Chitosan Modification and Pharmaceutical/Biomedical Applications
Abstract
:1. Introduction
2. Structure and Characterization of Chitosan
3. Enzymatic Preparation of LMWC and COS and Their Hypocholesterolemic and Immunoenhancing Effects
3.1. Non-Specific Enzymatic Preparations of LMWCs and Their Hypocholesterolemic Effects
3.1.1. Non-specific enzymatic preparations of LMWCs
3.1.1.1. Cellulase-treated chitosan
3.1.1.2. Lipase-treated chitosan
3.1.1.3. Papain-treated chitosan
3.1.2. The hypocholesterolemic effects of different prepared LMWCs
3.1.3. The hypocholesterolemic mechanism of LMWCs via adsorption, electrostatic force and entrapment
3.2. Enzymatic Preparations of COSs by Specific Chitosanases and Their Immuno-Modulating Effects
3.2.1. Enzymatic Preparations of COSs by fungi specific chitosanases
3.2.2. The immuno-modulating effect and mechanism of COSs
4. Hemostasis Effects of Chitosan and Its Derivatives
4.1. Quality Requirements for Chitosan as a Hemostasis Material
4.2. The Hemostatic Effect of Chitosan and Its Derivatives
4.2.1. The hemostatic effect of chitin and chitosan
4.2.2. The hemostatic effect of chitosan derivatives
4.3. The Hemostatic Mechanism of Chitosan and Its Derivatives
4.3.1. The hemostatic mechanism of chitosan
4.3.2. The hemostatic mechanism of chitosan derivatives
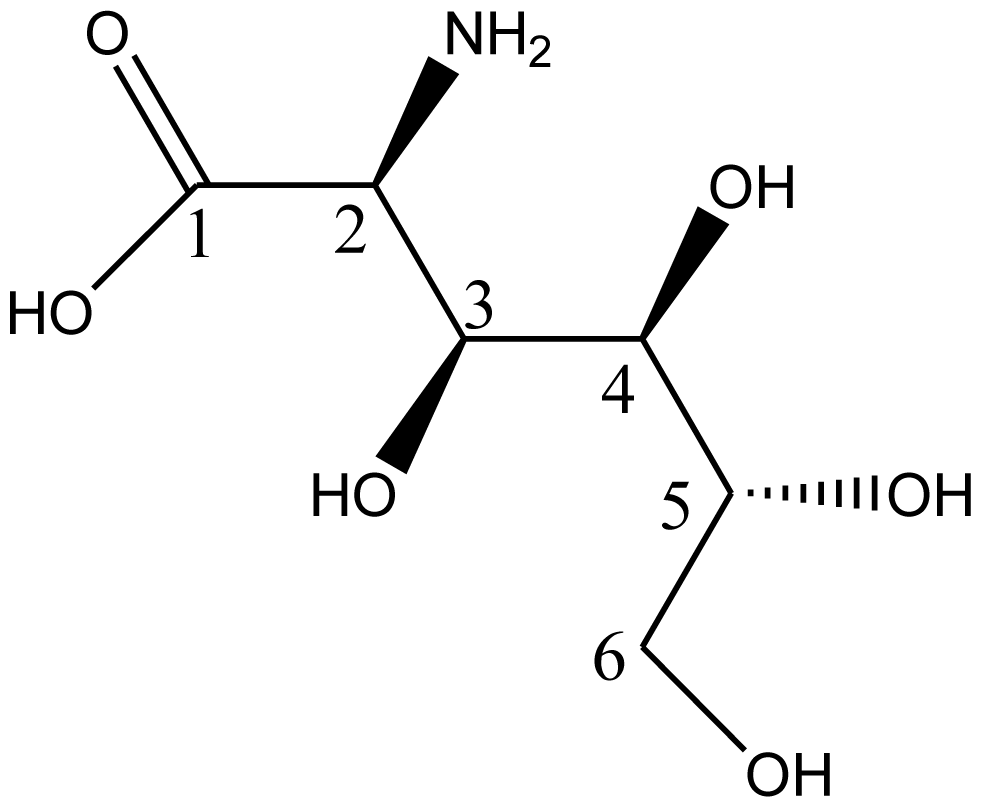
5. Synthesis of D-Glucosaminic Acid by Oxidation from D-Glucosamine: A Useful Metal Chelate for Anticancer and Anti-Diabetic Purposes
5.1. Biological Activities of D-Glusosaminic Acid
5.2. Synthesis of D-Glucosaminic Acid by Oxidation from D-Glucosamine
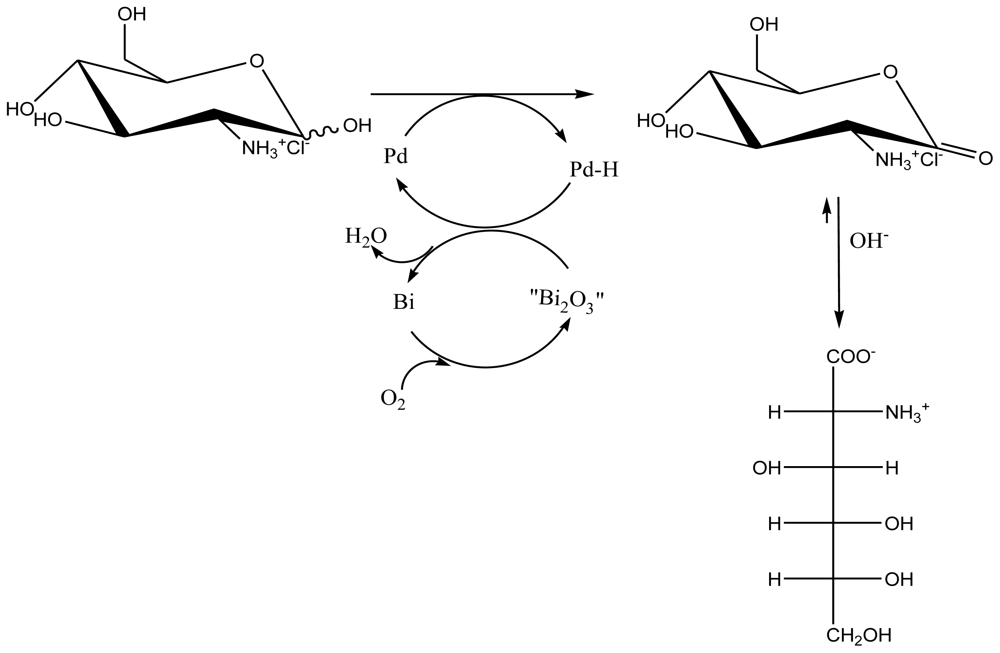
5.2.1. Metallic catalytic oxidation method
5.2.1.1. Oxidations with HgO and Hg(Ac)2
5.2.1.2. Platinum and Palladium Catalyzed Oxidations
5.2.2. Biosynthesis methods
5.2.3. Electrocatalytical oxidation
Acknowledgements
Reference
- Ravi Kumar, MNV; Muzzarelli, RAA; Muzzarelli, C; Sashiwa, H; Domb, AJ. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev 2004, 104, 6017–6084. [Google Scholar]
- Shahidi, F; Arachchi, JKV; Jeon, YJ. Food application of chitin and chitosan. Trends Food Sci. Technol 1999, 10, 37–51. [Google Scholar]
- Dodane, V; Vilivalam, VD. Pharmaceutical applications of chitosan. Pharm. Sci. Technol. Today 1998, 1, 246–253. [Google Scholar]
- Jeon, YJ; Shahidi, F; Kim, SK. Preparation of chitin and chitosan oligomers and thir application in physiological functional foods. Food Rev. Int 2000, 16, 159–176. [Google Scholar]
- Xia, WS. Physiological activities of chitosan and its application in functional foods. J. Chin. Inst. Food Sci. Technol 2003, 3(1), 77–81. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci 2006, 31, 603–632. [Google Scholar]
- Kurita, K. Chemistry and application of chitin and chitosan. Polym. Degrad. Stab 1998, 59(1–3), 117–120. [Google Scholar]
- Park, JK; Shimono, K; Ochiai, N; Shigeru, K; Kurita, M; Ohta, Y; Tanaka, K; Matsuda, H; Kawamukai, M. Purification, characterization, and gene analysis of a chitosanase (ChoA) from Matsuebacter Chitosanotabidus 3001. J. Bacteriol 1999, 181, 6642–6649. [Google Scholar]
- Abdel-Aziz, SM; Mostafa, YA; Moafi, FE. Partial Purification and Some Properties of the Chitosanases Produced by Bacillus Alvei Nrc-14. J. Appl. Sci. Res 2008, 4(10), 1285–1290. [Google Scholar]
- Chen, X; Xia, WS; Yu, X. Purification and characterization of two types of chitosanase from Aspergillus sp. CJ22–326. Food Res. Int 2005, 38, 315–322. [Google Scholar]
- Zhang, XY; Zhang, XK; Kuroiwa, K; Kodaira, R; Shimosaka, M; Okazaki, M; Dai, AL. Purification and characterization of chitosanase and exo-β-D glucosaminidase from a Koji Mold, Aspergillus oryzae IAM2660. Biosci. Biotech. Biochem 2000, 64, 1896–1902. [Google Scholar]
- Ouakfaoui, SE; Asselin, A. Diversity of chitosanase activity in cucumber. Plant Sci 1992, 85, 33–41. [Google Scholar]
