Neurotoxic Alkaloids: Saxitoxin and Its Analogs
Abstract
:1. Introduction
2. Saxitoxin and Its Analogs, the Paralytic Shellfish Toxins
3. Biotransformation of the Paralytic Shellfish Toxins
Detoxification of the paralytic shellfish toxins within mammals
4. A Genetic Basis for the Paralytic Shellfish Toxins
4.1. The saxitoxin biosynthetic gene cluster
4.2. Pharmaceutical potential of the paralytic shellfish toxins
5. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- Schantz, EJ; Mold, J; Stanger, D; Shavel, J; Riel, F; Bowden, J; Lynch, J; Wyler, R; Riegel, B; Sommer, H. Paralytic shellfish poison VI. A procedure for the isolation and purification of the poison from toxic clams and mussel tissues. J. Am. Chem. Soc 1957, 79, 5230–5235. [Google Scholar]
- Anderson, DM; Cembella, AD; Hallegraeff, GM. Physiological Ecology of Harmful Algal Blooms, 1st ed; Springer: Berlin, Germany, 1998; p. 662. [Google Scholar]
- Anderson, DM; Glibert, PM; Burkholder, JM. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar]
- Sellner, KG; Doucette, GJ; Kirkpatrick, GJ. Harmful algal blooms: Causes, impacts and detection. J. Ind. Microbiol. Biotechnol 2003, 30, 383–406. [Google Scholar]
- Zingone, A; Enevoldsen, HO. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manage 2000, 43, 725–748. [Google Scholar]
- Van Dolah, FM. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Perspect 2000, 108, 133–141. [Google Scholar]
- Llewellyn, LE. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep 2006, 23, 200–222. [Google Scholar]
- Lefebvre, KA; Bill, BD; Erickson, A; Baugh, KA; O'Rourke, L; Costa, PR; Nance, S; Trainer, VL. Characterization of intracellular and extracellular saxitoxin levels in both field and cultured Alexandrium spp. samples from Sequim Bay, Washington. Mar. Drugs 2008, 6, 103–116. [Google Scholar]
- Oshima, Y; Blackburn, SI; Hallegraeff, GM. Comparative study on paralytic shellfish toxin profiles of the dinoflagellate Gymnodinium catenatum from three different countries. Mar. Biol 1993, 116, 471–476. [Google Scholar]
- Usup, G; Kulis, DM; Anderson, DM. Growth and toxin production of the toxic dinoflagellate Pyrodinium bahamense var. compressum in laboratory cultures. Nat. Toxins 1994, 2, 254–262. [Google Scholar]
- Gainey, L; Shumway, J; Shumway, S. A compendium of the responses of bivalve molluscs to toxic dinoflagellates. J. Shellfish Res 1988, 7, 623–628. [Google Scholar]
- Shumway, SE. Phycotoxin-related shellfish poisoning: Bivalve molluscs are not the only vectors. Rev. Fish. Sci 1995, 3, 1–31. [Google Scholar]
- Deeds, J; Landsberg, J; Etheridge, S; Pitcher, G; Longan, S. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar]
- Carmichael, WW; Evans, WR; Yin, QQ; Bell, P; Moczydlowski, E. Evidence for paralytic shellfish poisons in the freshwater cyanobacterium Lyngbya wollei (Farlow ex Gomont) comb. nov. Appl. Environ. Microbiol 1997, 63, 3104–3110. [Google Scholar]
- Hoeger, SJ; Shaw, G; Hitzfeld, BC; Dietrich, DR. Occurrence and elimination of cyanobacterial toxins in two Australian drinking water treatment plants. Toxicon 2004, 43, 639–649. [Google Scholar]
- Molica, RJR; Oliveira, EJA; Carvalho, PVVC; Costa, ANSF; Cunha, MCC; Melo, GL; Azevedo, SMFO. Occurrence of saxitoxins and an anatoxin-a(s)-like anticholinesterase in a Brazilian drinking water supply. Harmful Algae 2005, 4, 743–753. [Google Scholar]
- Clemente, Z; Busato, RH; Oliveira Ribeiro, CA; Cestari, MM; Ramsdorf, WA; Magalhães, VF; Wosiack, AC; Silva de Assis, HC. Analyses of paralytic shellfish toxins and biomarkers in a southern Brazilian reservoir. Toxicon 2010, 55, 396–406. [Google Scholar]
- Liu, Y; Chen, W; Li, D; Shen, Y; Li, G; Liu, Y. First report of aphantoxins in China—waterblooms of toxigenic Aphanizomenon flos-aquae in Lake Dianchi. Ecotoxicol. Environ. Saf 2006, 65, 84–92. [Google Scholar]
- Berry, JP; Lind, O. First evidence of "paralytic shellfish toxins" and cylindrospermopsin in a Mexican freshwater system, Lago Catemaco, and apparent bioaccumulation of the toxins in "tegogolo" snails (Pomacea patula catemacensis). Toxicon 2010, 55, 930–938. [Google Scholar]
- Ballot, A; Fastner, J; Wiedner, C. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in Northeast Germany. Appl. Environ. Microbiol 2010, 76, 1173–1180. [Google Scholar]
- Codd, GA. Cyanobacterial toxins: occurrence, properties and biological significance. Water Sci. Technol 1995, 32, 149–156. [Google Scholar]
- Sivonen, K; Jones, G. Chorus, I, Bartram, J, Eds.; Cyanobacterial toxins. In Toxin Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; WHO E & FN Spon: London, UK, 1999; pp. 41–111. [Google Scholar]
- Kao, CY. Falconer, ER, Ed.; Paralytic shellfish poisoning. In Algal Toxins in Seafood and Drinking Water; Academic: London, UK, 1993; p. 75. [Google Scholar]
- Anderson, DM; Kulis, DM; Qi, Y; Zheng, L; Lu, S; Lin, Y. Paralytic shellfish poisoning in Southern China. Toxicon 1996, 34, 579–590. [Google Scholar]
- Rodrigue, DC; Etzel, RA; Hall, S; de Porras, E; Velasquez, OH; Tauxe, RV; Kilbourne, EM; Blake, PA. Lethal paralytic shellfish poisoning in Guatemala. Am. J. Trop. Med. Hyg 1990, 42, 267–271. [Google Scholar]
- Garcia, C; del Carmen Bravo, M; Lagos, M; Lagos, N. Paralytic shellfish poisoning: Post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon 2004, 43, 149–158. [Google Scholar]
