Cytotoxicity on Human Cancer Cells of Ophidiacerebrosides Isolated from the African Starfish Narcissia canariensis
Abstract
:1. Introduction
2. Results and Discussion
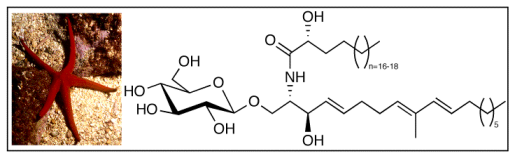
2.1. Glycolipid Isolation and Structure Determination
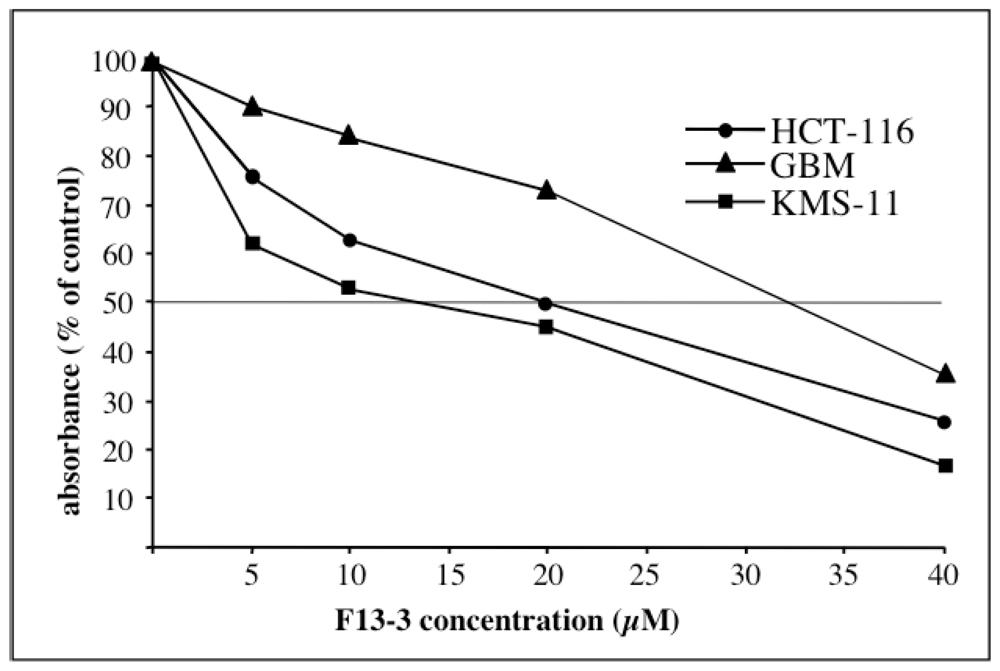
2.2. Cytotoxic Activity
3. Experimental Section
3.1. General Procedures
3.2. Animal Material
3.3. Lipid Extraction and F13-3 Isolation
3.4. Acetylation of F13-3
3.5. Methanolysis of F13-3
3.6. Cell Cultures
3.7. Neutral Red Assay
3.8. MTT Assay
Acknowledgements
References
- Mitsiades, CS; Hayden, PJ; Anderson, KC; Richardson, PG. From the bench to the bedside: Emerging new treatments in multiple myeloma. Best Pract Res Clin Haematol 2007, 20, 797–816. [Google Scholar]
- Harousseau, JL. Optimising patient outcomes in myeloma. Cancer Treat Rev 2010, 36, 33–35. [Google Scholar]
- Decaux, O; Clément, M; Magrangeas, F; Gouraud, W; Charbonnel, C; Campion, L; Loiseau, HA; Minvielle, S. Inhibition of mTORC1 activity by REDD1 induction in myeloma cells resistant to bortezomib cytotoxicity. Cancer Sci 2010, 101, 889–897. [Google Scholar]
- Chan, TA; Weingart, JD; Parisi, M; Hughes, MA; Olivi, A; Borzillary, S; Alahakone, D; Detorie, NA; Wharam, MD; Kleinberg, L. Treatment of recurrent glioblastoma multiforme with glia site brachytherapy. Radiat Oncol Biol Phys 2005, 62, 1133–1139. [Google Scholar]
- Towler, B; Irwig, L; Glasziou, P; Kewenter, J; Weller, D; Silagy, C. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, Hemoccult. Br Med J 1998, 29, 559–565. [Google Scholar]
- Fattorusso, E; Mangoni, A. Marine glycolipids. Prog Chem Org Nat Prod 1997, 72, 215–301. [Google Scholar]
- Costantino, V; Fattorusso, E; Imperatore, C; Mangoni, A. Glycolipids from sponges. 13.1 clarhamnoside, the first rhamnosylated α-galactosylceramide from Agelas clathrodes. Improving spectral strategies for glycoconjugate structure determination. J Org Chem 2004, 69, 1174–1179. [Google Scholar]
- Mansoor, TA; Shinde, PB; Luo, X; Hong, J; Lee, CO; Sim, X; Son, BW; Jung, JH. Renierosides, cerebrosides from a marine sponge Haliclona (Reniera) sp. J Nat Prod 2007, 70, 1481–1486. [Google Scholar]
- Costantino, V; Fattorusso, E; Imperatore, C; Mangoni, A; Freigang, S; Teyton, L. Corrugoside: A new immunostimulatory α-galactoglycosphingolipid from the marine sponge Axinella corrugata. Bioorg Med Chem 2008, 16, 2077–2085. [Google Scholar]