- Park, YM; Ghim, SY. Enhancement of the activity and pH-performance of chitosanase from Bacillus cereus strains by DNA shuffling. Biotechnol. Lett 2009, 31, 1463–1467. [Google Scholar]
- Charles-Rodriguez, AV; Mauricio-Benavides, JE; Garza-Garcia, Y; Aguilar, CN; Rodriguez-Herrera, R; Rodriguez, J; Contreras-Esquivel, JC. Chitosanase Production by a New Bacterial Sources. Res. J. Biol. Sci 2008, 2, 957–963. [Google Scholar]
- Yun, C; Amakata, D; Matsuo, Y; Matsuda, H; Kawamukai, M. New chitosan-degrading strains that produce chitosanases similar to ChoA of Mitsuaria chitosanitabida. Appl. Environ. Microbiol 2005, 71, 5138–5144. [Google Scholar]
- Johnsen, MG; Hansen, OC; Stougaard, P. Isolation, characterization and heterologous expression of a novel chitosanase from Janthinobacterium sp. strain 4239. Microb. Cell Fact 2010, 9, 5. [Google Scholar]
- Zhou, W; Yuan, H; Wang, J; Yao, J. Production, purification and characterization of chitosanase produced by Gongronella sp. JG. Lett. Appl. Microbiol 2008, 46, 49–54. [Google Scholar]
- Xie, Y; Wei, Y; Hu, J. Depolymerization of chitosan with a crude cellulase preparation from Aspergillus niger. Appl. Biochem. Biotechnol 2010, 160, 1074–1083. [Google Scholar]
- Roncal, T; Oviedo, A; López de Armentia, I; Fernández, L; Villarán, MC. High yield production of monomer-free chitosan oligosaccharides by pepsin catalyzed hydrolysis of a high deacetylation degree chitosan. Carbohydr. Res 2007, 342, 2750–2756. [Google Scholar]
- Tanabe, T; Morinaga, K; Fukamizo, T; Mitsutomi, M. Novel chitosanase from Streptomyces griseus HUT 6037 with transglycosylation activity. Biosci. Biotechnol. Biochem 2003, 67, 354–364. [Google Scholar]
- Kumar, VAB; Gowda, LR; Tharanathan, RN. A comparative study on depolymerization of chitosan by proteolytic enzymes. Carbohydr. Polym 2004, 58, 275–283. [Google Scholar]
- Pantaleone, D; Yalpani, M; Scollar, M. Unusual susceptibility of chitosan to enzymatic hydrolysis. Carbohydr. Res 1992, 237, 325–332. [Google Scholar]
- Muzzarelli, RAA; Xia, WS; Tomasetti, M; Ilari, P. Depolymerization of chitosan and substituted chitosans with the aid of a wheat germ lipase preparation. Enzyme Microbial. Technol 1995, 17, 541–545. [Google Scholar]
- Lin, SB; Lin, YC; Chen, HH. Low molecular weight chitosan prepared with the aid of cellulase, lysozyme and chitinase: Characterisation and antibacterial activity. Food Chem 2009, 116(1), 47–53. [Google Scholar]
- Qin, CQ; Du, YM. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int. J. Biol. Macromol 2002, 31, 111–117. [Google Scholar]
- Qin, CQ; Zhou, B; Zeng, LT; Zhang, ZH; Liu, Y; Du, YM; Xiao, L. The physicochemical properties and antitumor activity of cellulase-treated chitosan. Food Chem 2004, 84, 107–115. [Google Scholar]
- Liu, YJ; Jiang, Y; Feng, YF; Han, DL. Study on the chitosan hydrolysis catalyzed by special cellulase and preparation of chitooligosaccharide. J. Func. Polym 2005, 18, 325–329. [Google Scholar]
- Liu, J; Xia, WS. Purification and characterization of a bifunctional enzyme with Chitosanase and cellulase activity from commercial cellulase. Biochem. Eng. J 2006, 30, 82–87. [Google Scholar]
- Liu, J; Xia, WS. Characterization of chitosanase from cellulase produced by Trichoderma viride. Chin. J. Biochem. Mol. Biol 2005, 21, 713–716. [Google Scholar]
- Liu, JN; Xia, WS; Zhang, JL. Effects of Chitosans Physico–chemical Properties on Binding Capacities of Lipid and Bile Salts in vitro. Chin. Food Sci 2008, 29(1), 45–49. [Google Scholar]
- Xia, WS; Liu, P; Liu, J. Advance in chitosan hydrolysis by nonspecific cellulases. Bioresour. Technol 2008, 99(15), 6751–6762. [Google Scholar]