- Landsberg, JH; Hall, S; Johannessen, JN; White, KD; Conrad, SM; Abbott, JP; Flewelling, LJ; Richardson, RW; Dickey, RW; Jester, EL; Etheridge, SM; Deeds, JR; Van Dolah, FM; Leighfield, TA; Zou, Y; Beaudry, CG; Benner, RA; Rogers, PL; Scott, PS; Kawabata, K; Wolny, JL; Steidinger, KA. Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Perspect 2006, 114, 1502–1507. [Google Scholar]
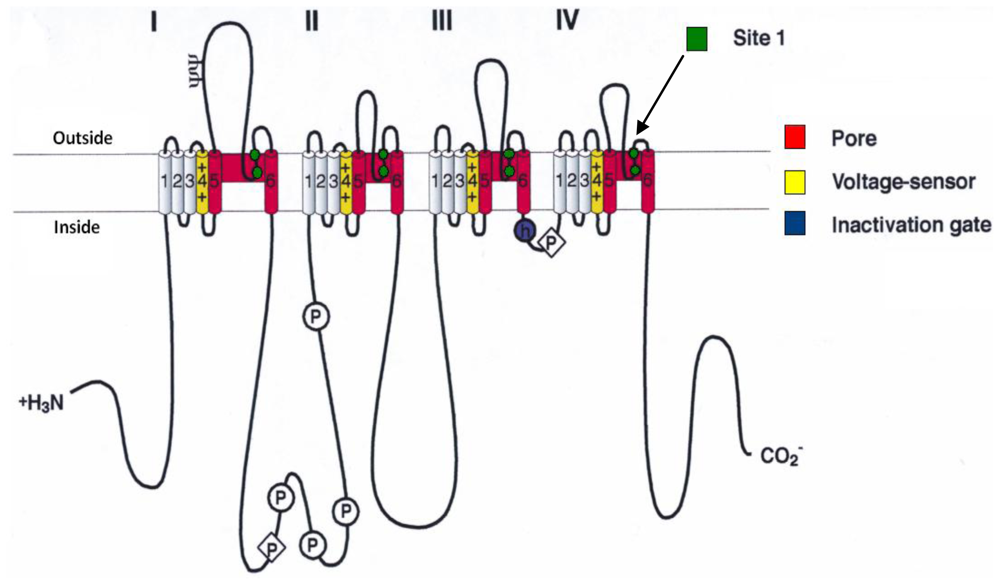
- Catterall, WA; Morrow, CS; Hartshorne, RP. Neurotoxin binding to receptor sites associated with voltage-sensitive sodium channels in intact, lysed, and detergent-solubilized brain membranes. J. Biol. Chem 1979, 254, 11379–11387. [Google Scholar]
- Catterall, WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol 1980, 20, 15–43. [Google Scholar]
- Cestèle, S; Catterall, WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 2000, 82, 883–892. [Google Scholar]
- Guy, AL; Griffin, G. Adopting alternatives for the regulatory monitoring of shellfish for paralytic shellfish poisoning in Canada: Interface between federal regulators, science and ethics. Regul. Toxicol. Pharmacol 2009, 54, 256–263. [Google Scholar]
- Stewart, I; Seawright, AA; Shaw, GR. Hudnell, HK, Ed.; Cyanobacterial poisoning in livestock, wild mammals and birds—an overview. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: New York, NY, USA, 2008; pp. 613–637. [Google Scholar]
- Kellmann, R; Mihali, TK; Jeon, YJ; Pickford, R; Pomati, F; Neilan, BA. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol 2008, 74, 4044–4053. [Google Scholar]
- Mihali, TK; Kellmann, R; Neilan, BA. Characterisation of the paralytic shellfish toxin biosynthesis gene clusters in Anabaena circinalis AWQC131C and Aphanizomenon sp. NH-5. BMC Biochem 2009, 10, 8. [Google Scholar]
- Llewellyn, LE; Negri, AP; Doyle, J; Baker, PD; Beltran, EC; Neilan, BA. Radioreceptor assays for sensitive detection and quantitation of saxitoxin and its analogues from strains of the freshwater cyanobacterium, Anabaena circinalis. Environ. Sci. Technol 2001, 35, 1445–1451. [Google Scholar]
- Lagos, N; Onodera, H; Zagatto, PA; Andrinolo, D; Azevedo, SMFQ; Oshima, Y. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon 1999, 37, 1359–1373. [Google Scholar]
- Ferreira, FMB; Soler, JMF; Fidalgo, ML; Fernández-Vila, P. PSP toxins from Aphanizomenon flos-aquae (cyanobacteria) collected in the Crestuma-Lever reservoir (Douro river, northern Portugal). Toxicon 2001, 39, 757–761. [Google Scholar]
- Hille, B. The receptor for tetrodotoxin and saxitoxin. A structural hypothesis. Biophys. J 1975, 15, 615–619. [Google Scholar]
- Adams, HJ; Blair, MR, Jr; Takman, BH. The local anesthetic activity of saxitoxin alone and with vasoconstrictor and local anesthetic agents. Arch. Int. Pharmacodyn. Ther 1976, 224, 275–282. [Google Scholar]
- Khosla, C; Keasling, JD. Metabolic engineering for drug discovery and development. Nat. Rev. Drug Discov 2003, 2, 1019–1025. [Google Scholar]
- Zhang, W; Tang, Y. Combinatorial biosynthesis of natural products. J. Med. Chem 2008, 51, 2629–2633. [Google Scholar]
- Bordner, J; Thiessen, WE; Bates, HA; Rapoport, H. Structure of a crystalline derivative of saxitoxin. Structure of saxitoxin. J. Am. Chem. Soc 1975, 97, 6008–6012. [Google Scholar]
- Schantz, EJ; Ghazarossian, VE; Schnoes, HK; Strong, FM; Springer, JP; Pezzanite, JO; Clardy, J. Structure of saxitoxin. J. Am. Chem. Soc 1975, 97, 1238–1239. [Google Scholar]
- Shimizu, Y. Botana, LM, Ed.; Chemistry and mechanism of action. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection; Marcel Dekker: New York, NY, USA, 2000; pp. 151–172. [Google Scholar]
- Berlinck, RGS; Kossuga, MH. Natural guanidine derivatives. Nat. Prod. Rep 2005, 22, 516–550. [Google Scholar]
- Ciminiello, P; Fattorusso, E; Forino, M; Montresor, M. Saxitoxin and neosaxitoxin as toxic principles of Alexandrium andersoni (Dinophyceae) from the Gulf of Naples, Italy. Toxicon 2000, 38, 1871–1877. [Google Scholar]
- Siu, G; Young, M; Chan, D. Environmental and nutritional factors which regulate population dynamics and toxin production in the dinoflagellate Alexandrium catenella. Hydrobiologia 1997, 352, 117–140. [Google Scholar]
- Sebastián, CR; Etheridge, SM; Cook, PA; O'Ryan, C; Pitcher, GC. Phylogenetic analysis of toxic Alexandrium (Dinophyceae) isolates from South Africa: implications for the global phylogeography of the Alexandrium tamarense species complex. Phycologia 2005, 44, 49–60. [Google Scholar]
- Krock, B; Seguel, CG; Cembella, AD. Toxin profile of Alexandrium catenella from the Chilean coast as determined by liquid chromatography with fluorescence detection and liquid chromatography coupled with tandem mass spectrometry. Harmful Algae 2007, 6, 734–744. [Google Scholar]
- Poulton, NJ; Keafer, BA; Anderson, DM. Toxin variability in natural populations of Alexandrium fundyense in Casco Bay, Maine—evidence of nitrogen limitation. Deep-Sea Res. PT. II 2005, 52, 2501–2521. [Google Scholar]
- Anderson, DM; Kulis, DM; Sullivan, JJ; Hall, S. Toxin composition variations in one isolate of the dinoflagellate Alexandrium fundyense. Toxicon 1990, 28, 885–893. [Google Scholar]
- Jaime, E; Gerdts, G; Luckas, B. In vitro transformation of PSP toxins by different shellfish tissues. Harmful Algae 2007, 6, 308–316. [Google Scholar]
