Production of Metabolites as Bacterial Responses to the Marine Environment
Abstract
:1. Introduction
2. Adaptive Mechanisms
2.1. Adaptation at the cellular membrane: Production of specialized lipids
2.2. Production of exopolymers and biosurfactants
2.3. Biofilm formation and quorum sensing
3. Production of Secondary Metabolites
3.1. Terpenes and terpenoids
3.2. Production of siderophores
4. Final Remarks
Acknowledgements
References and Notes
- Macleod, RA. Question of existence of specific marine bacteria. Bacteriol Rev 1965, 29, 9–23. [Google Scholar]
- Bowers, K; Mesbah, N; Wiegel, J. Biodiversity of poly-extremophilic Bacteria: Does combining the extremes of high salt, alkaline pH and elevated temperature approach a physicochemical boundary for life. Saline Syst 2009, 5, 9. [Google Scholar]
- Wai, SN; Mizunoe, Y; Yoshida, S. How Vibrio cholerae survive during starvation. FEMS Microbiol Lett 1999, 180, 123–131. [Google Scholar]
- Delong, EF; Yayanos, AA. Properties of the glucose-transport system in some deep-sea bacteria. Appl Environ Microbiol 1987, 53, 527–532. [Google Scholar]
- Reichelt, JL; Nealson, K; Hastings, JW. The specificity of symbiosis: Pony fish and luminescent bacteria. Arch Microbiol 1977, 112, 157–161. [Google Scholar]
- Jensen, PR; Fenical, W. Marine bacterial diversity as a resource for novel microbial products. J Ind Microb Biotechnol 1996, 17, 346–351. [Google Scholar]
- Valentine, DL. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nature Rev Microbiol 2007, 5, 316–323. [Google Scholar]
- Okazaki, T; Kitahara, T; Okami, Y. Studies on marine microorganisms .4. New antibiotic Ss-228 Y produced by Chainia isolated from shallow sea mud. J Antibiot 1975, 28, 176–184. [Google Scholar]
- Proksch, P; Edrada, RA; Ebel, R. Drugs from the seas - current status and microbiological implications. Appl Microbiol Biotechnol 2002, 59, 125–134. [Google Scholar]
- Williams, PG. Panning for chemical gold: marine bacteria as a source of new therapeutics. Trends Biotechnol 2009, 27, 45–52. [Google Scholar]
- Bull, AT; Stach, JEM. Marine actinobacteria: new opportunities for natural product search and discovery. Trends Microbiol 2007, 15, 491–499. [Google Scholar]
- Sikkema, J; Debont, JAM; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 1995, 59, 201–222. [Google Scholar]
- Heipieper, HJ; Weber, FJ; Sikkema, J; Keweloh, H; Debont, JAM. Mechanisms of resistance of whole cells to toxic organic-solvents. Trends Biotechnol 1994, 12, 409–415. [Google Scholar]
- Weber, FJ; deBont, JAM. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim Biophys Acta Rev Biomembr 1996, 1286, 225–245. [Google Scholar]
- Sinensky, M. Homeoviscous adaptation–homeostatic process that regulates viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci 1974, 71, 522–525. [Google Scholar]
- Segura, A; Duque, E; Mosqueda, G; Ramos, JL; Junker, F. Multiple responses of Gram-negative bacteria to organic solvents. Environ Microbiol 1999, 1, 191–198. [Google Scholar]
- Sardessai, Y; Bhosle, S. Tolerance of bacteria to organic solvents. Res Microbiol 2002, 153, 263–268. [Google Scholar]
- de Carvalho, C; Wick, LY; Heipieper, HJ. Cell wall adaptations of planktonic and biofilm Rhodococcus erythropolis cells to growth on C5 to C16 n-alkane hydrocarbons. Appl Microbiol Biotechnol 2009, 82, 311–320. [Google Scholar]
- Vreeland, RH; Anderson, R; Murray, RG. Cell wall and phospholipid composition and their contribution to the salt tolerance of Halomonas elongata. J Bacteriol 1984, 160, 879–883. [Google Scholar]
- Russell, NJ. Adaptive modifications in membranes of halotolerant and halophilic microorganisms. J Bioenerg Biomembr 1989, 21, 93–113. [Google Scholar]
- Leone, S; Silipo, A; Nazarenko, EL; Lanzetta, R; Parrilli, M; Molinaro, A. Molecular structure of endotoxins from gram-negative marine bacteria: An update. Mar Drugs 2007, 5, 85–112. [Google Scholar]
- Brown, GR; Sutcliffe, IC; Cummings, SP. Combined solvent and water activity stresses on turgor regulation and membrane adaptation in Oceanimonas baumannii ATCC 700832. Anton Leeuw 2003, 83, 275–283. [Google Scholar]
- Montgomery, MT; Boyd, TJ; Osburn, CL; Plummer, RE; Masutani, SM; Coffin, RB. Desalination technology waste streams: Effect of pH and salinity on metabolism of marine microbial assemblages. Desalination 2009, 249, 861–864. [Google Scholar]
