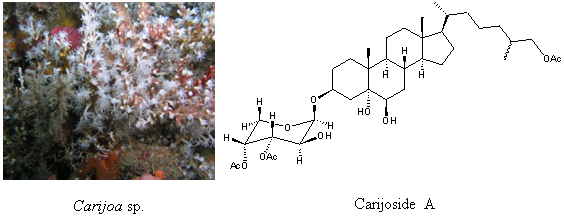
Carijoside A, a Bioactive Sterol Glycoside from an Octocoral Carijoa sp. (Clavulariidae)
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Human Neutrophil Superoxide Anion Generation and Elastase Release
3.5. Cytotoxicity Testing
Acknowledgements
- Samples Availability: Not available.
References and Notes
- Liyanage, GK; Schmitz, FJ. Cytotoxic amides from the octocoral Telesto riisei. J. Nat. Prod 1996, 59, 148–151. [Google Scholar]
- Dorta, E; Díaz-Marrero, AR; Cueto, M; D’Croz, L; Maté, JL; San-Martín, A; Darias, J. Unusual chlorinated pregnanes from the eastern Pacific octocoral Carijoa multiflora. Tetrahedron Lett 2004, 45, 915–918. [Google Scholar]
- Ciavatta, ML; Gresa, MPL; Manzo, E; Gavagnin, M; Wahidulla, S; Cimino, G. New C21 Δ20 pregnanes, inhibitors of mitochondrial respiratory chain, from Indopacific octocoral Carijoa sp. Tetrahedron Lett 2004, 45, 7745–7748. [Google Scholar]
- Maia, LF; Epifanio, RA; Fenical, W. New cytotoxic sterol glycosides from the octocoral Carijoa (Telesto) riisei. J. Nat. Prod 2000, 63, 1427–1430. [Google Scholar]
- Dorta, E; Díaz-Marrero, AR; Cueto, M; D’Croz, L; Maté, JL; Darias, J. Carijenone, a novel class of bicyclic prostanoid from the eastern Pacific octocoral Carijoa multiflora. Org. Lett 2004, 6, 2229–2232. [Google Scholar]
- Diphenylene indonium (DPI) was used as a positive control in anti-inflammatory activity testing. This compound displayed inhibitory effect on superoxide anion generation by human neutrophils (IC50 = 0.8 μM).
- Elastatinal was used as a positive control in anti-inflammatory activity testing. This compound displayed inhibitory effects on elastase release by human neutrophils (IC50 = 30.8 μM).
- Doxorubicin was used as a positive control in cytotoxicity testing. This compound exhibited cytotoxicity toward DLD-1, P388D1, HL-60, and CCRF-CEM tumor cells (ED50 = 0.06, 0.37, 0.08, and 0.02 μg/mL), respectively.
- Fabricius, K; Alderslade, P. Soft Corals and Sea Fans-A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed; Australian Institute of Marine Science: Queensland, Australia, 2001; pp. 70–71. [Google Scholar]
- Hwang, T-L; Li, G-L; Lan, Y-H; Chia, Y-C; Hsieh, P-W; Wu, Y-H; Wu, Y-C. Potent inhibitors of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med 2009, 46, 520–528. [Google Scholar]