- Parkhill, J; Cembella, A. Effects of salinity, light and inorganic nitrogen on growth and toxigenicity of the marine dinoflagellate Alexandrium tamarense from northeastern Canada. J. Plankton Res 1999, 21, 939–955. [Google Scholar]
- Yu, R; Hummert, C; Luckas, B; Qian, P; Li, J; Zhou, M. A modified HPLC method for analysis of PSP toxins in algae and shellfish from china. Chromatographia 1998, 48, 671–676. [Google Scholar]
- Ichimi, K; Suzuki, T; Ito, A. Variety of PSP toxin profiles in various culture strains of Alexandrium tamarense and change of toxin profile in natural A. tamarense population. J. Exp. Mar. Biol. Ecol 2002, 273, 51–60. [Google Scholar]
- Dell'Aversano, C; Walter, JA; Burton, IW; Stirling, DJ; Fattorusso, E; Quilliam, MA. Isolation and structure elucidation of new and unusual saxitoxin analogues from mussels. J. Nat. Prod 2008, 71, 1518–1523. [Google Scholar]
- Velzeboer, RMA; Baker, PD; Rositano, J; Heresztyn, T; Codd, GA; Raggett, SL. Geographical patterns of occurrence and composition of saxitoxins in the cyanobacterial genus Anabaena (Nostocales, Cyanophyta) in Australia. Phycologia 2000, 39, 395–407. [Google Scholar]
- Testé, V; Briand, J-F; Nicholson, BC; Puiseux-Dao, S. Comparison of changes in toxicity during growth of Anabaena circinalis (cyanobacteria) determined by mouse neuroblastoma bioassay and HPLC. J. Appl. Phycol 2002, 14, 399–407. [Google Scholar]
- Negri, AP; Jones, GJ. Bioaccumulation of paralytic shellfish poisoning (PSP) toxins from the cyanobacterium Anabaena circinalis by the freshwater mussel Alathyria condola. Toxicon 1995, 33, 667–678. [Google Scholar]
- Dias, E; Pereira, P; Franca, S. Production of the paralytic shellfish toxins by Aphanizomenon sp. LMECYA 31 (cyanobacteria). J. Phycol 2002, 38, 705–712. [Google Scholar]
- Ikawa, M; Wegener, K; Foxall, TL; Sasner, JJ, Jr. Comparison of the toxins of the blue-green alga Aphanizomenon flos-aquae with the Gonyaulax toxins. Toxicon 1982, 20, 747–752. [Google Scholar]
- Mahmood, NA; Carmichael, WW. Paralytic shellfish poisons produced by the freshwater cyanobacterium Aphanizomenon flos-aquae NH-5. Toxicon 1986, 24, 175–186. [Google Scholar]
- Pereira, P; Onodera, H; Andrinolo, D; Franca, S; Araújo, F; Lagos, N; Oshima, Y. Paralytic shellfish toxins in the freshwater cyanobacterium Aphanizomenon flos-aquae, isolated from Montargil reservoir, Portugal. Toxicon 2000, 38, 1689–1702. [Google Scholar]
- Pereira, P; Li, R; Carmichael, W; Dias, E; Franca, S. Taxonomy and production of paralytic shellfish toxins by the freshwater cyanobacterium Aphanizomenon gracile LMECYA40. Eur. J. Phycol 2004, 39, 361–368. [Google Scholar]
- Nogueira, ICG; Pereira, P; Dias, E; Pflugmacher, S; Wiegand, C; Franca, S; Vasconcelos, VM. Accumulation of paralytic shellfish toxins (PST) from the cyanobacterium Aphanizomenon issatschenkoi by the cladoceran Daphnia magna. Toxicon 2004, 44, 773–780. [Google Scholar]
- Rapala, J; Robertson, A; Negri, AP; Berg, KA; Tuomi, P; Lyra, C; Erkomaa, K; Lahti, K; Hoppu, K; Lepistö, L. First report of saxitoxin in Finnish lakes and possible associated effects on human health. Environ. Toxicol 2005, 20, 331–340. [Google Scholar]
- Castro, D; Vera, D; Lagos, N; García, C; Vásquez, M. The effect of temperature on growth and production of paralytic shellfish poisoning toxins by the cyanobacterium Cylindrospermopsis raciborskii C10. Toxicon 2004, 44, 483–489. [Google Scholar]
- Pomati, F; Moffitt, MC; Cavaliere, R; Neilan, BA. Evidence for differences in the metabolism of saxitoxin and C1+2 toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii T3. BBA-Gen. Subjects 2004, 1674, 60–67. [Google Scholar]
- Molica, R; Onodbra, H; Garcia, C; Rivas, M; Andrinolo, D; Nascimento, S; Meguro, H; Oshimo, Y; Azevedo, S; Lagos, N. Toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii (Cyanophyceae) isolated from Tabocas reservoir in Caruaru, Brazil, including demonstration of a new saxitoxin analogue. Phycologia 2002, 41, 606–611. [Google Scholar]
- Holmes, MJ; Bolch, CJS; Green, DH; Cembella, AD; Teo, SLM. Singapore isolates of the dinoflagellate Gymnodinium catenatum (Dinophyceae) produce a unique profile of paralytic shellfish poisoning toxins. J. Phycol 2002, 38, 96–106. [Google Scholar]
- Gárate-Lizárraga, I; Bustillos-Guzmán, JJ; Morquecho, L; Band-Schmidt, CJ; Alonso-Rodríguez, R; Erler, K; Luckas, B; Reyes-Salinas, A; Góngora-González, DT. Comparative paralytic shellfish toxin profiles in the strains of Gymnodinium catenatum Graham from the Gulf of California, Mexico. Mar. Pollut. Bull 2005, 50, 211–217. [Google Scholar]
- Negri, AP; Bolch, CJS; Geier, S; Green, DH; Park, T-G; Blackburn, SI. Widespread presence of hydrophobic paralytic shellfish toxins in Gymnodinium catenatum. Harmful Algae 2007, 6, 774–780. [Google Scholar]
- Pomati, F; Sacchi, S; Rossetti, C; Giovannardi, S; Onodera, H; Oshima, Y; Neilan, BA. The freshwater cyanobacterium Planktothrix sp. FP1: Molecular identification and detection of paralytic shellfish poisoning toxins. J. Phycol 2000, 36, 553–562. [Google Scholar]
- Liu, Y; Chen, W; Li, D; Shen, Y; Liu, Y; Song, L. Analysis of paralytic shellfish toxins in Aphanizomenon DC-1 from Lake Dianchi, China. Environ. Toxicol 2006, 21, 289–295. [Google Scholar]