- de Carvalho, C; da Fonseca, MMR. Degradation of hydrocarbons and alcohols at different temperatures and salinities by Rhodococcus erythropolis DCL 14. FEMS Microbiol Ecol 2005, 51, 389–399. [Google Scholar]
- Yano, Y; Nakayama, A; Yoshida, K. Distribution of polyunsaturated fatty acids in bacteria present in intestines of deep-sea fish and shallow-sea poikilothermic animals. Appl Environ Microbiol 1997, 63, 2572–2577. [Google Scholar]
- Valentine, RC; Valentine, DL. Omega-3 fatty acids in cellular membranes: a unified concept. Prog Lipid Res 2004, 43, 383–402. [Google Scholar]
- Hayashida-Soiza, G; Uchida, A; Mori, N; Kuwahara, Y; Ishida, Y. Purification and characterization of antibacterial substances produced by a marine bacterium Pseudoalteromonas haloplanktis strain. J Appl Microbiol 2008, 105, 1672–1677. [Google Scholar]
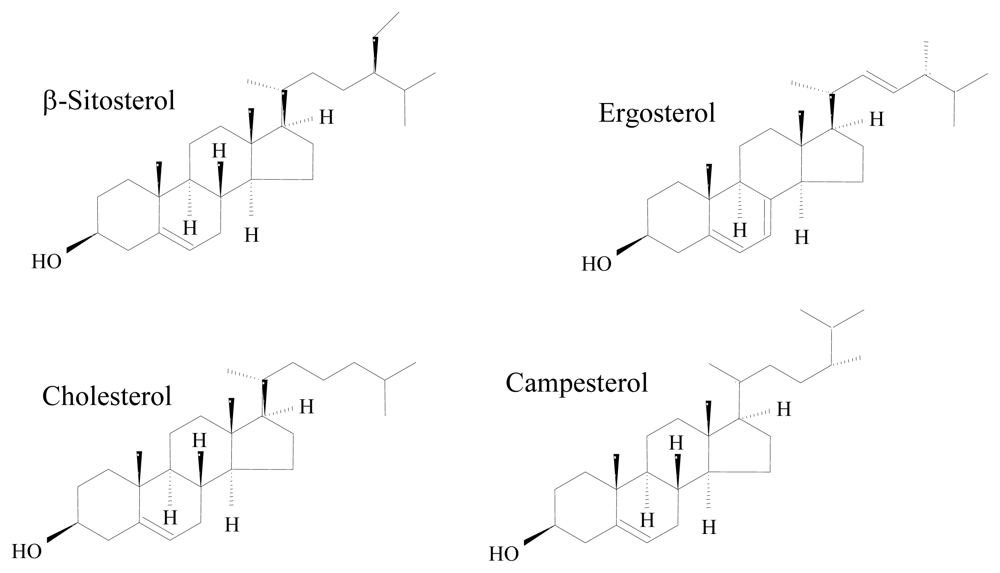
- Baker, BJ; Kerr, RG. Biosynthesis of marine sterols. Top Curr Chem 1993, 167, 1–31. [Google Scholar]
- Hartmann, MA. Plant sterols and the membrane environment. Trends Plant Sci 1998, 3, 170–175. [Google Scholar]
- Volkman, JK. Sterols in microorganisms. Appl Microbiol Biotechnol 2003, 60, 495–506. [Google Scholar]
- Fabregas, J; Aran, J; Morales, ED; Lamela, T; Otero, A. Modification of sterol concentration in marine microalgae. Phytochemistry 1997, 46, 1189–1191. [Google Scholar]
- Kannenberg, EL; Poralla, K. Hopanoid biosynthesis and function in bacteria. Naturwissenschaften 1999, 86, 168–176. [Google Scholar]
- Schouten, S; Bowman, JP; Rijpstra, WIC; Damste, JSS. Sterols in a psychrophilic methanotroph Methylosphaera hansonii. FEMS Microbiol Lett 2000, 186, 193–195. [Google Scholar]
- Poralla, K; Kannenberg, E; Blume, A. A Glycolipid containing hopane isolated from the acidophilic, thermophilic Bacillus acidocaldarius, has a cholesterol-like function in membranes. Febs Lett 1980, 113, 107–110. [Google Scholar]
- Joyeux, C; Fouchard, S; Llopiz, P; Neunlist, S. Influence of the temperature and the growth phase on the hopanoids and fatty acids content of Frateuria aurantia (DSMZ 6220. Fems Microbiol. Ecol 2004, 47, 371–379. [Google Scholar]
- Schmidt, EW. From chemical structure to environmental biosynthetic pathways: navigating marine invertebrate-bacteria associations. Trends Biotechnol 2005, 23, 437–440. [Google Scholar]
- Pearson, A; Page, SRF; Jorgenson, TL; Fischer, WW; Higgins, MB. Novel hopanoid cyclases from the environment. Environ Microbiol 2007, 9, 2175–2188. [Google Scholar]
- Talbot, HM; Summons, RE; Jahnke, LL; Cockell, CS; Rohmer, M; Farrimond, P. Cyanobacterial bacteriohopanepolyol signatures from cultures and natural environmental settings. Org Geochem 2008, 39, 232–263. [Google Scholar]
- Blumenberg, M; Oppermann, BI; Guyoneaud, R; Michaelis, W. Hopanoid production by Desulfovibrio bastinii isolated from oilfield formation water. FEMS Microbiol Lett 2009, 293, 73–78. [Google Scholar]
- Flemming, HC; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs) - Part I: structural and ecological aspects. Water Sci Technol 2001, 43, 1–8. [Google Scholar]
- van der Merwe, P; Lannuzel, D; Nichols, CAM; Meiners, K; Heil, P; Norman, L; Thomas, DN; Bowie, AR. Biogeochemical observations during the winter-spring transition in East Antarctic sea ice: evidence of iron and exopolysaccharide controls. Mar Chem 2009, 115, 163–175. [Google Scholar]
- Gutiérrez, T; Shimmield, T; Haidon, C; Black, K; Green, DH. Emulsifying and metal ion binding activity of a glycoprotein exopolymer produced by Pseudoalteromonas sp. strain TG12. Appl Environ Microbiol 2008, 74, 4867–4876. [Google Scholar]
- Harder, W; Dijkhuizen, L. Physiological-responses to nutrient limitation. Annu Rev Microbiol 1983, 37, 1–23. [Google Scholar]
- Manca, MCLL; Improta, R; Esposito, A; Gambacorta, A; Nicolaus, B. Chemical composition of two exopolysaccharides from Bacillus thermoantarcticus. Appl Environ Microbiol 1996, 62, 3265–3269. [Google Scholar]
- Sutherland, IW. Bacterial exopolysaccharides. Adv Microb Physiol 1972, 8, 143–213. [Google Scholar]
- Debnath, M; Paul, AK; Bisen, PS. Natural bioactive compounds and biotechnological potential of marine bacteria. Curr Pharm Biotechnol 2007, 8, 253–260. [Google Scholar]