- Durán, R; Zubia, E; Ortega, MJ; Naranjo, S; Salvá, J. Phallusides, New Glucosphingolipids from the Ascidian Phallusia fumigata. Tetrahedron 1998, 54, 14597–14602. [Google Scholar]
- Cheng, SY; Wen, ZH; Chiou, SF; Tsai, CW; Wang, SK; Hsu, CH; Dai, CF; Chiang, MY; Wang, WH; Duh, CY. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J Nat Prod 2009, 72, 465–468. [Google Scholar]
- Kawano, Y; Higuchi, R; Isobe, R; Komori, T. Isolation and structure of six new cerebrosides. Liebigs Ann Chem 1988, 1988, 19–24. [Google Scholar]
- Higuchi, R; Kagoshima, M; Komori, T. Structure of three new cerebrosides, Astrocerebrosides A, B, and C and of related nearly homogeneous cerebrosides. Liebigs Ann Chem 1990, 1990, 659–663. [Google Scholar]
- Higuchi, R; Natori, T; Komori, T. Isolation and characterization of Acanthacerebroside B and structure elucidation of related, nearly homogeneous cerebrosides. Liebigs Ann Chem 1990, 1990, 51–55. [Google Scholar]
- Higuchi, R; Jhou, J; Inukai, K; Komori, T. Isolation and structure of six new cerebrosides, Asteriacerebrosides A–F, and two known cerebrosides, Astrocerebroside A and Acanthacerebroside C. Liebigs Ann Chem 1991, 1991, 745–752. [Google Scholar]
- Jin, W; Rinehart, KL; Jares-Erijman, EA. Ophidiacerebrosides: Cytotoxic glycosphingolipids containing a novel sphingosine from a sea star. J Org Chem 1994, 59, 144–147. [Google Scholar]
- Higuchi, R; Harano, Y; Mitsuyuki, M; Isobe, R; Yamada, K; Miyamoto, T; Komori, T. Isolation and structure of cerebrosides from the starfish Stellaster equestris. Liebigs Ann 1996, 1996, 593–599. [Google Scholar]
- Inagaki, M; Isobe, R; Kawano, Y; Miyamoto, T; Komori, T; Higuchi, R. Isolation and structure of three new ceramides from the starfish Acanthaster planci. Eur J Org Chem 1998, 1998, 129–131. [Google Scholar]
- Maier, MS; Kuriss, A; Seldes, AM. Isolation and structure of glucosylceramides from the starfish Cosmasterias lurida. Lipids 1998, 33, 825–827. [Google Scholar]
- Díaz de Vivar, ME; Seldes, AM; Maier, MS. Two novel glucosylceramides from gonads and body walls of the Patagonian starfish Allostichaster inaequalis. Lipids 2002, 37, 597–603. [Google Scholar]
- Kawatake, S; Nakamura, K; Inagaki, M; Higuchi, R. Isolation and structural determination of six glucocerebrosides from the starfish Luidia maculata. Chem Pharm Bull 2002, 50, 1091–1096. [Google Scholar]
- Chludil, HD; Seldes, AM; Maier, MSD. Anasterocerebroside A, a new glucosylceramide from the Patagonian starfish Anasterias minuta. Z Naturforsch 2003, 58c, 433–440. [Google Scholar]