- Xia, WS; Liu, P. Columbus, F, Ed.; Characterization and mechanism of chitosan hydrolysis by nonspecific enzymes. In Handbook of Carbohydrate Polymers: Development, Properties and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2010; Volume Chapter 2. [Google Scholar]
- Shin, SS; Lee, YC; Lee, C. The degradation of chitosan with the aid of lipase from Rhizopus japonicus for the production of soluble chitosan. J. Food Biochem 2001, 25, 307–321. [Google Scholar]
- Xia, WS; Lee, DX. Purification and characterization of exo-β-D-glucosaminidase from commercial lipase. Carbohydr. Polym 2008, 74(3), 544–551. [Google Scholar]
- Lee, DX; Xia, WS; Zhang, JL. Enzymatic preparation of chitooligosaccharides by commercial lipase. Food Chem 2008, 111, 291–295. [Google Scholar]
- Muzzarelli, RAA; Tomasetti, M; Ilari, P. Deploymerization of chitosan with the aid of papain. Enzyme Microbial. Technol 1994, 16(2), 110–114. [Google Scholar]
- Terbojevich, M; Cosani, A; Muzzarelli, RAA. Molecular parameters of chitosans depolymerized with the aid of papain. Carbohydr. Polym 1996, 29(1), 63–68. [Google Scholar]
- Su, C; Xia, WS; Yao, HY. The relationship between structure of chitosan and papain activity. J. Wuxi Univ. Light Ind 2002, 21, 112–115. [Google Scholar]
- Sugano, M; Fujikawa, T; Hiratsuji, Y; Nakashima, K; Fukuda, N; Hasegawa, Y. A novel use of chitosan as a hypocholesterolemic agent in rats. Am. J. Clin. Nutr 1980, 33, 787–793. [Google Scholar]
- Maezaki, Y; Tsuji, K; Nakagawa, Y; Kawai, Y; Akimoto, M; Tsugita, T. Hypochloesterolemic effect of chitosan in adult males. Biosci. Biotech. Biochem 1993, 57, 1439–1444. [Google Scholar]
- Fukada, Y; Kimura, K; Ayaki, Y. Effect of chitosan feeding on intestinal bile acid metabolism in rats. Lipids 1991, 26, 395–939. [Google Scholar]
- Ikeda, I; Sugano, M; Yoshida, K; Sasaki, E; Iwamoto, Y; Hatano, K. Effects of chitosan hydrolysates on lipid absorption and on serum and liver lipid concentration in rats. J. Agric. Food Chem 1993, 41, 431–435. [Google Scholar]
- Cho, YI; No, HK; Meyers, SP. Physicochemical characteristics and functional properties of various commercial chitin and chitosan products. J. Agric. Food Chem 1998, 46, 3839–3843. [Google Scholar]
- Zhou, K; Xia, WS; Zhang, C; Yu, L. In vitro binding of bile acids and triglycerides by selected chitosan preparations and their physicochemical properties. LWT–Food Sci. Technol 2006, 39, 1087–1092. [Google Scholar]
- Razdan, A; Pettersson, D. Effect of chitin and chitosan on nutrient digestibility and plasma lipid concentrations in broiler chickens. Br. J. Nutr 1994, 72, 277–288. [Google Scholar]
- Liu, JN; Zhang, JL; Xia, WS. Hypocholesterolemic effects of different chitosan samples in vitro and in vivo. Food Chem 2008, 107, 419–425. [Google Scholar]
- Deuchi, K; Kanauchi, O; Shizukuish, M; Kobayashi, E. Continuous and massive intake of chitosan effects mineral and fat–soluble vitamin status in rats fed on a high–fat diet. Biosci. Biotech. Biochem 1995, 59, 1211–1216. [Google Scholar]
- Li, L; Zhang, JL; Liu, JN; Xia, WS. Effects of chitosan on serum lipid and fat liver. Chin. J. Mar. Drugs 2007, 26(2), 7–9. [Google Scholar]
- Ahmad, A; Sumathi, S; Hameed, B. Residual oil and suspended solid removal using natural adsorbents chitosan, bentonite and activated carbon: a comparative study. Chem. Eng. J 2005, 108(1), 179–185. [Google Scholar]
- Deuchi, K; Kanauchi, O; Shizukuish, M; Kobayashi, E. Continuous and massive intake of chitosan effects mineral and fat–soluble vitamin status in rats fed on a high–fat diet. Biosci. Biotech. Biochem 1995, 59, 1211–1216. [Google Scholar]
- Kittur, FS; Kumar, ABV; Gowda, LR; Tharanathan, RN. Chitosanolysis by a pectinase isozyme of Aspergillus niger—A non-specific activity. Carbohydr. Polym 2003, 53, 191–196. [Google Scholar]
- Jung, WJ; Kuk, JH; Kim, KY; Jung, KC; Park, RD. Purification and characterization of exo-β-D-glucosaminidase from Aspergillus fumigatus S-26. Protein Expression Purif 2006, 45, 125–131. [Google Scholar]