- Chi, Z; Fang, Y. Exopolysaccharides from marine bacteria. J Ocean Univ Chin 2005, 4, 67–74. [Google Scholar]
- Yakimov, MM; Golyshin, PN; Lang, S; Wagner, F; Moore, E; Abraham, WR; Timmis, KN. New moderate halophilic marine strain MM1 produces novel class of biosurfactants, abstr. 182. Abstracts of the First International Congress on Extremophiles, Estoril, Portugal; 1996. [Google Scholar]
- Pfiffner, SM; McInerney, MJ; Jenneman, GE; Knapp, RM. Isolation of halotolerant, thermotolerant, facultative polymer-producing bacteria and characterization of the exopolymer. Appl Environ Microbiol 1986, 51, 1224–1229. [Google Scholar]
- Decho, AW; Herndl, GJ. Microbial activities and the transformation of organic-matter within mucilaginous material. Sci Total Environ 1995, 165, 33–42. [Google Scholar]
- Colliec-Jouault, S; Zanchetta, P; Helley, D; Ratiskol, J; Sinquin, C; Fischer, AM; Guezennec, J. Exopolysaccharides produced by bacteria isolated from deep-sea hydrothermal vents: new agents with therapeutic potential. Pathol Biol 2004, 52, 127–130. [Google Scholar]
- Das, P; Mukherjee, S; Sen, R. Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J Appl Microbiol 2008, 104, 1675–1684. [Google Scholar]
- Egan, S; James, S; Kjelleberg, S. Identification and characterization of a putative transcriptional regulator controlling the expression of fouling inhibitors in Pseudoalteromonas tunicata. Appl Environ Microbiol 2002, 68, 372–378. [Google Scholar]
- Sangnoi, Y; Srisukchayakul, P; Arunpairojana, V; Kanjana-Opas, A. Diversity of marine gliding bacteria in Thailand and their cytotoxicity. Electron J Biotechnol 2009, 12. [Google Scholar] [CrossRef]
- Gutiérrez, T; Mulloy, B; Bavington, C; Black, K; Green, DH. Partial purification and chemical characterization of a glycoprotein (putative hydrocolloid) emulsifier produced by a marine bacterium. Antarctobacter Appl Microbiol Biotechnol 2007, 76, 1017–1026. [Google Scholar]
- Quesada, E; Bejar, V; Calvo, C. Exopolysaccharide production by Volcaniella eurihalina. Experientia 1993, 49, 1037–1041. [Google Scholar]
- Gutiérrez, T; Mulloy, B; Black, K; Green, DH. Glycoprotein emulsifiers from two marine Halomonas species: chemical and physical characterization. J Appl Microbiol 2007, 103, 1716–1727. [Google Scholar]
- Costerton, JW. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev 1974, 38, 87–110. [Google Scholar]
- Decho, AW. Microbial biofilms in intertidal systems: an overview. Cont Shelf Res 2000, 20, 1257–1273. [Google Scholar]
- Matz, C; Webb, JS; Schupp, PJ; Phang, SY; Penesyan, A; Egan, S; Steinberg, P; Kjelleberg, S. Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS ONE 2008, 3, e2744. [Google Scholar]
- Yildiz, FH; Visick, KL. Vibrio biofilms: so much the same yet so different. Trends Microbiol 2009, 17, 109–118. [Google Scholar]
- Fuqua, C; Winans, SC; Greenberg, EP. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum sensing transcriptional regulators. Annu Rev Microbiol 1996, 50, 727–751. [Google Scholar]
- Lyon, P. From quorum to cooperation: lessons from bacterial sociality for evolutionary theory. Stud Hist Phil Biol Biomed Sci 2007, 38, 820–833. [Google Scholar]
- Wilson, BA; Salyers, AA. Is the evolution of bacterial pathogens an out-of-body experience. Trends Microbiol 2003, 11, 347–350. [Google Scholar]
- Milton, DL. Quorum sensing in vibrios: Complexity for diversification. Int J Med Microbiol 2006, 296, 61–71. [Google Scholar]
- Teasdale, ME; Liu, JY; Wallace, J; Akhlaghi, F; Rowley, DC. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in Gram-negative bacteria. Appl Environ Microbiol 2009, 75, 567–572. [Google Scholar]
- de Carvalho, C; da Fonseca, MMR. Biotransformation of terpenes. Biotechnol Adv 2006, 24, 134–142. [Google Scholar]
- Gershenzon, J; Dudareva, N. The function of terpene natural products in the natural world. Nat Chem Biol 2007, 3, 408–414. [Google Scholar]
- Courington, DP; Goodwin, TW. A survey of the pigments of a number of chromogenic marine bacteria, with special reference to the carotenoids. J Bacteriol 1955, 70, 568–571. [Google Scholar]