- Inagaki, M; Nakamura, K; Kawatake, S; Higuchi, R. Isolation and structural determination of four new ceramide lactosides from the starfish Luidia maculata. Eur J Org Chem 2003, 2003, 325–331. [Google Scholar]
- Inagaki, M; Nakata, T; Higuchi, R. Isolation and structure of galactocerebroside molecular species from the starfish Culcita novaguineae. Chem Pharm Bull 2005, 54, 260–261. [Google Scholar]
- Constantino, V; de Rosa, C; Fattorusso, E; Imperatore, C; Mangoni, A; Irace, C; Maffettone, C; Capasso, D; Malorni, L; Palumbo, R; Pedone, C. Oreacerebrosides: Bioactive cerebrosides with a triunsaturated sphingoid base from the sea star Oreaster reticulatus. Eur J Org Chem 2007, 2007, 5277–5283. [Google Scholar]
- Namba, M; Ohtsuki, T; Mori, M; Togawa, A; Wada, H; Sugihara, T; Yawata, Y; Kimoto, T. Establishment of five human myeloma cell lines. In Vitro Cell Dev Biol 1989, 25, 723–729. [Google Scholar]
- Cartron, JP; Juin, P; Oliver, L; Martin, S; Meflah, K; Vallette, FM. Non-redundant role of Bax and Bak in Bid-mediated apoptosis. Mol Cell Biol 2003, 23, 4701–4712. [Google Scholar]
- Brattain, MG; Fine, WD; Khaled, FM; Thompson, J; Brattain, DE. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res 1981, 41, 1751–1756. [Google Scholar]
- El Kihel, L; Clément, M; Bazin, MA; Descamps, G; Khalid, M; Rault, S. New lithocholic and chenodeoxycholic piperazinylcarboxamides with antiproliferative and pro-apoptic effects on human cancer cell lines. Bioorg Med Chem 2008, 16, 8737–8744. [Google Scholar]
- Black, FJ; Kocienski, PJ. Synthesis of phalluside-1 and Sch II using 1,2-metallate rearrangements. Org Biomol Chem 2010, 8, 1188–1193. [Google Scholar]
- Denizot, F; Lang, R. Rapid colorimetric assay for cell growth and survival-modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986, 89, 271–277. [Google Scholar]
Abbreviations
| COSY | homonuclear correlation spectroscopy |
| FAME | fatty acid methyl ester |
| GC-MS | gas chromatography-mass spectrometry |
| GL | glycolipid(s) |
| GSL | glycosphingolipid(s) |
| HMBC | heteronuclear multiple bond coherence |
| HSQC | heteronuclear single quantum coherence |
| IC50 | 50% inhibitory concentration |
| ESI-MS | electrospray ionization mass spectrometry |
| TLC | thin layer chromatography |
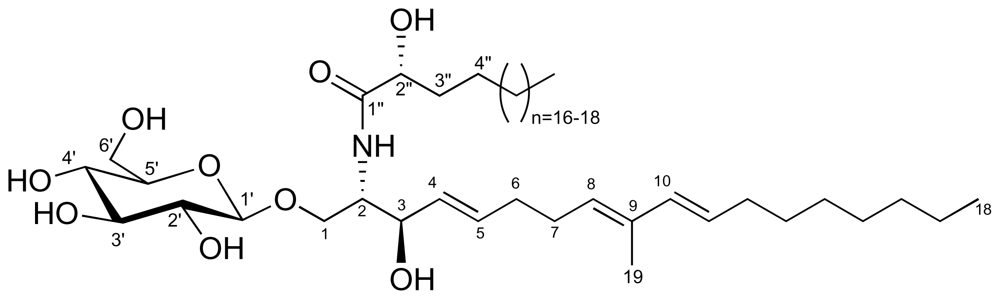
| Position | δH ppm, mult., J in Hz | δC ppm | cosy correlation |
|---|---|---|---|
| NH | 6.35 (d, J = 9.0) | - | |