- Hwang, T-L; Su, Y-C; Chang, H-L; Leu, Y-L; Chung, P-J; Kuo, L-M; Chang, Y-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res 2009, 50, 1395–1408. [Google Scholar]
- Alley, MC; Scudiero, DA; Monks, A; Hursey, ML; Czerwinski, MJ; Fine, DL; Abbott, BJ; Mayo, JG; Shoemaker, RH; Boyd, MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 1988, 48, 589–601. [Google Scholar]
- Scudiero, DA; Shoemaker, RH; Paull, KD; Monks, A; Tierney, S; Nofziger, TH; Currens, MJ; Seniff, D; Boyd, MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 1988, 48, 4827–4833. [Google Scholar]


| C/H | 1 | 2a | ||
|---|---|---|---|---|
| 1Hb/δ | 13Cc/δ | 1Hd/δ | 13Ce/δ | |
| 1 | 1.42, m; 1.60 m | 32.2 (CH2) | 1.40 m; 1.52 m | 32.3 (CH2) |
| 2 | 1.86 m (2H) | 28.6 (CH2) | 1.86 m | 30.7 (CH2) |
| 3 | 4.07 m | 74.9 (CH) | 4.08 m | 67.6 (CH) |
| 4 | 1.69 m; 2.10 m | 37.3 (CH2) | 1.63 m; 2.08 m | 40.6 (CH2) |
| 5 | 75.9 (C) | 76.0 (C) | ||
| 6 | 3.54 br s | 76.0 (CH) | 3.52 br s | 75.8 (CH) |
| 7 | 1.59 m (2H) | 34.6 (CH2) | 1.60 m | 34.3 (CH2) |
| 8 | 1.74 m | 30.1 (CH) | 1.72 m | 30.2 (CH) |
| 9 | 1.16 m | 45.9 (CH) | 1.25j | 45.7 (CH) |
| 10 | 38.4 (C) | 38.2 (C) | ||
| 11 | 1.38 m (2H) | 21.1 (CH2) | 1.37 m | 21.1 (CH2) |
| 12 | 1.16 m; 2.02 m | 39.9 (CH2) | 1.16 m; 1.98 m | 39.9 (CH2) |
| 13 | 42.7 (C) | 42.7 (C) | ||
| 14 | 1.08 m | 55.9 (CH) | 1.08 m | 55.9 (CH) |
| 15 | 1.08 m; 1.59 m | 24.1 (CH2) | 1.08 m; 1.56 m | 24.1 (CH2) |
| 16 | 1.24 m; 1.77 m | 28.2 (CH2) | 1.22 m; 1.83 m | 28.2 (CH2) |
| 17 | 1.10 m | 56.2 (CH) | 1.11 m | 56.2 (CH) |
| 18 | 0.68 s | 12.1 (CH3) | 0.67 s | 12.1 (CH3) |
| 19 | 1.20 s | 17.0 (CH3) | 1.17 s | 17.0 (CH3) |
| 20 | 1.36 m | 35.7 (CH) | 1.37 m | 36.0 (CH) |
| 21 | 0.90 d (6.0)f | 18.7 (CH3) | 0.92 d (7.0) | 18.6 (CH3) |
| 22 | 1.00 m; 1.33 m | 36.0 (CH2) | 1.00 m; 1.37 m | 35.7 (CH2) |
| 23 | 1.33 m (2H) | 23.3 (CH2) | 1.37 m | 23.3 (CH2) |
| 24 | 1.13 m; 1.27 mg | 33.9 (CH2) | 1.72 m | 33.9 (CH2) |
| 33.7 | 33.7 | |||
| 25 | 1.76 m | 32.5 (CH) | 1.77 m | 32.5 (CH) |
| 32.5 | 32.4 | |||
| 26a | 3.82 dd (10.4, 1.6) | 69.6 (CH2) | 3.82 dd (2.5, 10.5) | 69.9 (CH2) |
| 3.85 dd (10.4, 1.6) | 69.4 | 3.84 dd (2.5, 10.5) | 69.5 | |
| b | 3.92 dd (10.4, 6.0)h | 3.93 dd (6.0, 10.5) | ||
| 3.93 dd (10.4, 6.0)h | 3.95 dd (6.0, 10.5) | |||
| 27 | 0.92 d (6.8) | 16.9 (CH3) | 0.90 d (6.0) | 16.8 (CH3) |
| 0.91 d (6.8) | 16.8 | 0.91 d (6.0) | ||