- Kittur, FS; Kumar, ABV; Varadaraj, MC; Tharanathan, RN. Chitooligosaccharides -preparation with the aid of pectinase isozyme from Aspergillus niger and their antibacterial activity. Carbohydr. Res 2005, 340, 1239–1245. [Google Scholar]
- Kittur, FS; Kumar, ABV; Varadaraj, MC; Tharanathan, RN. Low molecular weight chitosans—preparation by depolymerization with Aspergillus niger pectinase, and characterization. Carbohydr. Res 2003, 338, 1283–1290. [Google Scholar]
- Li, SL; Wang, C; Xia, WS. Expression, purification and characterization of exo-β-D-glucosaminidase of Aspergillus sp. CJ22–326 from Escherichia coli. Carbohydr. Res 2009, 344, 1046–1049. [Google Scholar]
- Li, SL; Chen, L; Wang, C; Xia, WS. Expression, purification and characterization of endo–type chitosanase of Aspergillus sp. CJ22–326 from Escherichia coli. Carbohydr. Res 2008, 343(17), 3001–3004. [Google Scholar]
- Shimosaka, M; Kumehara, M; Zhang, XY; Nogawa, M; Okazaki, M. Cloning and characterization of a chitosanase gene from the plant pathogenic fungus Fusarium solani. J. Ferment. Bioeng 1996, 82(5), 426–431. [Google Scholar]
- Shimosaka, M; Sato, K; Nishiwaki, N; Miyazawa, T; Okazaki, M. Analysis of essential carboxylic amino acid residues for catalytic activity of fungal chitosanases by site-directed mutagenesis. J. Biosci. Bioeng 2005, 100(5), 545–550. [Google Scholar]
- Wei, XL; Wang, YF; Xiao, JB; Xia, WS. Separation of chitooligosaccharides and the potent effects on gene expression of cell surface receptor CR3. Int. J. Biol. Macromol 2009, 45, 432–436. [Google Scholar]
- Jeon, YJ; Shahidi, F; Kim, SK. Preparation of chitin and chitosan oligomers and their applications in physiological functional foods. Food Rev. Int 2000, 16, 159–176. [Google Scholar]
- Jeon, YJ; Kim, SK. Potential immuno–stimulating effect of antitumoral fraction of chitosan oligosaccharides. J. Chitin Chitosan 2001, 6, 163–167. [Google Scholar]
- Wu, GJ; Tsai, GJ. Cellulase degradation of shrimp chitosan for the preparation of a water–soluble hydrolysate with immunoactivity. Fish. Sci 2004, 70, 1113–1120. [Google Scholar]
- Feng, J; Zhao, LH; Yu, QQ. Receptor–mediated stimulatory effect of oligochitosan in macrophages. Biochem. Biophys. Res. Commun 2004, 317, 414–420. [Google Scholar]
- Le Cabec, V; Cols, C; Maridonneau–Parini, I. Nonopsonic Phagocytosis of Zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) Involves Distinct Molecular Determinants and is or is Not Coupled with NADPH Oxidase Activation. Infect. Immunol 2000, 68, 4736–4745. [Google Scholar]
- Wei, XL; Wang, YF; Zhu, Q; Xiao, JB; Xia, WS. Effects of chitosan pentamer and chitosan hexamer in vivo and in vitro on gene expression and secretion of cytokines. Food Agric. Immunol 2009, 20(3), 269–280. [Google Scholar]
- Tokoro, A; Suzuki, K; Matsumoto, T; Mikami, T; Suzuki, S; Suzuki, M. Chemotactic response of human neutrophils to N–cetyl chitohexaose in vitro. Microbiol. Immunol 1988, 32, 387. [Google Scholar]
- Tsukada, K; Matsumoto, T; Aizawa, K; Tokoro, A; Naruse, R; Suzuki, S; Suzuki, M. Antimetastatic and growth-inhibitory effects of N-acetylchitohexaose in mice bearing Lewis lung carcinoma. Jpn. J. Cancer Res 1990, 81, 259–265. [Google Scholar]
- Tokoro, A; Kobayashi, M; Tatewaki, N; Suzuki, K; Okawa, Y; Mikami, T; Suzuki, S; Suzuki, M. Protective effect of N-acetyl-chito-hexaose on Listeria monocytogenes infection in mice. Microbiol. Immunol 1989, 33, 357–367. [Google Scholar]
- Wang, FY; He, YS. Study on antitumor effect of water-soluble chitosan. J. Chin. Biochem. Drug 2001, 22, 21–22. [Google Scholar]
- Khor, E. Chitin: Fulfilling a Biomaterials Promise; Elsevier Science: London, UK, 2001. [Google Scholar]
- Jayakumar, R; Nwe, N; Tokura, S; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol 2007, 40, 175–181. [Google Scholar]