- Sabehi, G; Loy, A; Jung, K.-H; Partha, R; Spudich, JL; Isaacson, T; Hirschberg, J; Wagner, M; Béjà, O. New insights into metabolic properties of marine bacteria encoding proteorhodopsins. PLoS Biol 2005, 3, e273. [Google Scholar]
- Lewin, RA. Saprospira grandis: A flexibacterium that can catch bacterial prey by “ixotrophy”. Microb Ecol 1997, 34, 232–236. [Google Scholar]
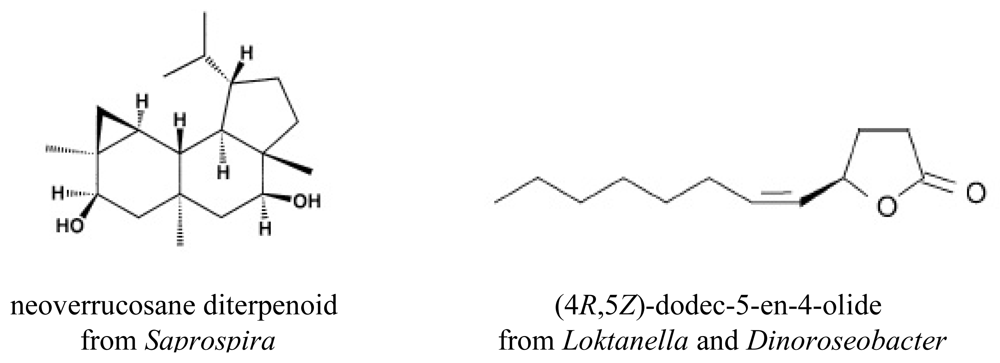
- Mincer, TJ; Spyere, A; Jensen, PR; Fenical, W. Phylogenetic analyses and diterpenoid production by marine bacteria of the Genus. Saprospira Curr Microbiol 2004, 49, 300–307. [Google Scholar]
- Spyere, A; Rowley, DC; Jensen, PR; Fenical, W. New neoverrucosane diterpenoids produced by the marine gliding bacterium Saprospira grandis. J Nat Prod 2003, 66, 818–822. [Google Scholar]
- Hefter, J; Richnow, HH; Fischer, U; Trendel, JM; Michaelis, W. (−)-Verrucosan-2-beta-ol from the phototrophic bacterium Chloroflexus aurantiacus–1st report of a verrucosan-type diterpenoid from a prokaryote. J Gen Microbiol 1993, 139, 2757–2761. [Google Scholar]
- Schulz, S; Dickschat, JS. Bacterial volatiles: the smell of small organisms. Nat Prod Rep 2007, 24, 814–842. [Google Scholar]
- Wu, J; Luther, GW, III. Spatial and temporal distribution of iron in the surface water of the northwestern Atlantic Ocean. Geochim Cosmochim Acta 1996, 60, 2729. [Google Scholar]
- De Baar, HJW; De Jong, JTM. Distribution, sources and sinks of iron in seawater. In The Biogeochemistry of Iron in Seawater; Turner, DR, Hunter, KA, Eds.; Wiley: New York, NY, USA, 2001; Chapter 5; pp. 123–253. [Google Scholar]
- Measures, CI; Landing, WM; Brown, MT; Buck, CS. High-resolution Al and Fe data from the Atlantic Ocean CLIVAR-CO2 repeat hydrography A16N transect: extensive linkages between atmospheric dust and upper ocean geochemistry. Global Biogeochem Cycles 2008, 22, GB1005. [Google Scholar]
- Neilands, JB. Siderophores of bacteria and fungi. Microbiol Sci 1984, 1, 9–14. [Google Scholar]
- Borer, PM; Sulzberger, B; Reichard, P; Kraemer, SM. Effect of siderophores on the light-induced dissolution of colloidal iron(III) (hydr)oxides. Mar Chem 2005, 93, 179–193. [Google Scholar]
- Rastogi, RP; Sinha, RP. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol Adv 2009, 27, 521–539. [Google Scholar]
- Mawji, E; Gledhill, M; Milton, JA; Tarran, GA; Ussher, S; Thompson, A; Wolff, GA; Worsfold, PJ; Achterberg, EP. Hydroxamate siderophores: occurrence and importance in the Atlantic Ocean. Environ Sci Technol 2008, 42, 8675–8680. [Google Scholar]
- Albrecht-Gary, AM; Crumbliss, AL. Coordination chemistry of siderophores: thermodynamics and kinetics of iron chelation and release. Met Ions Biol Syst 1998, 35, 239–327. [Google Scholar]
- Borer, PM; Sulzberger, B; Reichard, P; Kraemer, SM. Effect of siderophores on the light-induced dissolution of colloidal iron(III) (hydr)oxides. Mar Chem 2005, 93, 179–193. [Google Scholar]
- Zhang, G; Amin, SA; Küpper, FC; Holt, PD; Carrano, CJ; Butler, A. Ferric stability constants of representative marine siderophores: marinobactins, aquachelins, and petrobactin. Inorg Chem 2009, 48, 11466–11473. [Google Scholar]
- Becker, JO; Messens, E; Hedges, RW. The influence of agrobactin on the uptake of ferric iron by plants. Fems Microbiol Ecol 1985, 31, 171–175. [Google Scholar]
- Crowley, DE; Reid, CPP; Szaniszlo, PJ. Utilization of microbial siderophores in iron acquisition by Oat. Plant Physiol 1988, 87, 680–685. [Google Scholar]
- Shenker, M; Oliver, I; Helmann, M; Hadar, Y; Chen, Y. Utilization by tomatoes of iron mediated by a siderophore produced by Rhizopus arrhizus. J Plant Nutr 1992, 15, 2173–2182. [Google Scholar]
- Sharma, A; Johri, BN. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiol Res 2003, 158, 243–248. [Google Scholar]
- Siebner-Freibach, H; Hadar, Y; Chen, Y. Siderophores sorbed on Ca-montmorillonite as an iron source for plants. Plant Soil 2003, 251, 115–124. [Google Scholar]
- Siebner-Freibach, H; Hadar, Y; Chen, Y. Interaction of iron chelating agents with clay minerals. Soil Sci Soc Am J 2004, 68, 470–480. [Google Scholar]
- Chincholkar, SB; Chaudhari, BL; Rane, MR. Microbial siderophores in human and plant health-care. In Microbial Siderophores; Springer: New York, NY, USA, 2007; pp. 205–217. [Google Scholar]
- Crosa, JH. Deferration using anguibactin siderophore. 1995. US Patent 5393777.