- Navarro, JM; Muñoz, MG; Contreras, AM. Temperature as a factor regulating growth and toxin content in the dinoflagellate Alexandrium catenella. Harmful Algae 2006, 5, 762–769. [Google Scholar]
- Samsur, M; Yamaguchi, Y; Sagara, T; Takatani, T; Arakawa, O; Noguchi, T. Accumulation and depuration profiles of PSP toxins in the short-necked clam Tapes japonica fed with the toxic dinoflagellate Alexandrium catenella. Toxicon 2006, 48, 323–330. [Google Scholar]
- Franco, J; Fernández, P; Reguera, B. Toxin profiles of natural populations and cultures of Alexandrium minutum Halim from Galician (Spain) coastal waters. J. Appl. Phycol 1994, 6, 275–279. [Google Scholar]
- Hwang, D-F; Lu, Y-H; Noguchi, T. Effects of exogenous polyamines on growth, toxicity, and toxin profile of dinoflagellate Alexandrium minutum. J. Food Hyg. Soc. Jpn 2003, 44, 49–53. [Google Scholar]
- Pitcher, GC; Cembella, AD; Joyce, LB; Larsen, J; Probyn, TA; Ruiz Sebastián, C. The dinoflagellate Alexandrium minutum in Cape Town harbour (South Africa): Bloom characteristics, phylogenetic analysis and toxin composition. Harmful Algae 2007, 6, 823–836. [Google Scholar]
- Hansen, PJ; Cembella, AD; Moestrup, Ø. The marine dinoflagellate Alexandrium ostenfeldii: Paralytic shellfish toxin concentration, composition and toxicity to a tintinnid cilliate. J. Phycol 1992, 28, 597–603. [Google Scholar]
- Vale, P. Metabolites of saxitoxin analogues in bivalves contaminated by Gymnodinium catenatum. Toxicon 2010, 55, 162–165. [Google Scholar]
- Onodera, H; Satake, M; Oshima, Y; Yasumoto, T; Carmichael, WW. New saxitoxin analogues from the freshwater filamentous cyanobacterium Lyngbya wollei. Nat. Toxins 1997, 5, 146–151. [Google Scholar]
- Negri, A; Stirling, D; Quilliam, M; Blackburn, S; Bolch, C; Burton, I; Eaglesham, G; Thomas, K; Walter, J; Willis, R. Three novel hydroxybenzoate saxitoxin analogues isolated from the dinoflagellate Gymnodinium catenatum. Chem. Res. Toxicol 2003, 16, 1029–1033. [Google Scholar]
- Oshima, Y; Sugino, K; Itakura, H; Hirota, M; Yasumoto, T. Grane'li, E, Sundstrom, B, Edler, L, Anderson, DM, Eds.; Comparative studies on paralytic shellfish toxin profile of dinoflagellates and bivalves. In Toxic Marine Phytoplankton; Elsevier Science Publishing: New York, NY, USA, 1990; pp. 391–396. [Google Scholar]
- Vale, P. Complex profiles of hydrophobic paralytic shellfish poisoning compounds in Gymnodinium catenatum identified by liquid chromatography with fluorescence detection and mass spectrometry. J. Chromatogr. A 2008, 1195, 85–93. [Google Scholar]
- Vale, P; Rangel, I; Silva, B; Coelho, P; Vilar, A. Atypical profiles of paralytic shellfish poisoning toxins in shellfish from Luanda and Mussulo bays, Angola. Toxicon 2009, 53, 176–183. [Google Scholar]
- Arakawa, O; Nishio, S; Noguchi, T; Shida, Y; Onoue, Y. A new saxitoxin analogue from a xanthid crab Atergatis floridus. Toxicon 1995, 33, 1577–1584. [Google Scholar]
- Zaman, L; Arakawa, O; Shimosu, A; Shida, Y; Onoue, Y. Occurrence of a methyl derivative of saxitoxin in Bangladeshi freshwater puffers. Toxicon 1998, 36, 627–630. [Google Scholar]
- Yotsu-Yamashita, M; Kim, YH; Dudley, SC; Choudhary, G; Pfahnl, A; Oshima, Y; Daly, JW. The structure of zetekitoxin AB, a saxitoxin analog from the Panamanian golden frog Atelopus zeteki: A potent sodium-channel blocker. Proc. Natl. Acad. Sci. USA 2004, 101, 4346–4351. [Google Scholar]
- van Apeldoorn, ME; van Egmond, HP; Speijers, GJA; Bakker, GJI. Toxins of cyanobacteria. Mol. Nutr. Food Res 2007, 51, 7–60. [Google Scholar]
- Negri, AP; Bolch, CJ; Blackbum, SI; Dickman, M; Llewellyn, LE; Mendez, S. Hallegraeff, G, Bolch, CJ, Blackburn, SI, Lewis, RJ, Eds.; Paralytic shellfish toxins in Gymnodinium catenatum strains from six countries. In Harmfull Algal Blooms 2000; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2001; pp. 210–213. [Google Scholar]
- Llewellyn, L; Negri, A; Quilliam, M. High affinity for the rat brain sodium channel of newly discovered hydroxybenzoate saxitoxin analogues from the dinoflagellate Gymnodinium catenatum. Toxicon 2004, 43, 101–104. [Google Scholar]
- Vale, P. Fate of benzoate paralytic shellfish poisoning toxins from Gymnodinium catenatum in shellfish and fish detected by pre-column oxidation and liquid chromatography with fluorescence detection. J. Chromatogr. A 2008, 1190, 191–197. [Google Scholar]
- Codd, GA; Morrison, LF; Metcalf, JS. Cyanobacterial toxins: risk management for health protection. Toxicol. Appl. Pharm 2005, 203, 264–272. [Google Scholar]
- Usleber, E; Donald, M; Straka, M; Märtlbauer, E. Comparison of enzyme immunoassay and mouse bioassay for determining paralytic shellfish poisoning toxins in shellfish. Food Addit. Contam 1997, 14, 193–198. [Google Scholar]
- Genenah, AA; Shimizu, Y. Specific toxicity of paralytic shellfish poisons. J. Agr. Food Chem 1981, 29, 1289–1291. [Google Scholar]
- Sullivan, JJ; Wekell, MM; Kentala, LL. Application of HPLC for the Determination of PSP Toxins in Shellfish. J. Food Sci 1985, 50, 26–29. [Google Scholar]
- Oshima, Y; Sugino, K; Yasumoto, T. Mycotoxins and Phycotoxins '88; Elsevier Applied Science: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Oshima, Y. Lassus, P, Arzul, G, Erard, E, Gentien, P, Marcaillou, C, Eds.; Chemical and enzymatic transformation of paralytic shellfish toxins in marine organisms. In Harmful Marine Algal Blooms; Lavoisier: Paris, France, 1995. [Google Scholar]