- Prashanth, KVH; Tharanathan, RN. Chitin/chitosan: modifications and their unlimited application potential— an overview. Trends Food Sci. Technol 2007, 18, 117–131. [Google Scholar]
- Qian, RQ; Glanville, RW. Methods for purifying chitosan. US Patent 6896809, 2005. [Google Scholar]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxic. Pharm 2010, 56, 290–299. [Google Scholar]
- Baker, S; Wiesmann, WP. Methods of making a chitosan product having an ultra–low endotoxin concentration and the ultra-low endotoxin chitosan product derived therefrom and method of accurately determining inflammatory and anti–inflammatory cellular response to such materials PCT/US2007/023850. 2008.
- Sannan, T; Tsuchida, S; Yoshinaga, S; Seki, M. Purified chitins and production process thereof. US Patent 6989440, 2003. [Google Scholar]
- Peniston, QP; Johnson, EL. Process for demineralization of crustacea shells. US Patent 4066735, 1978. [Google Scholar]
- Percot, A; Viton, C; Domard, A. Characterization of shrimp shell deproteinization. Biomacromolecules 2003, 4(5), 1380–1385. [Google Scholar]
- No, HK; Meyers, JSP; Lee, KS. Isolation and Characterization of Chitin from Crawfish Shell Waste. J. Agric. Food Chem 1989, 37(3), 575–579. [Google Scholar]
- Hung, WM; Bergbauer, KL; Su, KC; Wang, G; Wages, S. Water–soluble chitosan having low endotoxin concentration and methods for making and using the same. US Patent 7125967, 2006. [Google Scholar]
- Cooper, JF. Prince, R, Ed.; Bacterial Endotoxins Test. In Microbiology in Pharmaceutical Manufacturing; PDA: Short Hills, NJ, USA, 2007; Volume Chapter 22. [Google Scholar]
- Shafaei, A; Ashtiani, FZ; Kaghazchi, T. Equilibrium studies of the sorption of Hg(II) ions onto chitosan. Chem. Eng. J 2007, 133, 311–316. [Google Scholar]
- Nguyen, S; Hisiger, S; Jolicoeur, M. Fractionation and characterization of chitosan by analytical SEC and 1H NMR after semi–preparative SEC. Carbohydr. Polym 2009, 75, 636–645. [Google Scholar]
- Lin, CW; Lin, JC. Characterization and blood coagulation evaluation of the water–soluble chitooligosaccharides prepared by a facile fractionation method. Biomacromolecules 2003, 4(6), 1691–1697. [Google Scholar]
- Abdou, ES; Nagy, KSA; Elsabee, MZ. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol 2008, 99, 1359–1367. [Google Scholar]
- Nguyen, S; Winnik, FM; Buschmann, MD. Improved reproducibility in the determination of the molecular weight of chitosan by analytical size exclusion chromatography. Carbohydr. Polym 2009, 75, 528–533. [Google Scholar]
- Trombotto, S; Ladaviere, C; Delolme, F. Chemical Preparation and Structural Characterization of a Homogeneous Series of Chitin/Chitosan Oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar]
- Van De Velde, K; Kiekens, P. Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT–IR spectroscopy and solid state 13C NMR. Carbohydr. Polym 2004, 58, 409–416. [Google Scholar]
- Muzzarelli, RAA. Chitin; Pergamon Press: Oxford, UK, 1977. [Google Scholar]
- Okamoto, Y; Yano, R; Miyatake, K; Tomohiro, I; Shigemasa, Y; Minami, S. Effects of chitin and chitosan on blood coagulation. Carbohydr. Polym 2003, 53, 337–342. [Google Scholar]
- Ishihara, M; Nakanishi, K; Ono, K; Sato, M; Kikuchi, M; Saito, Y; Yura, H; Matsui, T; Hattori, H; Uenoyama, M; Kurita, A. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar]
- Wang, X; Yan, Y; Zhang, R. A comparison of chitosan and collagen sponges as hemostatic dressings. J. Bioact. Compat. Polym 2006, 21, 39–54. [Google Scholar]
- Gu, R; Sun, W; Zhou, H; Wu, Z; Meng, Z; Zhu, X; Tang, Q; Dong, J; Dou, G. The performance of a fly–larva shell–derived chitosan sponge as an absorbable surgical hemostatic agent. Biomaterials 2010, 31(6), 1270–1277. [Google Scholar]