- Lindsey, WT; Olin, BR. Deferasirox for transfusion-related iron overload: A clinical review. Clin Ther 2007, 29, 2154–2166. [Google Scholar]
- Cappellini, MD; Pattoneri, P. Oral iron chelators. Annu Rev Med 2009, 60, 25–38. [Google Scholar]
- Taher, A; El-Beshlawy, A; Elalfy, MS; Al Zir, K; Daar, S; Habr, D; Kriemler-Krahn, U; Hmissi, A; Al Jefri, A. Efficacy and safety of Deferasirox, an oral iron chelator, in heavily iron-overloaded patients with beta-thalassaemia: the ESCALATOR study. Eur J Haematol 2009, 82, 458–465. [Google Scholar]
- Manning, T; Kean, G; Thomas, J; Thomas, K; Corbitt, M; Gosnell, D; Ware, R; Fulp, S; Jarrard, J; Phillips, D. Iron chelators in medicinal applications–chemical equilibrium considerations in pharmaceutical activity. Curr Med Chem 2009, 16, 2416–2429. [Google Scholar]
- Pahl, PMB; Horwitz, MA; Horwitz, KB; Horwitz, LD. Desferri-exochelin induces death by apoptosis in human breast cancer cells but does not kill normal breast cells. Breast Cancer Res Treat 2001, 69, 69–79. [Google Scholar]
- Rotheneder, A; Fritsche, G; Heinisch, L; Mollmann, U; Heggemann, S; Larcher, C; Weiss, G. Effects of synthetic siderophores on proliferation of Plasmodium falciparum in infected human erythrocytes. Antimicrob Agents Chemother 2002, 46, 2010–2013. [Google Scholar]
- Budzikiewicz, H. Siderophore-antibiotic conjugates used as Trojan Horses against Pseudomonas aeruginosa. Curr Top Med Chem 2001, 1, 73–82. [Google Scholar]
- Voest, EE; Vreugdenhil, G; Marx, JJM. Iron-chelating agents in non-iron overload conditions. Ann Intern Med 1994, 120, 490–499. [Google Scholar]
- Sandy, M; Butler, A. Microbial iron acquisition: marine and terrestrial siderophores. Chem Rev 2009, 109, 4580–4595. [Google Scholar]
- Guan, LL; Onuki, H; Kamino, K. Bacterial growth stimulation with exogenous siderophore and synthetic N-acyl homoserine lactone autoinducers under iron-limited and low-nutrient conditions. Appl Environ Microbiol 2000, 66, 2797–2803. [Google Scholar]
- Guan, LL; Kanoh, K; Kamino, K. Effect of exogenous siderophores on iron uptake activity of marine bacteria under iron-limited conditions. Appl Environ Microbiol 2001, 67, 1710–1717. [Google Scholar]
- Lamont, IL; Beare, PA; Ochsner, U; Vasil, AI; Vasil, ML. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc Natl Acad Sci 2002, 99, 7072–7077. [Google Scholar]
- Mullen, LGC; Czerwinski, K. Complexation of uranium(VI) with the siderophore desferrioxamine B. J Radioanal Nucl Chem 2007, 273, 683–688. [Google Scholar]
- Amin, SA; Kupper, FC; Green, DH; Harris, WR; Carrano, CJ. Boron binding by a siderophore isolated from marine bacteria associated with the toxic dinoflagellate Gymnodinium catenatum. J Am Chem Soc 2007, 129, 478–479. [Google Scholar]
- Harris, WR; Amin, SA; Kupper, FC; Green, DH; Carrano, CJ. Borate binding to siderophores: Structure and stability. J Am Chem Soc 2007, 129, 12263–12271. [Google Scholar]
- Carrano, CJ; Schellenberg, S; Amin, SA; Green, DH; Kupper, FC. Boron and marine life: a new look at an enigmatic bioelement. Mar Biotechnol 2009, 11, 431–440. [Google Scholar]
- Zurcher, S; Wackerlin, D; Bethuel, Y; Malisova, B; Textor, M; Tosatti, S; Gademann, K. Biomimetic surface modifications based on the cyanobacterial iron chelator anachelin. J Am Chem Soc 2006, 128, 1064–1065. [Google Scholar]
- Martinez, JS; Carter-Franklin, JN; Mann, EL; Martin, JD; Haygood, MG; Butler, A. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc Natl Acad Sci 2003, 100, 3754–3759. [Google Scholar]
- Vraspir, JM; Butler, A. Chemistry of marine ligands and siderophores. Annu Rev Mar Sci 2009, 1, 43–63. [Google Scholar]
- Mawji, E; Gledhill, M; Milton, JA; Tarran, GA; Ussher, S; Thompson, A; Wolff, GA; Worsfold, PJ; Achterberg, EP. Hydroxamate siderophores: occurrence and importance in the Atlantic Ocean. Environ Sci Technol 2008, 42, 8675–8680. [Google Scholar]
- Barbeau, K; Rue, EL; Trick, CG; Bruland, KT; Butler, A. Photochemical reactivity of siderophores produced by marine heterotrophic bacteria and cyanobacteria based on characteristic Fe(III) binding groups. Limnol Oceanogr 2003, 48, 1069–1078. [Google Scholar]
- Yoshida, T; Hayashi, K; Ohmoto, H. Dissolution of iron hydroxides by marine bacterial siderophore. Chem Geol 2002, 184, 1–9. [Google Scholar]