- Asakawa, M; Miyazawa, K; Takayama, H; Noguchi, T. Dinoflagellate Alexandrium tamarense as the source of paralytic shellfish poison (PSP) contained in bivalves from Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon 1995, 33, 691–697. [Google Scholar]
- Bricelj, VM; Lee, JH; Cembella, AD. Influence of dinoflagellate cell toxicity on uptake and loss of paralytic shellfish toxins in the northern quahog Mercenaria mercenaria. Mar. Ecol. Prog. Ser 1991, 74, 33–46. [Google Scholar]
- Bricelj, VM; Lee, JH; Cembella, AD; Anderson, DM. Uptake kinetics of paralytic shellfish toxins from the dinoflagellate Alexandrium fundyense in the mussel Mytilus edulis. Mar. Ecol. Prog. Ser 1990, 63, 177–188. [Google Scholar]
- Cembella, AD; Shumway, SE; Lewis, NI. Anatomical distribution and spatio-temporal variation in paralytic shellfish toxin composition in two bivalve species from the Gulf of Maine. J. Shellfish Res 1993, 12, 389–403. [Google Scholar]
- Lassus, P; Bardouill, M; Massselin, P; Naviner, P; Truquet, P. Comparative efficiencies of different non-toxic microalgal diets in detoxification of PSP-contaminated oysters (Crassoatrea gigas Thunberg). J. Nat. Toxins 2000, 9, 1–12. [Google Scholar]
- Oshima, Y; Fallon, WE; Shimizu, Y; Noguchi, T; Hashimoto, Y. Toxins of the Gonyaulax sp. and infested bivalves in Owase Bay. Bull. Jpn. Soc. Sci. Fish 1976, 42, 851–856. [Google Scholar]
- Sullivan, JJ; Iwaoka, WT; Liston, J. Enzymatic transformation of PSP toxins in the littleneck clam (Protothaca staminea). Biochem. Biophys. Res. Commun 1983, 114, 465–472. [Google Scholar]
- Shimizu, Y; Yoshioka, M. Transformation of paralytic shellfish toxins as demonstrated in scallop homogenates. Science 1981, 212, 547–549. [Google Scholar]
- Lu, Y; Hwang, D. Effects of toxic dinoflagellates and toxin biotransformation in bivalves. J. Nat. Toxins 2002, 11, 315–322. [Google Scholar]
- Fast, MD; Cembella, AD; Ross, NW. In vitro transformation of paralytic shellfish toxins in the clams Mya arenaria and Protothaca staminea. Harmful Algae 2006, 5, 79–90. [Google Scholar]
- Kotaki, Y; Oshima, Y; Yasumoto, T. Bacterial transformation of paralytic shellfish toxins in coral reef crabs and a marine snail. Nippon Suisan Gakk 1985, 51, 1009–1013. [Google Scholar]
- Kotaki, Y. Screening of bacteria which convert gonyautoxin 2,3 to saxitoxin. Nippon Suisan Gakk 1989, 55, 1293. [Google Scholar]
- Sugawara, A; Imamura, T; Aso, S; Ebitani, K. Change of paralytic shellfish poison by the marine bacteria living in the intestine of the Japanese surf clam, Pseudocardium sybillae, and the brown sole, Pleuronectes herensteini. Sci. Rep. Hokkaido Fish. Exp. Stat 1997, 50, 35–42. [Google Scholar]
- Smith, EA; Grant, F; Ferguson, CMJ; Gallacher, S. Biotransformations of paralytic shellfish toxins by bacteria isolated from bivalve molluscs. Appl. Environ. Microbiol 2001, 67, 2345–2353. [Google Scholar]
- Donovan, CJ; Garduno, RA; Kalmokoff, M; Ku, JC; Quilliam, MA; Gill, TA. Pseudoalteromonas bacteria are capable of degrading paralytic shellfish toxins. Appl. Environ. Microbiol 2009, 75, 6919–6923. [Google Scholar]
- Donovan, CJ; Ku, JC; Quilliam, MA; Gill, TA. Bacterial degradation of paralytic shellfish toxins. Toxicon 2008, 52, 91–100. [Google Scholar]
- Kayal, N; Newcombe, G; Ho, L. Investigating the fate of saxitoxins in biologically active water treatment plant filters. Environ. Toxicol 2008, 23, 751–755. [Google Scholar]
- García, C; Rodriguez-Navarro, A; Díaz, JC; Torres, R; Lagos, N. Evidence of in vitro glucuronidation and enzymatic transformation of paralytic shellfish toxins by healthy human liver microsomes fraction. Toxicon 2009, 53, 206–213. [Google Scholar]
- Andrinolo, D; Michea, LF; Lagos, N. Toxic effects, pharmacokinetics and clearance of saxitoxin, a component of paralytic shellfish poison (PSP), in cats. Toxicon 1999, 37, 447–464. [Google Scholar]
- Kasper, BC; Henton, D. Jakob, WB, Ed.; Glucuronidation. In Enzymatic Basis of Detoxication; Academic: New York, NY, USA, 1960; Volume 1. [Google Scholar]
- Stafford, RG; Hines, HB. Urinary elimination of saxitoxin after intravenous injection. Toxicon 1995, 33, 1501–1510. [Google Scholar]
- Hines, H; Naseem, S; Wannemacher, RJ. 3H-Saxitoxinol metabolism and elimination in the rat. Toxicon 1993, 31, 905–908. [Google Scholar]
- Gessner, BD; Bell, P; Doucette, GJ; Moczydlowski, E; Poli, MA; Van Dolah, F; Hall, S. Hypertension and identification of toxin in human urine and serum following a cluster of mussel-associated paralytic shellfish poisoning outbreaks. Toxicon 1997, 35, 711–722. [Google Scholar]
- Johnson, RC; Zhou, Y; Statler, K; Thomas, J; Cox, F; Hall, S; Barr, JR. Quantification of saxitoxin and neosaxitoxin in human urine utilizing isotope dilution tandem mass spectrometry. J. Anal. Toxicol 2009, 33, 8–14. [Google Scholar]
- Kellmann, R; Neilan, BA. Biochemical characterization of paralytic shellfish toxin biosynthesis in vitro. J. Phycol 2007, 43, 497–508. [Google Scholar]
- Mihali, TK; Carmichael, WW; Neilan, BA. Biosynthesis of the Lyngbya wollei (Farlow ex Gomont) paralytic shellfish toxins - natural biocombinatorics. PLoS One 2010. Submitted for publication. [Google Scholar]