- Owens, M; Senrud, A; Teach, J; Gregory, K. A device for the deployment of internal esophageal chitosan bandage. Gastrointestinal Endoscopy GIE 2006, 63(5), AB237. [Google Scholar]
- Janvikul, W; Uppanan, P; Thavornyutikarn, B; Krewraing, J; Prateepasen, R. In vitro comparative hemostatic studies of chitin, chitosan, and their derivatives. J. Appl. Polym. Sci 2006, 102, 445–451. [Google Scholar]
- Bochicchio, G; Kilbourne, M; Kuehn, R; Keledjian, K; Hess, J; Scalea, T. Use of a modified chitosan dressing in a hypothermic Coagulopathic grade V liver injury model. Am. J. Surg 2009, 198, 617–622. [Google Scholar]
- Ishihara, M; Ono, K; Saito, Y; Yura, H; Hattori, H; Matsui, T; Kurita, A. Photocrosslinkable chitosan: an effective adhesive with surgical applications. Int. Cong. Ser 2001, 1223, 251–257. [Google Scholar]
- Hayashi, T; Matsuyama, T; Hanada, K; Nakanishi, K; Uenoyama, M; Fujita, M; Ishihara, M; Kikuchi, M; Ikeda, T; Tajiri, H. Usefulness of photocrosslinkable chitosan for endoscopic cancer treatment in alimentary tract. J. Biomed. Mater. Res. Part B Appl. Biomater 2004, 71B, 367–372. [Google Scholar]
- Ono, K; Ishihara, M; Ozeki, Y; Deguchi, H; Sato, M; Saito, Y; Yura, H; Sato, M; Kikuchi, M; Kurita, A; Maehara, T. Experimental evaluation of photocrosslinkable chitosan as a biologic adhesive with surgical applications. Surgery 2001, 130, 844–850. [Google Scholar]
- Peng, HT; Shek, PN. Development of in situ–forming hydrogels for hemorrhage control. J. Mater. Sci. Mater. Med 2009, 20, 1753–1762. [Google Scholar]
- Ong, SY; Wu, J; Moochhala, SM; Tan, MH; Lu, J. Development of a chitosan–based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar]
- Hoemann, CD; Hurtig, M; Rossomacha, E; Sun, J; Chevrier, A; Shive, MS; Buschmann, MD. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J. Bone Joint. Surg. Am 2005, 87(12), 2671–2686. [Google Scholar]
- Hoemann, CD; Sun, J; McKee, MD; Chevrier, A; Rossomacha, E; Rivard, GE; Hurtig, M; Buschmann, MD. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthr. Cartilage 2007, 15(1), 78–89. [Google Scholar]
- Iliescu, M; Hoemann, CD; Shive, MS; Chenite, A; Buschmann, MD. Ultrastructure of Hybrid Chitosan-Glycerol Phosphate Blood Clots by Environmental Scanning Electron Microscopy. Microsc. Res. Tech 2008, 71, 236–247. [Google Scholar]
- Buschmann, MD; Hoemann, CD; Hurtig, M; Shive, MS. Williams, RJ, Ed.; Cartilage Repair with Chitosan/Glycerol-Phosphate Stabilised Blood Clots. In Cartilage Repair Strategies; Humana Press: Totowa, NJ, USA, 2007; pp. 85–104. [Google Scholar]
- Ogino, A; Kral, M; Yamashita, M; Nagatsu, M. Effects of low–temperature surface–wave plasma treatment with various gases on surface modification of chitosan. Appl. Surf. Sci 2008, 255, 2347–2352. [Google Scholar]
- Muzzarelli, RAA. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym 2009, 76, 167–182. [Google Scholar]
- Hirsch, JA; Reddy, SA; Capasso, WC; Linfante, I. Non-invasive hemostatic closure devices: “patches and pads”. Tech. Vasc. Intervent. Radiol 2003, 6(2), 92–95. [Google Scholar]
- Muzzarelli, RAA; Muzzarelli, C. Chitosan chemistry: relevance to the biomedical science. Adv. Polym. Sci 2005, 186, 151–209. [Google Scholar]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol 2010, 56(3), 290–299. [Google Scholar]
- Yang, J; Tian, F; Wang, Z; Wang, Q; Zeng, YJ; Chen, SQ. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. Part B Appl. Biomater 2008, 84B, 131–137. [Google Scholar]
- Benesch, J; Tengvall, P. Blood protein adsorption onto chitosan. Biomaterials 2002, 23, 2561–2568. [Google Scholar]
- Wu, Y; Hu, Y; Cai, J; Ma, S; Wang, X. Coagulation property of hyaluronic acid–collagen/chitosan complex film. J. Mater. Sci. Mater. Med 2008, 19, 3621–3629. [Google Scholar]