- Butler, A; Theisen, RM. Iron(III)-siderophore coordination chemistry: Reactivity of marine siderophores. Coord Chem Rev 2010, 254, 288–296. [Google Scholar]
- Homann, VV; Edwards, KJ; Webb, EA; Butler, A. Siderophores of Marinobacter aquaeolei: petrobactin and its sulfonated derivatives. Biometals 2009, 22, 565–571. [Google Scholar]
- Stackebrandt, E; Murray, RGE; Trüper, HG. Proteobacteria classis nov., a name for the phylogenetic taxon that includes the “Purple Bacteria and Their Relatives”. Int J Syst Bacteriol 1988, 38, 321–325. [Google Scholar]
- Armstrong, JE; Van Baalen, C. Iron transport in microalgae: the isolation and biological activity of a hydroxamate siderophore from the blue-green alga Agmenellum quadruplicatum. J Gen Microbiol 1979, 111, 253–262. [Google Scholar]
- Wilhelm, SW; Trick, CG. Iron-limited growth of cyanobacteria: multiple siderophore production is a common response. Limnol Oceanogr 1994, 39, 1979–1984. [Google Scholar]
- Ito, Y; Butler, A. Structure of synechobactins, new siderophores of the marine cyanobacterium Synechococcus sp. PCC 7002. Limnol. Oceanogr 2005, 50, 1918–1923. [Google Scholar]
- Itou, Y; Okada, S; Murakami, M. Two structural isomeric siderophores from the freshwater cyanobacterium Anabaena cylindrica (NIES-19). Tetrahedron 2001, 57, 9093–9099. [Google Scholar]
- Ito, Y; Ishida, K; Okada, S; Murakami, M. The absolute stereochemistry of anachelins, siderophores from the cyanobacterium Anabaena cylindrica. Tetrahedron 2004, 60, 9075–9080. [Google Scholar]
- Hopkinson, BM; Morel, FMM. The role of siderophores in iron acquisition by photosynthetic marine microorganisms. Biometals 2009, 22, 659–669. [Google Scholar]
- Brown, CM; Trick, CG. Response of the cyanobacterium, Oscillatoria tenuis, to low iron environments: the effect on growth rate and evidence for siderophore production. Arch Microbiol 1992, 157, 349–354. [Google Scholar]
- Li, J; Chi, Z. Siderophores from marine microorganisms and their applications. J Ocean Univ Chin 2004, 3, 40–47. [Google Scholar]
- Holinsworth, B; Martin, J. Siderophore production by marine-derived fungi. BioMetals 2009, 22, 625–632. [Google Scholar]
- Scanlan, DJ; Ostrowski, M; Mazard, S; Dufresne, A; Garczarek, L; Hess, WR; Post, AF; Hagemann, M; Paulsen, I; Partensky, F. Ecological genomics of marine Picocyanobacteria. Microbiol Mol Biol Rev 2009, 73, 249–299. [Google Scholar]
- Capon, RJ; Stewart, M; Ratnayake, R; Lacey, E; Gill, JH. Citromycetins and bilains A-C: new aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J Nat Prod 2007, 70, 1746–1752. [Google Scholar]
- Haygood, MG; Holt, PD; Butler, A. Aerobactin production by a planktonic marine Vibrio sp. Limnol Oceanogr 1993, 38, 1091–1097. [Google Scholar]
- Martinez, JS; Carter-Franklin, JN; Mann, EL; Martin, JD; Haygood, MG; Butler, A. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc Natl Acad Sci 2003, 100, 3754–3759. [Google Scholar]
- Jalal, MAF; Hossain, MB; Vanderhelm, D; Sandersloehr, J; Actis, LA; Crosa, JH. Structure of anguibactin, a unique plasmid-related bacterial siderophore from the fish pathogen Vibrio anguillarum. J Am Chem Soc 1989, 111, 292–296. [Google Scholar]
- Barbeau, K; Rue, EL; Bruland, KW; Butler, A. Photochemical cycling of iron in the surface ocean mediated by microbial iron(III)-binding ligands. Nature 2001, 413, 409–413. [Google Scholar]
- Martinez, JS; Zhang, GP; Holt, PD; Jung, HT; Carrano, CJ; Haygood, MG; Butler, A. Self-assembling amphiphilic siderophores from marine bacteria. Science 2000, 287, 1245–1247. [Google Scholar]
- Takahashi, A; Nakamura, H; Kameyama, T; Kurasawa, S; Naganawa, H; Okami, Y; Takeuchi, T; Umezawa, H. Bisucaberin, a new siderophore, sensitizing tumor-cells to macrophage-mediated cytolysis. 2. Physicochemical properties and structure determination. J Antibiot 1987, 40, 1671–1676. [Google Scholar]
- Winkelmann, G; Schmid, DG; Nicholson, G; Jung, G; Colquhoun, DJ. Bisucaberin–A dihydroxamate siderophore isolated from Vibrio salmonicida, an important pathogen of farmed Atlantic salmon (Salmo salar). BioMetals 2002, 15, 153–160. [Google Scholar]
- Martinez, JSH; MG; Butler, A. Identification of a natural desferrioxamine siderophore produced by a marine bacterium. Limnol Oceanogr 2001, 46, 420–424. [Google Scholar]