- Prol, MJ; Guisande, C; Barreiro, A; Miguez, B; de la Iglesia, P; Villar, A; Gago-Martinez, A; Combarro, MP. Evaluation of the production of paralytic shellfish poisoning toxins by extracellular bacteria isolated from the toxic dinoflagellate Alexandrium minutum. Can. J. Microbiol 2009, 55, 943–954. [Google Scholar]
- Baker, TR; Doucette, GJ; Powell, CL; Boyer, GL; Plumley, FG. GTX4 imposters: Characterization of fluorescent compounds synthesized by Pseudomonas stutzeri SF/PS and Pseudomonas/Alteromonas PTB-1, symbionts of saxitoxin-producing Alexandrium spp. Toxicon 2003, 41, 339–347. [Google Scholar]
- Gallacher, S; Flynn, K; Franco, J; Brueggemann, E; Hines, H. Evidence for production of paralytic shellfish toxins by bacteria associated with Alexandrium spp. (Dinophyta) in culture. Appl. Environ. Microbiol 1997, 63, 239–245. [Google Scholar]
- Moustafa, A; Loram, JE; Hackett, JD; Anderson, DM; Plumley, FG; Bhattacharya, D. Origin of saxitoxin biosynthetic genes in cyanobacteria. PLoS ONE 2009, 4, e5758. [Google Scholar]
- Hill, AM. The biosynthesis, molecular genetics and enzymology of the polyketide-derived metabolites. Nat. Prod. Rep 2006, 23, 256–320. [Google Scholar]
- Wolf, E; Vassilev, A; Makino, Y; Sali, A; Nakatani, Y; Burley, SK. Crystal Structure of a GCN5-Related N-acetyltransferase: Serratia marcescens Aminoglycoside 3-N-acetyltransferase. Cell 1998, 94, 439–449. [Google Scholar]
- Kagan, RM; Clarke, S. Widespread occurrence of three sequence motifs in diverse S-adenosylmethionine-dependent methyltransferases suggests a common structure for these enzymes. Arch. Biochem. Biophys 1994, 310, 417–427. [Google Scholar]
- Alexander, FW; Sandmeier, E; Mehta, PK; Christen, P. Evolutionary relationships among pyridoxal-5′-phosphate-dependent enzymes. Eur. J. Biochem 1994, 219, 953–960. [Google Scholar]
- Alexander, DC; Jensen, SE. Investigation of the Streptomyces clavuligerus cephamycin C gene cluster and its regulation by the CcaR protein. J. Bacteriol 1998, 180, 4068–4079. [Google Scholar]
- Yoshida, T; Sako, Y; Uchida, A; Kakutani, T; Arakawa, O; Noguchi, T; Ishida, Y. Purification and characterization of sulfotransferase specific to O-22 of 11-hydroxy saxitoxin from the toxic dinoflagellate Gymnodinium catenatum (dinophyceae). Fish. Sci 2002, 68, 634–642. [Google Scholar]
- Sako, Y; Yoshida, T; Uchida, A; Arakawa, O; Noguchi, T; Ishida, Y. Purification and characterization of a sulfotransferase specific to N-21 of saxitoxin and gonyautosin 2+3 from the toxic dinoflagellate Gymnodinium catenatum (Dinophyceae). J. Phycol 2001, 37, 1044–1051. [Google Scholar]
- Shimizu, Y. Microalgal metabolites. Chem. Rev 1993, 93, 1685–1698. [Google Scholar]
- Harlow, LD; Koutoulis, A; Hallegraeff, GM. S-adenosylmethionine synthetase genes from eleven marine dinoflagellates. Phycologia 2007, 46, 46–53. [Google Scholar]
- Altschul, SF; Madden, TL; Schaffer, AA; Zhang, J; Zhang, Z; Miller, W; Lipman, DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997, 25, 3389–3402. [Google Scholar]
- Kellmann, R; Mihali, TK; Neilan, BA. Identification of a saxitoxin biosynthesis gene with a history of frequent horizontal gene transfers. J. Mol. Evol 2008, 67, 526–538. [Google Scholar]
- Yang, I; John, U; Beszteri, S; Glockner, G; Krock, B; Goesmann, A; Cembella, A. Comparative gene expression in toxic versus non-toxic strains of the marine dinoflagellate Alexandrium minutum. BMC Genomics 2010, 11, 248. [Google Scholar]
- Halai, R; Craik, DJ. Conotoxins: Natural product drug leads. Nat. Prod. Rep 2009, 26, 526–536. [Google Scholar]
- Klotz, U. Ziconotide—a novel neuron-specific calcium channel blocker for the intrathecal treatment of severe chronic pain—a short review. Int. J. Clin. Pharmacol. Ther 2006, 44, 478–483. [Google Scholar]
- Glaser, KB; Mayer, AMS. A renaissance in marine pharmacology: From preclinical curiosity to clinical reality. Biochem. Pharmacol 2009, 78, 440–448. [Google Scholar]
- Olivera, BM; Cruz, LJ; de Santos, V; LeCheminant, GW; Griffin, D; Zeikus, R; McIntosh, M; Galyean, R; Varga, J; Gray, WR; Rivier, J. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry 1987, 26, 2086–2090. [Google Scholar]
- Molinski, TF; Dalisay, DS; Lievens, SL; Saludes, JP. Drug development from marine natural products. Nat. Rev. Drug Discov 2009, 8, 69–85. [Google Scholar]
- Kohane, DS; Yieh, J; Lu, NT; Langer, R; Strichartz, GR; Berde, CB. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology 1998, 89, 119–131. [Google Scholar]
- Barnet, CS; Tse, JY; Kohane, DS. Site 1 sodium channel blockers prolong the duration of sciatic nerve blockade from tricyclic antidepressants. Pain 2004, 110, 432–438. [Google Scholar]
- Kohane, DS; Lu, NT; Gökgöl-Kline, AC; Shubina, M; Kuang, Y; Hall, S; Strichartz, GR; Berde, CB. The local anesthetic properties and toxicity of saxitonin homologues for rat sciatic nerve block in vivo. Reg. Anesth. Pain Med 2000, 25, 52–59. [Google Scholar]
- Epstein-Barash, H; Shichor, I; Kwon, AH; Hall, S; Lawlor, MW; Langer, R; Kohane, DS. Prolonged duration local anesthesia with minimal toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 7125–7130. [Google Scholar]
- Chorny, M; Levy, RJ. Site-specific analgesia with sustained release liposomes. Proc. Natl. Acad. Sci. USA 2009, 106, 6891–6892. [Google Scholar]