- Buschmann, MD; Hoemann, CD; Hurting, MB; Shive, MS. Williams, RJ, III, Ed.; Cartilage repair with chitosan–glycerol phosphate–stabilized blood clots. In Cartilage Repair Strategies; Humana Press: Totowa, NJ, USA, 2007; pp. 85–104. [Google Scholar]
- Chou, TC; Fu, E; Wu, CJ; Yeh, JH. Chitosan enhances platelet adhesion and aggregation. Biochem. Biophys. Res. Commun 2003, 302, 480–483. [Google Scholar]
- Klokkevold, PR; Fukayama, H; Sung, EC; Bertolami, CN. The effect of chitosan (poly–N–acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J. Oral. Maxillofac. Surg 1999, 57, 49–52. [Google Scholar]
- Kim, SW; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohydr. Polym 2005, 62, 357–368. [Google Scholar]
- Smith, CJ; Vournakis, JN; Demcheva, M; Fischer, TH. Differential effect of materials for surface hemostasis on red blood cell morphology. Microsc. Res. Tech 2008, 71, 721–729. [Google Scholar]
- Marchand, C; Rivard, GE; Sun, J; Hoemann, CD. Solidification mechanisms of chitosan–glycerol phosphate/blood implant for articular cartilage repair. Osteoarthr Cartilage 2009, 17(7), 953–960. [Google Scholar]
- Wipff, G; Weiner, P; Kollman, P. A molecular–mechanics study of 18–crown–6 and its alkali complexes: an analysis of structural flexibility, ligand specificity, and the macrocyclic effect. J. Am. Chem. Soc 1982, 104(12), 3249–3258. [Google Scholar]
- Varela, O; Nin, AP; De Lederkremer, R. M.A short synthesis of (2S,4S,5R)-4,5,6-trihydroxynorleucine. Tetrahedron Lett 1994, 35(50), 9359–9362. [Google Scholar]
- Gerspacher, M; Rapoport, H. 2–Amino–2–deoxyhexoses as chiral educts for hydroxylated indolizidines. Synthesis of (+)-castanospermine and (+)-6-epicastanospermine. J. Org. Chem 1991, 56(11), 3700–3706. [Google Scholar]
- Bhat, UR; Forsberg, LS; Carlson, RW. Structure of lipid A component of Rhizobium leguminosarum bv phaseoli lipopolysaccharide. Unique nonphosph–orylated lipid A containing 2–amino–2–deoxygluconate galacturonate, and glucosamine. J. Biol. Chem 1994, 269(20), 14402–14410. [Google Scholar]
- Kanekiyo, Y; Aizawa, S; Koshino, N; Funahashi, S. Complexation equilibria of oxy-acid-2-amino-2-deoxy-D-gluconic acid–metal(II) ion ternary systems in aqueous solution as studied by potentiometry. Binding characteristics of borate and germanate. Inorg. Chim. Acta 2000, 298(2), 154–164. [Google Scholar]
- Yu, CJ; Zhao, JJ; Guo, YG; Lu, CL; Ma, X; Gu, ZW. A novel method to prepare water-dispersible magnetic nanoparticles and their biomedical applications: Magnetic capture probe and specific cellular uptake. J. Biomed. Mater. Res. Part A 2008, 87A(2), 364–372. [Google Scholar]
- Junicke, H; Arendt, Y; Steinborn, D. Synthesis and characterization of novel platinum (IV) complexes with functionalized carbohydrates. Inorg. Chim. Acta 2000, 304(2), 224–229. [Google Scholar]
- Ou, SJ; Chen, G; Lin, ZH; Bai, ZP; Duan, CY; Mao, CP. Chromium(III) Complexes of D-Glucosaminic Acid and their Effect on Decreasing Blood Sugar in vivo. Arch. Pharmazie 2006, 339(9), 527–530. [Google Scholar]
- Gu, WX; Xia, WS. Catalytic Synthesis of D-Glucosaminic Acid from D-Glucosamine on Active Charcoal–Supported Pd–Bi Catalysts. J. Carbohydr. Chem 2006, 25(4), 297–301. [Google Scholar]
- Pringsheim, H; Ruschman, G. Preparation of glucosaminic acid. Berichte der Deutschen Chemischen Gesellschaft 1915, 48, 680–682. [Google Scholar]
- Wolfrom, ML; Dacons, JC. The Polymer–homologous Series of Oligosaccharides from Cellulose. J. Am. Chem. Soc 1952, 74, 5331–5333. [Google Scholar]
- De Wilt, HGJ. Oxidation of glucose to gluconic acid. Ind. Eng. Chem. Prod. Res. Dev 1972, 11(4), 370–373. [Google Scholar]
- Smits, PCC; Kuster, BFM; Van Der Wiele, K; Van Der Baan, HS. The Selective Oxidation of Aldoses and Aldonic Acids to 2-Ketoaldonic Acids with Leadmodified Platinum-on-Carbon Catalysts. Carbohydr. Res 1986, 153, 227–235. [Google Scholar]