| 26-OAc | 171.3 (C) | 171.3 (C) | ||
| 2.05 s | 20.9 (CH3)i | 2.05 s | 21.0 (CH3) | |
| 1′ | 5.06 d (4.0) | 97.4 (CH) | ||
| 2′ | 3.93 dd (10.4, 4.0) | 67.3 (CH) | ||
| 3′ | 5.13 dd (10.4, 3.2) | 70.5 (CH) | ||
| 4′ | 5.25 br s | 69.4 (CH) | ||
| 5′ | 3.65 dd (12.8, 2.0); 3.97 mh | 60.9 (CH2) | ||
| 3′-OAc | 170.9 (C) | |||
| 2.07 s | 21.0 (CH3)i | |||
| 4′-OAc | 170.4 (C) | |||
| 2.13 s | 21.0 (CH3)i | |||
| C/H | 1 | 3a | 4a | |||
|---|---|---|---|---|---|---|
| 1H/δ | 13C/δ | 1H/δ | 13C/δ | 1H/δ | 13C/δ | |
| 1′ | 5.06 d (4.0) | 97.4 (CH) | 5.04 d (4.5) | 97.8 (CH) | 5.01 d (3.5) | 97.6 (CH) |
| 2′ | 3.93 dd (10.4, 4.0) | 67.3 (CH) | 3.94 m | 67.3 (CH) | 3.80 dd (3.5, 10.5) | 68.8 (CH) |
| 3′ | 5.13 dd (10.4, 3.2) | 70.5 (CH) | 5.07 dd (3.0, 9.8) | 73.1 (CH) | 3.94 m | 67.8 (CH) |
| 4′ | 5.25 br s | 69.4 (CH) | 4.03 br s | 68.3 (CH) | 5.15 br s | 71.1 (CH) |
| 5′ | 3.65 dd (12.8, 2.0) | 60.9 (CH2) | 3.66 dd (2.0, 12.5) | 62.6 (CH2) | 3.68 br d (11.0) | 60.5 (CH2) |
| 3.97 m | 3.94 m | 3.93 m | ||||
| 3′-OAc | 170.9 (C) | 170.9 (C) | ||||
| 2.07 s | 21.0 (CH3) | 2.17 s | 21.2 (CH3) | |||
| 4′-OAc | 170.4 (C) | 170.9 (C) | ||||
| 2.13 s | 21.0 (CH3) | 2.17 s | 21.2 (CH3) | |||
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, C.-Y.; Hwang, T.-L.; Lin, M.-R.; Chen, Y.-H.; Chang, Y.-C.; Fang, L.-S.; Wang, W.-H.; Wu, Y.-C.; Sung, P.-J. Carijoside A, a Bioactive Sterol Glycoside from an Octocoral Carijoa sp. (Clavulariidae). Mar. Drugs 2010, 8, 2014-2020. https://doi.org/10.3390/md8072014
Liu C-Y, Hwang T-L, Lin M-R, Chen Y-H, Chang Y-C, Fang L-S, Wang W-H, Wu Y-C, Sung P-J. Carijoside A, a Bioactive Sterol Glycoside from an Octocoral Carijoa sp. (Clavulariidae). Marine Drugs. 2010; 8(7):2014-2020. https://doi.org/10.3390/md8072014
Chicago/Turabian StyleLiu, Chih-Yang, Tsong-Long Hwang, Mei-Ru Lin, Yung-Husan Chen, Yu-Chia Chang, Lee-Shing Fang, Wei-Hsien Wang, Yang-Chang Wu, and Ping-Jyun Sung. 2010. "Carijoside A, a Bioactive Sterol Glycoside from an Octocoral Carijoa sp. (Clavulariidae)" Marine Drugs 8, no. 7: 2014-2020. https://doi.org/10.3390/md8072014
APA StyleLiu, C.-Y., Hwang, T.-L., Lin, M.-R., Chen, Y.-H., Chang, Y.-C., Fang, L.-S., Wang, W.-H., Wu, Y.-C., & Sung, P.-J. (2010). Carijoside A, a Bioactive Sterol Glycoside from an Octocoral Carijoa sp. (Clavulariidae). Marine Drugs, 8(7), 2014-2020. https://doi.org/10.3390/md8072014