- Wang, WL; Chi, Z; Liu, GL; Buzdar, MA; Chi, ZM; Gu, QQ. Chemical and biological characterization of siderophore produced by the marine-derived Aureobasidium pullulans HN6.2 and its antibacterial activity. Biometals 2009, 22, 965–972. [Google Scholar]
- Homann, VV; Sandy, M; Tincu, JA; Templeton, AS; Tebo, BM; Butler, A. Loihichelins A-F, a suite of amphiphilic siderophores produced by the marine bacterium Halomonas LOB-5. J Nat Prod 2009, 72, 884–888. [Google Scholar]
- Martin, JD; Ito, Y; Homann, VV; Haygood, MG; Butler, A. Structure and membrane affinity of new amphiphilic siderophores produced by Ochrobactrum sp SP18. J Biol Inorg Chem 2006, 11, 633–641. [Google Scholar]
- Barbeau, K; Zhang, GP; Live, DH; Butler, A. Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. J Am Chem Soc 2002, 124, 378–379. [Google Scholar]
- Hickford, SJH; Kupper, FC; Zhang, GP; Carrano, CJ; Blunt, JW; Butler, A. Petrobactin sulfonate, a new siderophore produced by the marine bacterium Marinobacter hydrocarbonoclasticus. J Nat Prod 2004, 67, 1897–1899. [Google Scholar]
- Manwar, AV; Khandelwal, SR; Chaudhari, BL; Meyer, JM; Chincholkar, SB. Siderophore production by a marine Pseudomonas aeruginosa and its antagonistic action against phytopathogenic fungi. Appl Biochem Biotechnol 2004, 118, 243–251. [Google Scholar]
- Simpson, FB; Neilands, JB. Siderochromes in cyanophyceae: isolation and characterization of schizokinen from Anabaena sp. J Phycol 1976, 12, 44–48. [Google Scholar]
- Okujo, N; Saito, M; Yamamoto, S; Yoshida, T; Miyoshi, S; Shinoda, S. Structure of Vulnibactin, a new polyamine-containing siderophore from Vibrio vulnificus. Biometals 1994, 7, 109–116. [Google Scholar]
- Renshaw, JC; Robson, GD; Trinci, APJ; Wiebe, MG; Livens, FR; Collison, D; Taylor, RJ. Fungal siderophores: structures, functions and applications. Mycol Res 2002, 106, 1123–1142. [Google Scholar]
- Armstrong, E; Granger, J; Mann, EL; Price, NM. Outer-membrane siderophore receptors of heterotrophic oceanic bacteria. Limnol Oceanogr 2004, 49, 579–587. [Google Scholar]
- Cornelis, P. Andrews, SC, Ed.; Iron Uptake and Homeostasis in Microorganisms; Caister Academic Press: Norwich, UK, 2010. [Google Scholar]
- Gademann, K. Cyanobacterial natural products for the inhibition of biofilm formation and biofouling. Chimia 2007, 61, 373–377. [Google Scholar]
| Examples of functions of EPS in bacterial cells | |||
|---|---|---|---|
| Type of EPS | Function | Bacterium | Reference |
| Glycolipid | Biosurfactant | Halomonadaceae sp. strain MM1 | [48] |
| Polysaccharide | Benefit during competition for space and nutrients on surfaces | Pseudoalteromonas tunicata | [46] |
| Polysaccharide | Allow survival in oilwells | Bacillus sp. | [49] |
| Polysaccharide and proteins | Helps microbial interactions | Nocardia amarae | [50] |
| Examples of applications of EPS from marine bacteria | |||
| Bacterium | Applications | Reference | |
| Alteromonas infernus | Bone-healing material | [51] | |
| Bacillus circulans | Biosurfactant; antimicrobial action | [52] | |
| Vibrio and Alteromonas | Tissue regeneration; antithrombotic effects | [46] | |
| P. tunicata | Antifouling activity | [53] | |
| Flavobacterium uliginosa | Antitumor activity | [54] | |
| Bacillus sp. | Pseudoplastic behavior | [49] | |
| Siderophore | Producer | Comments | Reference |
|---|---|---|---|
| Aerobactin | Vibrio sp. DS40M5, SD004, SD101, SD102, SD248 | - | [130] |
| Amphibactins | Vibrio sp. R-10 | Amphiphilic cell associated siderophores. This feature can be ascribed to the membrane affinities of amphibactins, which range from 3.8 × 103 to 8.3 × 102 M−1, clearly exceeding those for other amphiphilic siderophores. | [131] |
| Anachelins | Anabaena cylindrica NIES-19 | - | [122,123] |
| Anguibactin | Vibrio anguillarum 775 | Backbone derived from ω-N-hydroxyhistamine, cysteine, and 2,3- dihydroxybenzoic acid. Producing strain is a fish pathogen | [132] |
| Aquachelins | Halomonas aquamarina DS40M3 | Contain a given peptidic head group that coordinates Fe(III), alongside with an appendage of a fatty acid moiety. Aquachelins display low critical micelle concentration. Production by open ocean bacteria | [133,134] |