- Heinen, AW; Peters, JA; Bekkum, HV. The oxidation of fructose on Pt/C catalysts. The formation of D-Threo-hexo-2,5-diulose and the effect of additives. Carbohydr. Res 1997, 304(2), 155–164. [Google Scholar]
- Jenzer, G; Schneider, MS; Wandeler, R; Mallat, T; Baiker, A. Palladium-Catalyzed Oxidation of Octyl Alcohols in “Supercritical” Carbon Dioxide. J. Catal 2001, 199(1), 141–148. [Google Scholar]
- Pezzotti, F; Therisod, H; Therisod, M. Enzymatic synthesis of D-glucosaminic acid from D-glucosamine. Carbohydr. Res 2005, 340, 139–141. [Google Scholar]
- Takiguchi, Y; Shiina, A; Yamaguchi, T. Bioconversion of D-glucosamine to D-glucosaminic acid. Nippon Nogeikagaku Kaishi 2003, 77(6), 576–578. [Google Scholar]
- Glucosaminic acid production by Aeromonas. JP Patent 59011188, 1984.
- D-glucosaminic acid production by a new Aerononas strain. JP Patent 59014785, 1984.
- Producton of D-glucosaminic acid by Aerononas xanthus 102–1. JP Patent 59014786, 1984.
- Production of D-glucosaminic acid by Aeromonas species. JP Patent 59014787, 1984.
- Glucosaminic acid production by Aeromonas. JP Patent 82120774, 1982.
- Yao, SJ; Appleby, AJ; Geisel, A; Cash, HR; Wolfson, SK. Anodic Oxidation of Carbohydrates and their Derivatives in Neutral Saline Solution. Nature 1969, 224(5222), 921–922. [Google Scholar]
- Appleby, AJ; Drunen, CJV. The oxygen evolution reaction on rhodium and iridium electrodes in 85% orthophosphoric acid. J. Electroanal. Chem 1975, 60(1), 101–108. [Google Scholar]
- Tominaga, M; Nagashima, M; Taniguchi, I. Controlled–potential electro- synthesis of glucosaminic acid from glucosamine at a gold electrode. Electrochem. Commun 2007, 9(5), 911–914. [Google Scholar]
| Commercial name | Company | Material and function |
|---|---|---|
| HemCon® | HemCon | Freeze-dried chitosan acetate salt, for emergency use to stop bleeding |
| Chitoflex® | HemCon | Based on chitosan, antibacterial, biocompatible wound dressing designed to be stuffed into a wound track to control moderate to severe bleeding |
| Chitoseal® | Abbott | Based on chitosan, backed with cellulose coating, for bleeding wounds |
| Clo-Sur® | Scion | Based on chitosan, a pressure pad applied topically to accelerate wound healing |
| TraumaStat® | Ore-Medix | Freeze-dried chitosan containing highly porous silica |
| Syvek-Patch® | Marine Polymer Technologies | Made of fully acetylated, high molecular-weight chitin in a crystalline, three-dimensional beta structure array, and isolated from the centric diatom Thalassiosira fluviatilis. It is claimed to be 7 times faster in achieving hemostasis than fibrin glue, because it agglutinates red blood cells, activates platelets whose pseudopodia make robust contact with chitin and promotes fibrin gel formation within the patch, thus acting in a redundant way even on heparinized patients |
| BST-CarGel® | Biosyntech company | chitosan-glycerophosphate hydrogels, a biodegradable gel for cartilage repair |
| Catalyst | Yield |
|---|---|
| PtO2 | 37% |
| Pd | 54–60% |
| Pd-Bi (yield: 70%) | 70% |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahi, T.; Gu, W.; Li, B. Chitosan Modification and Pharmaceutical/Biomedical Applications. Mar. Drugs 2010, 8, 1962-1987. https://doi.org/10.3390/md8071962
Zhang J, Xia W, Liu P, Cheng Q, Tahi T, Gu W, Li B. Chitosan Modification and Pharmaceutical/Biomedical Applications. Marine Drugs. 2010; 8(7):1962-1987. https://doi.org/10.3390/md8071962
Chicago/Turabian StyleZhang, Jiali, Wenshui Xia, Ping Liu, Qinyuan Cheng, Talba Tahi, Wenxiu Gu, and Bo Li. 2010. "Chitosan Modification and Pharmaceutical/Biomedical Applications" Marine Drugs 8, no. 7: 1962-1987. https://doi.org/10.3390/md8071962
APA StyleZhang, J., Xia, W., Liu, P., Cheng, Q., Tahi, T., Gu, W., & Li, B. (2010). Chitosan Modification and Pharmaceutical/Biomedical Applications. Marine Drugs, 8(7), 1962-1987. https://doi.org/10.3390/md8071962