- Garrido, R; Lagos, N; Lattes, K; Azolas, CG; Bocic, G; Cuneo, A; Chiong, H; Jensen, C; Henriquez, AI; Fernandez, C. The gonyautoxin 2/3 epimers reduces anal tone when injected in the anal sphincter of healthy adults. Biol. Res 2004, 37, 395–403. [Google Scholar]
- Garrido, R; Lagos, N; Lattes, K; Abedrapo, M; Bocic, G; Cuneo, A; Chiong, H; Jensen, C; Azolas, R; Henriquez, A; Garcia, C. Gonyautoxin: New treatment for healing acute and chronic anal fissures. Dis. Colon Rectum 2005, 48, 335–343. [Google Scholar]
- Garrido, R; Lagos, N; Lagos, M; Rodríguez-Navarro, AJ; Garcia, C; Truan, D; Henriquez, A. Treatment of chronic anal fissure by gonyautoxin. Colorectal Dis 2007, 9, 619–624. [Google Scholar]
- Eisenhammer, S. The surgical correction of chronic internal anal (sphincteric) contracture. S. Afr. Med. J 1951, 25, 486–489. [Google Scholar]
- Khubchandani, IT; Reed, JF. Sequelae of internal sphincterotomy for chronic fissure in ano. Br. J. Surg 1989, 76, 431–434. [Google Scholar]
- Hsu, T-C; MacKeigan, J. Surgical treatment of chronic anal fissure. Dis. Colon Rectum 1984, 27, 475–478. [Google Scholar]
- Ezri, T; Susmallian, S. Topical nifedipine vs. topical glyceryl trinitrate for treatment of chronic anal fissure. Dis. Colon Rectum 2003, 46, 805–808. [Google Scholar]
- Maria, G; Brisinda, G; Bentivoglio, AR; Cassetta, E; Gui, D; Albanese, A. Botulinum toxin injections in the internal anal sphincter for the treatment of chronic anal fissure: Long-term results after two different dosage regimens. Ann. Surg 1998, 228, 664–669. [Google Scholar]
- Gorfine, SR. Topical nitroglycerin therapy for anal fissures and ulcers. N. Engl. J. Med 1995, 333, 1156–1157. [Google Scholar]
- Lattes, K; Venegas, P; Lagos, N; Lagos, M; Pedraza, L; Rodriguez-Navarro, AJ; Garcia, C. Local infiltration of gonyautoxin is safe and effective in treatment of chronic tension-type headache. Neurol. Res 2009, 31, 228–233. [Google Scholar]
- Yan, T; Fu, M; Li, J; Yu, R; Zhou, M. Accumulation, transformation and elimination of PSP in Mytilus edulis. Oceanol. Limnol. Sin 2001, 32, 421–427. [Google Scholar]
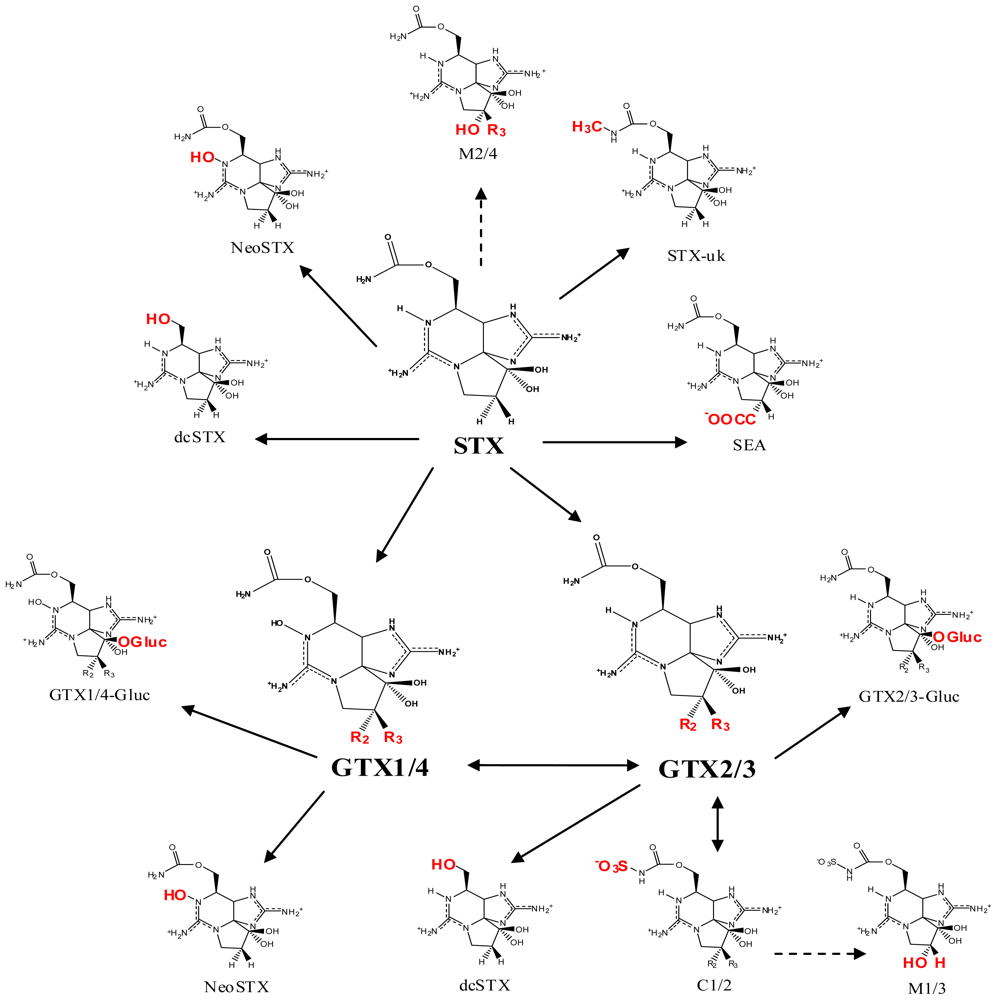
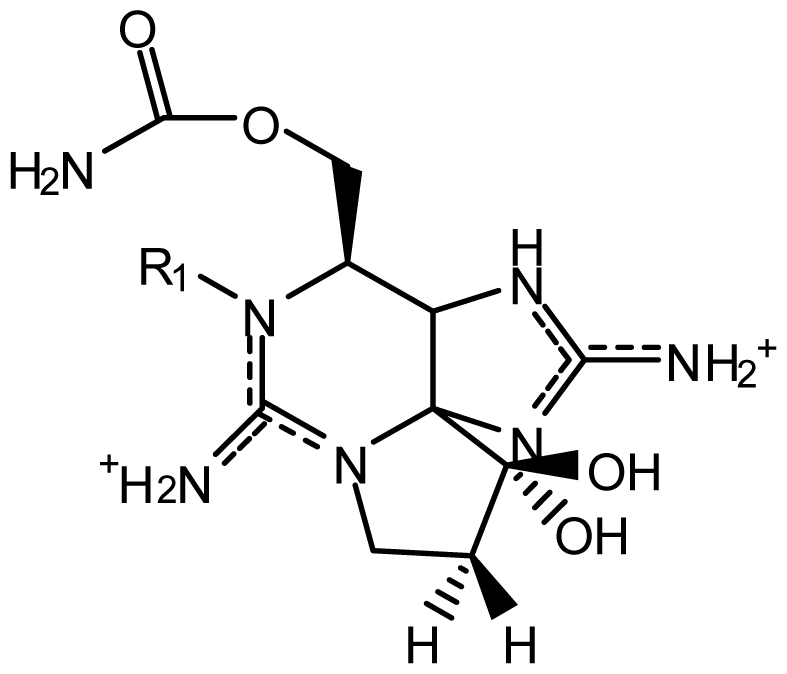
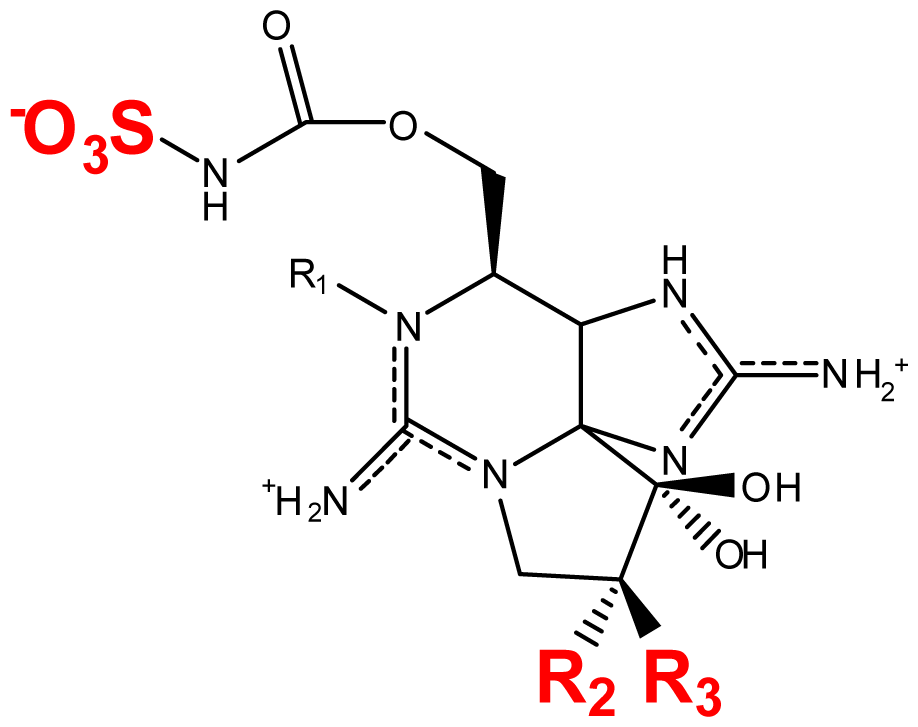
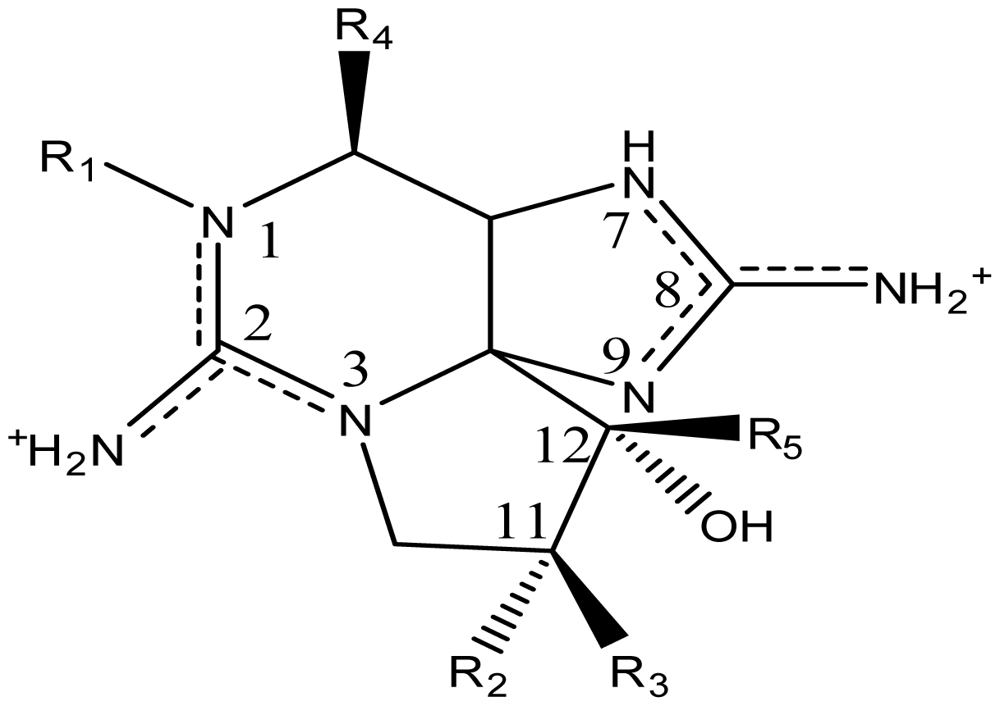 | |||||||
|---|---|---|---|---|---|---|---|
| Toxin | R1 | R2 | R3 | Ω R4 | R5 | Origin | Ref. |
| STX | H | H | H | OCONH2 | OH | Alexandrium andersoni | [46] |
| A. catenella | [47–49] | ||||||
| A. fundyense | [50–52] | ||||||
| A. tamarense | [53–56] | ||||||
| A. circinalis | [35,57–59] | ||||||
| Aphanizomenon flos-aquae | [60–63] | ||||||
| Aph. gracile | [20,64] | ||||||
| Aph. issatschenkoi | [65] | ||||||
| Anabaena lemmermannii | [66] | ||||||
| C. raciborskii | [16,36,67–69] | ||||||
| Gymnodinium catenatum | [70–72] | ||||||
| Pyrodinium bahamense | [10] | ||||||
| Planktothrix sp. | [73] | ||||||
| neoSTX | OH | H | H | OCONH2 | OH | A. andersoni | [46] |
| A. catenella | [47–49] | ||||||
| A. fundyense | [50–52] | ||||||
| A. tamarense | [53–56] | ||||||
| Aph. flos-aquae | [60–63] | ||||||
| Aph. gracile | [20,64] | ||||||
| Aph. issatschenkoi | [65] | ||||||
| Aph. sp. | [74] | ||||||
| C. raciborskii | [16,36,69] | ||||||
| G. catenatum | [70,71] | ||||||
| P. bahamense | [10] | ||||||
| Mono-Sulfated | |||||||
| GTX1 | OH | H | OSO3− | OCONH2 | OH | A. catenella | [47–49,75,76] |
| A. fundyense | [50–52] | ||||||
| A. minutum | [77–79] | ||||||
| A. tamarense | [53–56] | ||||||
| Aph. flos-aquae | [37] | ||||||
| G. catenatum | [9,70,72] | ||||||
| GTX2 | H | H | OSO3− | OCONH2 | OH | A. catenella | [48,49] |
| A. fundyense | [50–52] | ||||||
| A. minutum | [77–79] | ||||||
| A. ostenfeldii | [80] | ||||||
| A. tamarense | [53–56] | ||||||
| A. circinalis | [35,57–59] | ||||||
| C. raciborskii | [36,67] | ||||||
| G. catenatum | [9,70,72] | ||||||
| GTX3 | H | OSO3− | H | OCONH2 | OH | A. catenella | 47–49] |
| A. fundyense | [50–52] | ||||||
| A. minutum | [77–79] | ||||||
| A. ostenfeldii | [80] | ||||||
| A. tamarense | [53–56] | ||||||
| A. circinalis | [35,57–59] | ||||||
| Aph. flos-aquae | [37] | ||||||
| C. raciborskii | [36,67] | ||||||
| G. catenatum | [9,70,72] | ||||||
| GTX4 | OH | OSO3− | H | OCONH2 | OH | A. catenella | [47–49,75,76] |
| A. fundyense | [50–52] | ||||||
| A. minutum | [77–79] | ||||||
| A. tamarense | [53–56] | ||||||
| Aph. flos-aquae | [37] | ||||||
| G. catenatum | [9,70,72] | ||||||
| GTX5 (B1) | H | H | H | OCONHSO3− | OH | A. catenella | [48,49,75,76] |
| A. fundyense | [50–52] | ||||||
| A. tamarense | [54,56] | ||||||
| A. circinalis | [35,57,59] | ||||||
| Aph. flos-aquae | [60,63] | ||||||
| Aph. gracile | [20] | ||||||
| Aph. issatschenkoi | [37,65] | ||||||
| G. catenatum | [9,71,81] | ||||||
| P. bahamense | [10] | ||||||
| GTX6 (B2) | OH | H | H | OCONHSO3− | OH | A. catenella | [47,49,75,76] |
| A. fundyense | [52] | ||||||
| A. ostenfeldii | [80] | ||||||
| A. tamarense | [54] | ||||||
| Aph. flos-aquae | [63] | ||||||
| C. raciborskii | [69] | ||||||
| G. catenatum | [9,71,72,81] | ||||||
| P. bahamense | [10] | ||||||
| Di-Sulfated | |||||||
| C1 | H | H | OSO3− | OCONHSO3− | OH | A. catenella | [48,49,75,76] |
| A. fundyense | [50–52] | ||||||
| A. ostenfeldii | [80] | ||||||
