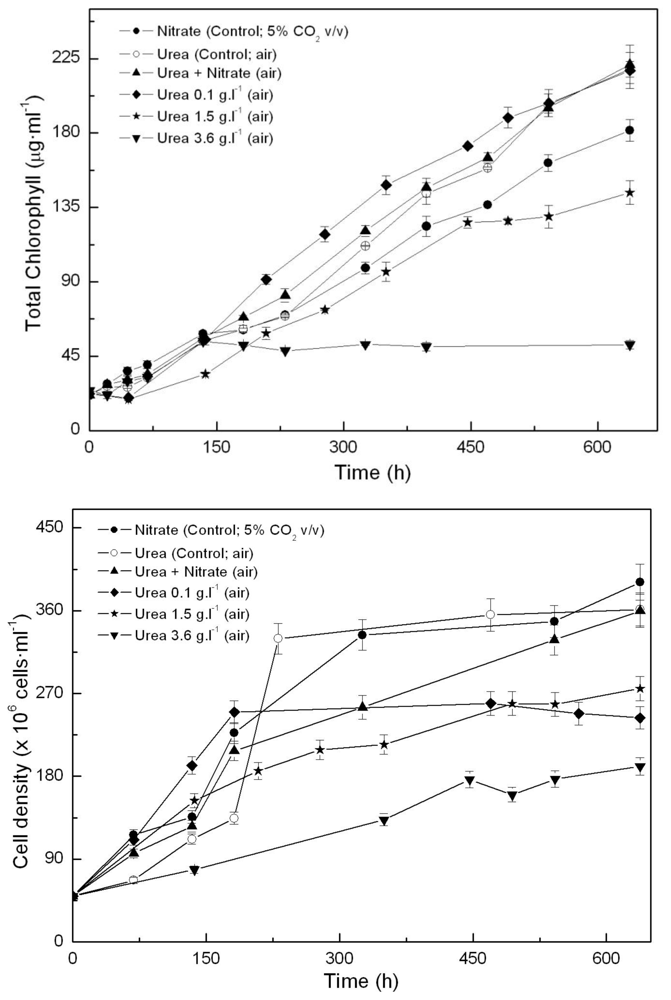
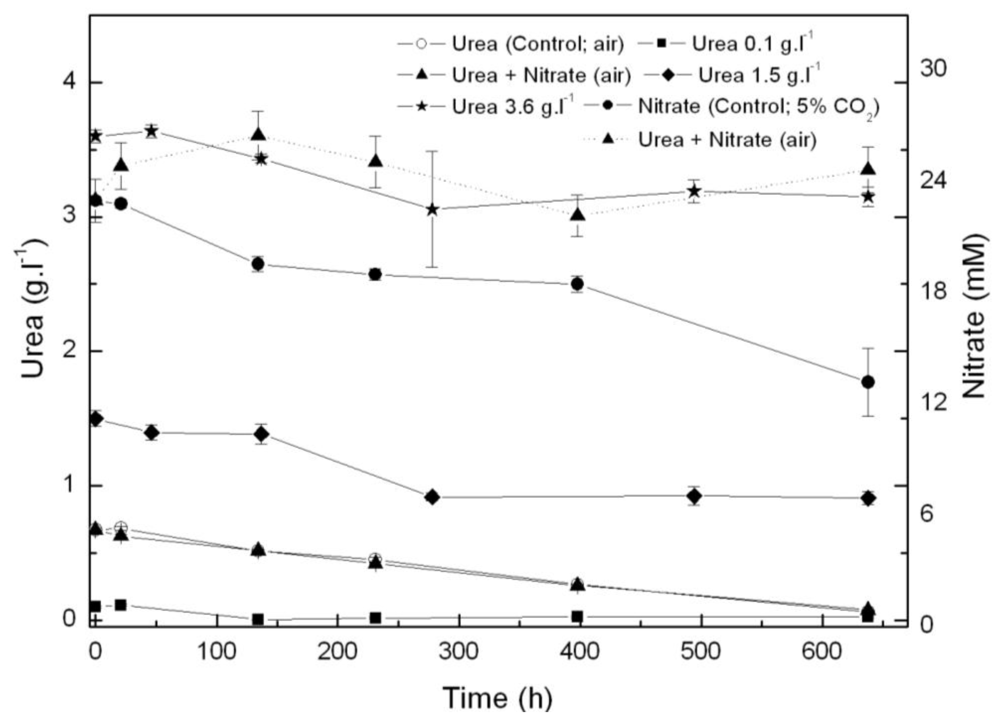
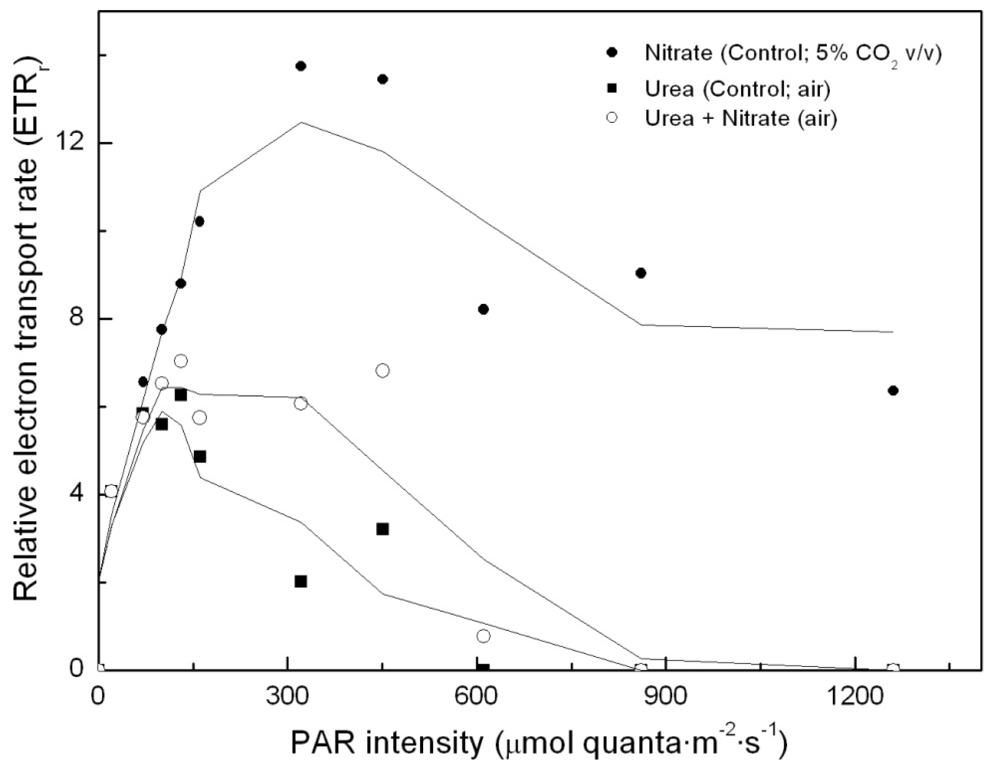
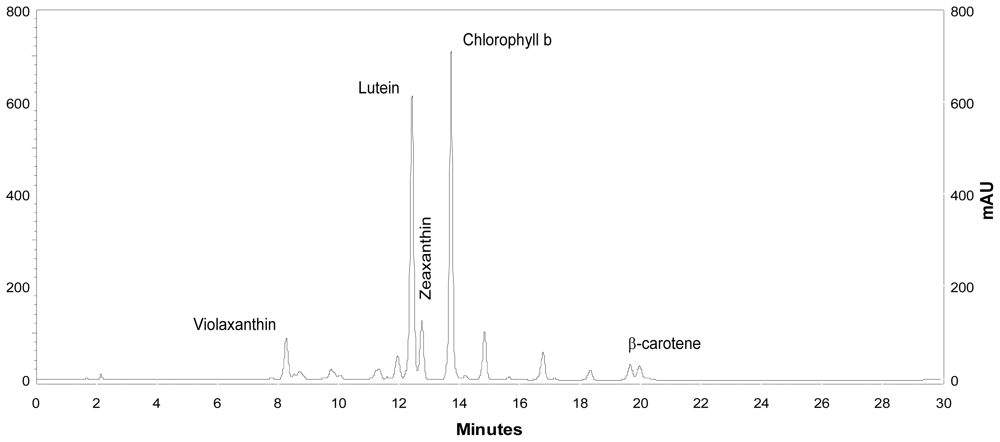
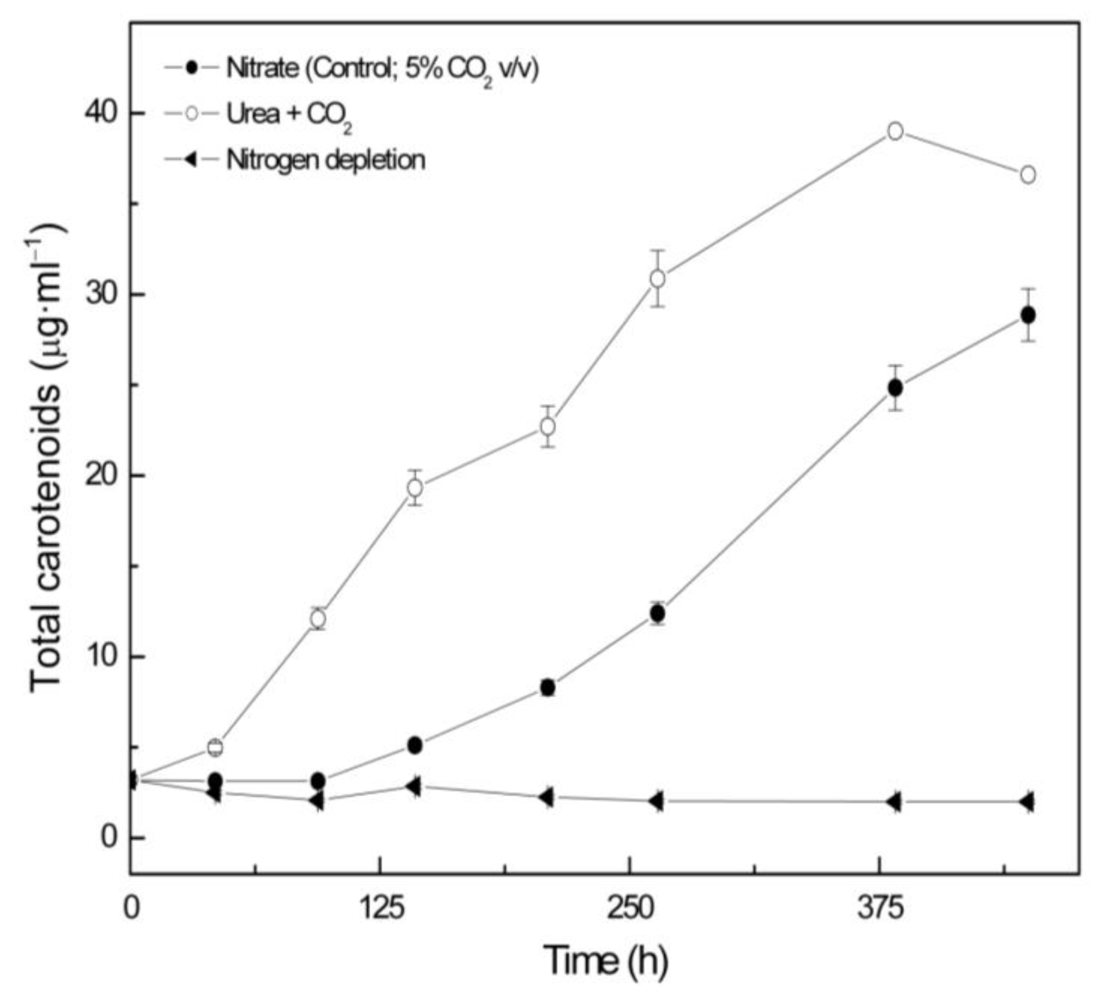
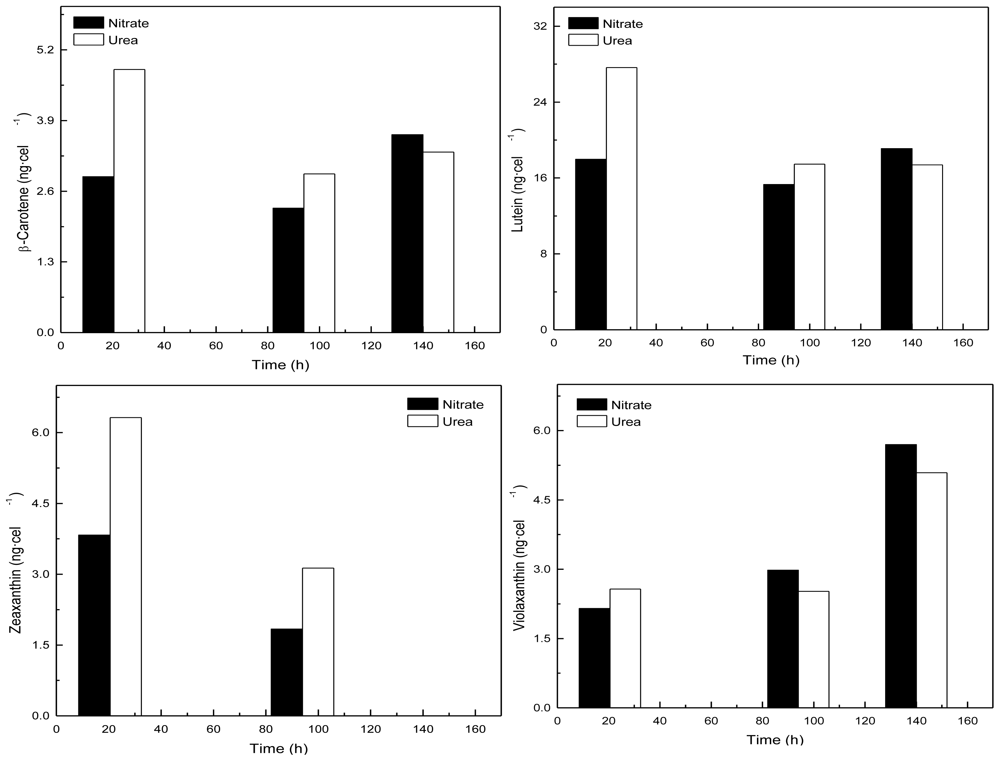
| Nitrogen source | Growth rate (d·1) | Maximum productivity (g·L·1·d·1) | Maximum cellular carotenoids content (pg·cell·1) |
|---|---|---|---|
| Nitrate | 0.27 | 0.23 | 0.084 |
| Nitrite | 0.02 | 0.13 | Non detectable |