| Bisucaberin | Alteromonas haloplanktis, Vibrio salmonicida (fish pathogen) | Anti-tumor activity | [135,136] |
| Desferrioxamine G | Strain BLI-41 | Structurally similar to desferrioxamine B, but for the substitution of a terminal methyl group by a propionic acid moiety | [137] |
| Fusigen | Aureobasidium pullulans HN6.2 | Anti-bacterial activity tested against the pathogen Vibrio anguillarum | [138] |
| Loihichelins A–F | Halomonas sp. LOB-5 | Potential role in the promotion of Mn(II) and Fe(II) oxidation | [139] |
| Marinobactins | Marinobacter sp. DS40M6 and DS40M8 | In the presence of Fe(III) marinobactins undergo a spontaneous phase change to form vesicles. In the absence of iron, they are present as micelles at concentration over the critical micelle concentration. | [133] |
| Ochrobactins A–C | Ochrobactrum sp. SP18 | Membrane-associated citrate-type photoreactive siderophore, amphiphilic derivatives of aerobactin | [140] |
| Petrobactin and sulfonated derivatives thereof | Marinobacter hydrocarbonoclasticus, Marinobacter aquaeolei | Sulfonation takes place in an aromatic ring of petrobactin and is considered to increase the water solubility of the aromatic compounds, as well as to reduce the oxidation of the catechol ring and affect the Fe(III) stability constant | [139,141,142] |
| Pistillarin | Penicillium bilaii | Rare siderophore, one of the two findings of this compound as a natural product. Contains the unusual 3,4-dihydroxycatechol moiety, that is also found in petrobactin | [129] |
| Pyoverdine | Pseudomonas aeruginosa ID 4365 | Inhibitory of the growth of fungal plant pathogens | [143] |
| Rhizoferrin | Cuninghamella elegans ATCC36112 | Polycarboxylate siderophore | [127] |
| Schizokinen | Anabaena sp. PCC 7120 | - | [144] |
| Synechobactins A–C | Synechococcus sp. PCC 7002 | Schizokinen derivatives with amphiphilic nature, where an hydroxamic acid is replaced by a long fatty acid, these siderophores are suggested to fix into the membrane of cyanobacteria, given the high affinity, therefore preventing its loss by diffusion into marine environments | [121,131] |
| Vibrioferrin | Marinobacter sp. DG870, 879, 893, and 979 | Stoichiometrical boron binding ability through the α-hydroxy- carboxylic acid groups. The photoproduct of this photosensitive siderophore lacks affinity for iron, hence leading to the destruction of the ligand, in contrast with other photosensitive siderophores, where the photoproduct can still coordinate and sequester Fe(III). This characteristic has been suggested to contribute for the mutualistic sharing of iron between marine bacteria and phytoplankton | [107,108] |
| Vulnibactin | Vibrio vulnificus (marine pathogen, causer of causing lethal septicemia or wound infections in humans) | - | [145] |
| Siderophore | Coordination with Fe(III) |
|---|---|
| Amphibactins | Through the hydroxamate groups |
| Aquachelins, marinobactins, ochrobactins, synechobactins | Through the oxygen atoms of each hydroxamate group and both oxygen atoms of the β-hydroxy aspartic acid (in the aquachelins and marinobactins) or of the citric acid (in the ochrobactins and synechobactins) |
| Alterobactins and pseudoalterobactins | Through the β-hydroxy aspartate moieties and a catecholate group |
| Petrobactin and sulfonate derivatives | Through the catecholates and the α-hydroxy acid portion of the citrate backbone |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Carvalho, C.C.C.R.; Fernandes, P. Production of Metabolites as Bacterial Responses to the Marine Environment. Mar. Drugs 2010, 8, 705-727. https://doi.org/10.3390/md8030705
De Carvalho CCCR, Fernandes P. Production of Metabolites as Bacterial Responses to the Marine Environment. Marine Drugs. 2010; 8(3):705-727. https://doi.org/10.3390/md8030705
Chicago/Turabian StyleDe Carvalho, Carla C. C. R., and Pedro Fernandes. 2010. "Production of Metabolites as Bacterial Responses to the Marine Environment" Marine Drugs 8, no. 3: 705-727. https://doi.org/10.3390/md8030705
APA StyleDe Carvalho, C. C. C. R., & Fernandes, P. (2010). Production of Metabolites as Bacterial Responses to the Marine Environment. Marine Drugs, 8(3), 705-727. https://doi.org/10.3390/md8030705