| A. tamarense | [53–56] | ||||||
| A. circinalis | [35,57–59] | ||||||
| C. raciborskii | [68] | ||||||
| G. catenatum | [9,71,72,81] | ||||||
| C2 | H | OSO3− | H | OCONHSO3− | OH | A. catenella | [48,49,75] |
| A. fundyense | [50–52] | ||||||
| A. ostenfeldii | [80] | ||||||
| A. tamarense | [53–56] | ||||||
| A. circinalis | [35,57–59] | ||||||
| C. raciborskii | [68] | ||||||
| G. catenatum | [9,71,72,81] | ||||||
| C3 | OH | H | OSO3− | OCONHSO3− | OH | A. catenella | [48,49,75,76] |
| G. catenatum | [9,72,81] | ||||||
| C4 | OH | OSO3− | H | OCONHSO3− | OH | A. catenella | [48,49,75,76] |
| G. catenatum | [9,72,81] | ||||||
| Decarbamoylated | |||||||
| dcSTX | H | H | H | OH | OH | A. catenella | [49] |
| A. circinalis | [35,59] | ||||||
| Aph. flos-aquae | [60,63] | ||||||
| Aph. gracile | [20] | ||||||
| Aph. issatschenkoi | [65] | ||||||
| Aph. sp. | [74] | ||||||
| C. raciborskii | [16,67,69] | ||||||
| Lyngbya wollei | [82] | ||||||
| G. catenatum | [9,71,72] | ||||||
| P. bahamense | [10] | ||||||
| dcneoSTX | OH | H | H | OH | OH | C. raciborskii | [69] |
| dcGTX1 | OH | H | OSO3− | OH | OH | G. catenatum | [83] |
| dcGTX2 | H | H | OSO3− | OH | OH | A. catenella | [49] |
| A. fundyense | [52] | ||||||
| A. circinalis | [35,57–59] | ||||||
| G. catenatum | [9,71] | ||||||
| L. wollei | [14,82] | ||||||
| dcGTX3 | H | OSO3− | H | OH | OH | A. catenella | [49] |
| A. fundyense | [50,52] | ||||||
| A. circinalis | [35,57–59] | ||||||
| Aphanizomenon sp. | [74] | ||||||
| L. wollei | [14,82] | ||||||
| G. catenatum | [9,71] | ||||||
| dcGTX4 | OH | OSO3− | H | OH | OH | G. catenatum | [83] |
| Deoxy-Decarbomoylated | |||||||
| doSTX | H | H | H | H | OH | G. catenatum | [9,84] |
| doGTX1 | OH | H | OSO3− | H | OH | G. catenatum | [9,84] |
| doGTX2 | H | H | OSO3− | H | OH | G. catenatum | [9,84] |
| L. wollei toxins | |||||||
| LWTX1 | H | H | OSO3− | OCOCH3 | H | L. wollei | [82] |
| LWTX2 | H | H | OSO3− | OCOCH3 | OH | L. wollei | [82] |
| LWTX3 | H | OSO3− | H | OCOCH3 | OH | L. wollei | [82] |
| LWTX4 | H | H | H | H | H | L. wollei | [82] |
| LWTX5 | H | H | H | OCOCH3 | OH | L. wollei | [82] |
| LWTX6 | H | H | H | OCOCH3 | H | L. wollei | [82] |
| Mono-Hydroxy-Benzoate Analogs | |||||||
| GC1 | H | H | OSO3− | OCOPhOH | OH | G. catenatum | [83] |
| GC2 | H | OSO3− | H | OCOPhOH | OH | G. catenatum | [83] |
| GC3 | H | H | H | OCOPhOH | OH | G. catenatum | [83] |
| *GC4 | OH | H | OSO3− | OCOPhOH | OH | G. catenatum | [85] |
| *GC5 | OH | OSO3− | H | OCOPhOH | OH | G. catenatum | [85] |
| *GC6 | OH | H | H | OCOPhOH | OH | G. catenatum | [85] |
| Di-Hydroxy Benzoate Analogs | |||||||
| ŧGC1a | H | H | OSO3− | DHB | OH | G. catenatum | [85] |
| ŧGC2a | H | OSO3− | H | DHB | OH | G. catenatum | [85] |
| ŧGC3a | H | H | H | DHB | OH | G. catenatum | [85] |
| ŧGC4a | OH | H | OSO3− | DHB | OH | G. catenatum | [85] |
| ŧGC5a | OH | OSO3− | H | DHB | OH | G. catenatum | [85] |
| ŧGC6a | OH | H | H | DHB | OH | G. catenatum | [85] |
| Sulfated Benzoate Analogs | |||||||
| ŧGC1b | H | H | OSO3− | SB | OH | G. catenatum | [85] |
| ŧGC2b | H | OSO3− | H | SB | OH | G. catenatum | [85] |
| ŧGC3b | H | H | H | SB | OH | G. catenatum | [85] |
| ŧGC4b | OH | H | OSO3− | SB | OH | G. catenatum | [85] |
| ŧGC5b | OH | OSO3− | H | SB | OH | G. catenatum | [85] |
| ŧGC6b | OH | H | H | SB | OH | G. catenatum | [85] |
| Other PST Analogs | |||||||
| M1 | H | OH | H | OCONHSO3− | OH | Metabolic transformation | [56,81] |
| M2 | H | OH | H | OCONH2 | OH | Metabolic transformation | [56] |
| M3 | H | OH | OH | OCONHSO3− | OH | Metabolic transformation | [56] |
| M4 | H | OH | OH | OCONH2 | OH | Metabolic transformation | [56] |
| *M5 | Metabolic transformation | [56] | |||||
| *A | Unknown | [86] | |||||
| *B | Unknown | [86] | |||||
| *C | Unknown | [86] | |||||
| *D | Unknown | [86] | |||||
| SEA | H | CCOO− | H | OCONH2 | OH | Atergatis floridus | [87] |
| STX-uk | H | H | H | OCONHCH3 | OH | Tetraodon cutcutia | [88] |
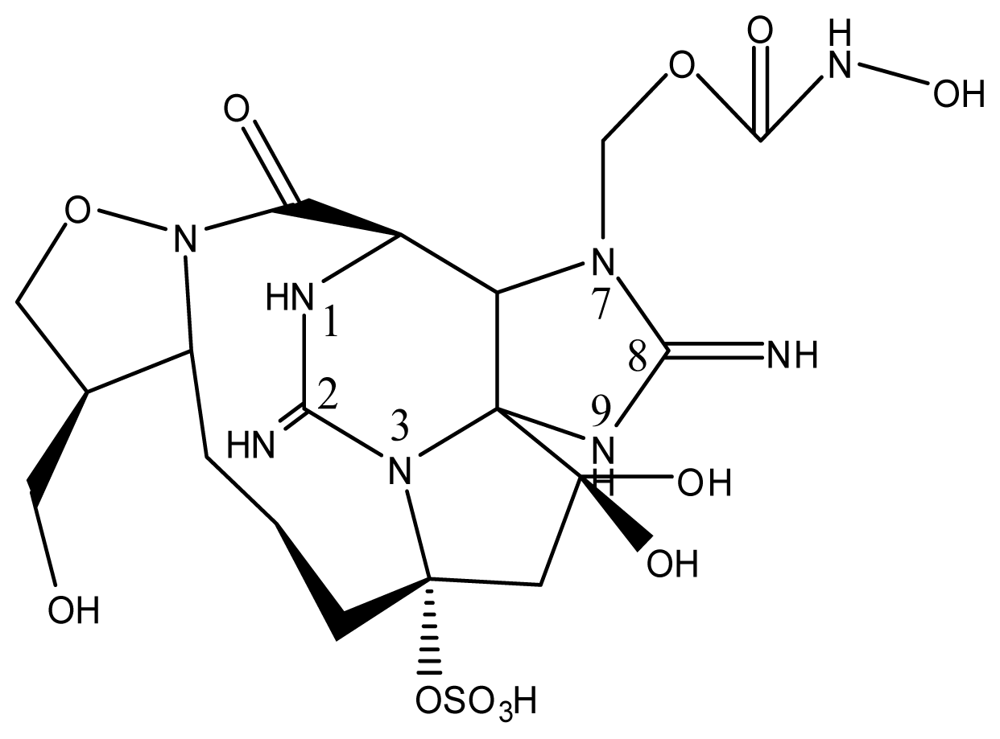
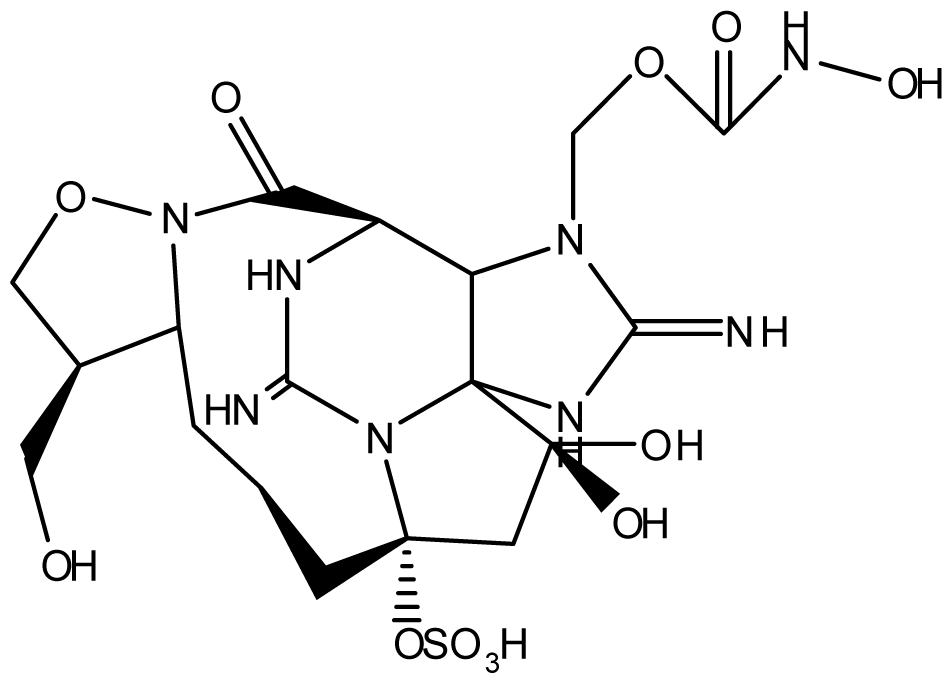
| Zetekitoxin AB | Atelopus zeteki | [89] | |||||
 | |||||||
| StructureΩ | Toxin | Relative toxicityΦ |
|---|---|---|
 | Zetekitoxin AB | 63, 160, 580ω |
 | Non-Sulfated | |
| STX NeoSTX | 1 05–1.1 | |
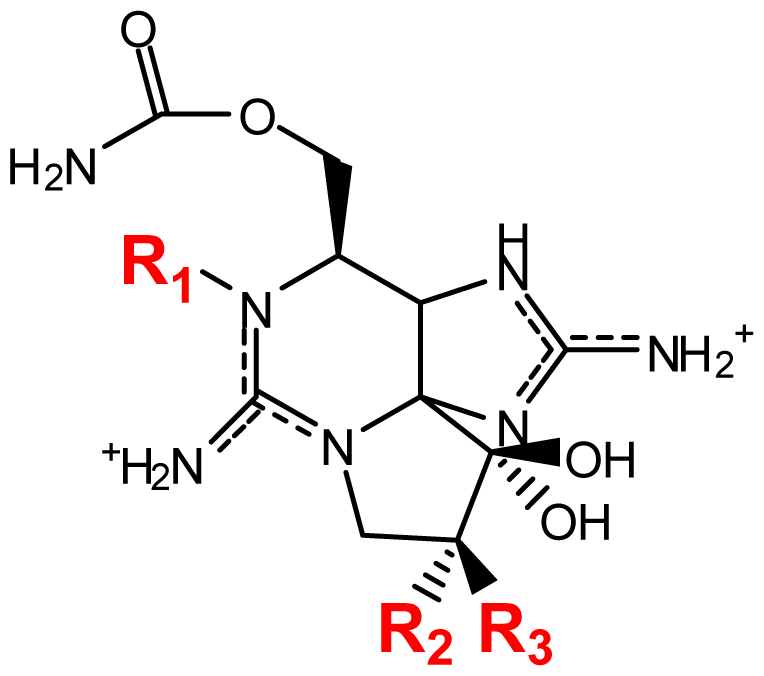 | Mono-sulfated | |
| GTX1/4¥ GTX2/3¥ | 0.39/1.09–0.48/0.76 0.8/0.33–0.9/0.9 | |
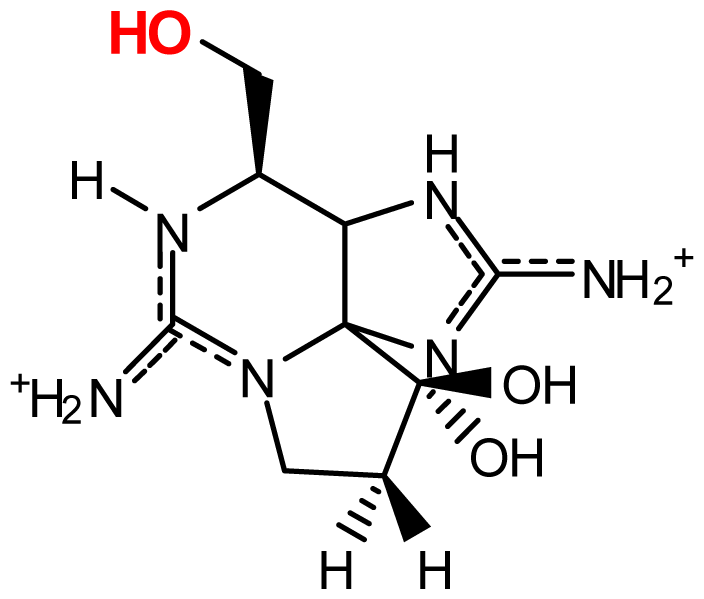 | Decarbamoylated | |
| dcSTX dcNeoSTX dcGTX1-4 | 0.43 0.43 0.18–0.45 | |
 | Di-sulfated | |
| C1-4 | <0.01–0.14 | |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8, 2185-2211. https://doi.org/10.3390/md8072185
Wiese M, D’Agostino PM, Mihali TK, Moffitt MC, Neilan BA. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Marine Drugs. 2010; 8(7):2185-2211. https://doi.org/10.3390/md8072185
Chicago/Turabian StyleWiese, Maria, Paul M. D’Agostino, Troco K. Mihali, Michelle C. Moffitt, and Brett A. Neilan. 2010. "Neurotoxic Alkaloids: Saxitoxin and Its Analogs" Marine Drugs 8, no. 7: 2185-2211. https://doi.org/10.3390/md8072185
APA StyleWiese, M., D’Agostino, P. M., Mihali, T. K., Moffitt, M. C., & Neilan, B. A. (2010). Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Marine Drugs, 8(7), 2185-2211. https://doi.org/10.3390/md8072185