| Ammonium | 0.31 | 0.24 | 0.055 |
| Urea | 0.34 | 0.25 | 0.104 |
| Microalga | Lutein (mg·g−1) | Lutein productivity (mg·L·1·d·1) | Cultivation system |
|---|---|---|---|
| Scenedesmus almeriensis | 5.5 4.5 | 4.9 mg·L·2·d·1 290 mg·m·2·d·1 | Laboratory, continuous culture, 2 L |
| Muriellopsis sp | 5.5 4.3 | 1.4 mg·L·2·d·1 7.2 mg·L·2·d·1 | Laboratory, batch, 0.2 L Outdoor, tubular systems, 55 L |
| Chlorella protothecoides | 4.6 | 10 mg·L·2·d·1 | Laboratory, batch, heterotrophic, 16 L |
| Coccomyxa acidophila | 6.1 | 2.0 mg·L·2·d·1 | Laboratory, batch, 2 L |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Casal, C.; Cuaresma, M.; Vega, J.M.; Vilchez, C. Enhanced Productivity of a Lutein-Enriched Novel Acidophile Microalga Grown on Urea. Mar. Drugs 2011, 9, 29-42. https://doi.org/10.3390/md9010029
Casal C, Cuaresma M, Vega JM, Vilchez C. Enhanced Productivity of a Lutein-Enriched Novel Acidophile Microalga Grown on Urea. Marine Drugs. 2011; 9(1):29-42. https://doi.org/10.3390/md9010029
Chicago/Turabian StyleCasal, Carlos, Maria Cuaresma, Jose Maria Vega, and Carlos Vilchez. 2011. "Enhanced Productivity of a Lutein-Enriched Novel Acidophile Microalga Grown on Urea" Marine Drugs 9, no. 1: 29-42. https://doi.org/10.3390/md9010029
APA StyleCasal, C., Cuaresma, M., Vega, J. M., & Vilchez, C. (2011). Enhanced Productivity of a Lutein-Enriched Novel Acidophile Microalga Grown on Urea. Marine Drugs, 9(1), 29-42. https://doi.org/10.3390/md9010029