| 1a | 3.95 (dd, J = 10.0/3.8) | 67.2 | 2, 1b |
| 1b | 3.62 (dd, J = 10.0/3.8) | 67.2 | 2, 1a |
| 2 | 4.32 (m) | 72.7 | 1a, 1b, 3, NH |
| 3 | 4.27 (d, J = 4.6) | 50.6 | 4, 2 |
| 4 | 5.34 (m) | 128.7 | 3, 5 |
| 5 | 5.43 (dd, J = 6.5/15.0) | 124.8 | 4, 6 |
| 6 | 2.07 (s) | 32.3 | 5, 7 |
| 7 | 2.20 (s) | 31.9 | 6, 8, 19 |
| 8 | 5.83 (m) | 136.4 | 7 |
| 9 | - | 134.2 | |
| 10 | 6.04 (d, J = 15.4) | 134.4 | 11 |
| 11 | 5.59 (m) | 127.8 | 10, 12 |
| 12 | 2.10 (m) | 32.9 | 11, 13 |
| 19 | 1.73 (s) | 12.5 | 7, 8 |
| CH2 | 1.27 (m) | 22.7–29.7 | |
| CH3 acetates | 2.02/2.04/2.05/2.08/2.11/2.19 (6s) | 20.6–21.0 | |
| C=O acetates | 169.3/169.4/169.6/160.7/169.8/169.9 | ||
| terminal CH3 | 0.90 (t, J = 6.8) | 14.1 | |
| 1′ | 4.49 (d, J = 7.9) | 100.5 | 2′ |
| 2′ | 4.97 (dd, J = 8.0/9.5) | 71.2 | 1′, 3′ |
| 3′ | 5.20 (t, J = 9.5) | 72.7 | 2′, 4′ |
| 4′ | 5.10 (t, J = 9.7) | 68.2 | 3′, 5′ |
| 5′ | 3.71 (m) | 71.9 | 4′, 6′a, 6′b |
| 6′a | 4.24 (d, J = 4.5) | 61.8 | 5′, 6′b |
| 6′b | 4.16 (dd, J = 12.5/2.3) | 61.8 | 5, 6a |
| 1″ | - | 172.2 | |
| 2″ | 5.36 (m) | 73.0 | |
| 3″ | 1.83 (m) | 31.8 | |
| 4″ | 1.40 (m) | 29.5 | |
| terminal CH3 | 0.83 (m) | 14.1 | |
| IC50 | KMS-11 | HCT-116 | GBM |
|---|---|---|---|
| F13-3 | 15.2 ± 4.0 | 18 ± 3.9 | 34.6 ± 5.1 |
© 2010 by the authors; license MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farokhi, F.; Wielgosz-Collin, G.; Clement, M.; Kornprobst, J.-M.; Barnathan, G. Cytotoxicity on Human Cancer Cells of Ophidiacerebrosides Isolated from the African Starfish Narcissia canariensis. Mar. Drugs 2010, 8, 2988-2998. https://doi.org/10.3390/md8122988
Farokhi F, Wielgosz-Collin G, Clement M, Kornprobst J-M, Barnathan G. Cytotoxicity on Human Cancer Cells of Ophidiacerebrosides Isolated from the African Starfish Narcissia canariensis. Marine Drugs. 2010; 8(12):2988-2998. https://doi.org/10.3390/md8122988
Chicago/Turabian StyleFarokhi, Fereshteh, Gaetane Wielgosz-Collin, Monique Clement, Jean-Michel Kornprobst, and Gilles Barnathan. 2010. "Cytotoxicity on Human Cancer Cells of Ophidiacerebrosides Isolated from the African Starfish Narcissia canariensis" Marine Drugs 8, no. 12: 2988-2998. https://doi.org/10.3390/md8122988
APA StyleFarokhi, F., Wielgosz-Collin, G., Clement, M., Kornprobst, J.-M., & Barnathan, G. (2010). Cytotoxicity on Human Cancer Cells of Ophidiacerebrosides Isolated from the African Starfish Narcissia canariensis. Marine Drugs, 8(12), 2988-2998. https://doi.org/10.3390/md8122988