Immense Essence of Excellence: Marine Microbial Bioactive Compounds
Abstract
:1. Introduction
2. Marine Environment as a Prolific Source of Bioactive Compounds

3. Marine Microorganisms as Producers of Bioactive Compounds
3.1. Marine bacteria


3.2. Marine fungi




3.3. Actinomycetes





3.4. Microalgae


3.5. Marine viruses
3.6. Symbiotic marine micro-organisms



4. Clinical Status of Marine Compounds of Microbial Origin


5. Future Perspectives and Concluding Remarks
Acknowledgements
References
- Cragg, GM; Newman, DJ. Medicinals for the millennia: the historical record. Ann. N.Y. Acad. Sci 2001, 953, 3–25. [Google Scholar]
- Finglas, PM; Wright, AJ; Wolfe, CA; Hart, DJ; Wright, DM; Dainty, JR. Is there more to folates than neural-tube defects? Proc. Nutr. Soc 2003, 62, 591–598. [Google Scholar]
- Berdy, J. Bioactive microbial metabolites. A personal view. J. Antibiot 2005, 58, 1–26. [Google Scholar]
- Ruiz, B; Chávez, A; Forero, A; García-Huante, Y; Romero, A; Sánchez, M; Rocha, D; Sánchez, B; Rodríguez-Sanoja, R; Sánchez, S; Langley, E. Production of microbial secondary metabolites: regulation by the carbon source. Crit. Rev. Microbiol 2010, 36, 146–67. [Google Scholar]
- Jha, RK; Zi-rong, X. Biomedical Compounds from Marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar]
- Blunt, JW; Copp, BR; Munro, MHG; Northcote, PT; Prinsep, MR. Marine Natural products. Nat. Prod. Rep 2004, 21, 1–49. [Google Scholar]
- Tan, LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar]
- Burja, AM; Banaigs, B; Abou-Mansour, E; Burgess, JG; Wright, PC. Marine cyanobacteria-a prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar]
- Gorajana, A; Venkatesan, M; Vinjamuri, S; Kurada, VVSNB; Peela, S; Jangam, P; Poluri, E; Zeeck, A. Resistoflavine, cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. Microbiol. Res 2007, 162, 322–327. [Google Scholar]
- Kwon, HC; Kauffman, CA; Jensen, PR; Fenical, W. Marinomycins A–D, Antitumor-Antibiotics of a New Structure Class from a Marine Actinomycete of the Recently Discovered Genus “Marinispora”. J. Am. Chem. Soc 2006, 128, 1622–1632. [Google Scholar]
- Asolkar, RN; Jensen, PR; Kauffman, CA; Fenical, W. Daryamides A–C, Weakly Cytotoxic Polyketides from a Marine-Derived Actinomycete of the Genus Streptomyces Strain CNQ-085. J. Nat. Prod 2006, 69, 1756–1759. [Google Scholar]
- Matz, C; Webb, JS; Schupp, PJ; Phang, SY; Penesyan, A; Egan, S; Steinberg, P; Kjelleberg, S. Marine Biofilm Bacteria Evade Eukaryotic Predation by Targeted Chemical Defense. PLoS One 2008, 3, e2744. [Google Scholar]
- Lu, XL; Xu, QZ; Shen, YH; Liu, XY; Jiao, BH; Zhang, WD; Ni, KY. Macrolactin S, a novel macrolactin antibiotic from marine Bacillus sp. Nat. prod. Res 2010, 22, 342–347. [Google Scholar]
- Maya, SP; Kong, F; Jeffrey, JE; Daniel, AA; Paola, SA; Valerie, BS; Peter, PJ; Weiss, WJ; Carter, G; Greenstein, M. Novel alpha-pyrones produced by a marine Pseudomonas sp. F92S91: taxonomy and biological activities. J. Antibiot 2003, 56, 1033–1044. [Google Scholar]
- Isnansetyo, A; Kamei, Y. Anti-methicillin-resistant Staphylococcus aureus (MRSA) activity of MC21-B, an antibacterial compound produced by the marine bacterium Pseudoalteromonas phenolica O-BC30T. Int. J. Antimicrob. Agents 2009b, 34, 131–135. [Google Scholar]
- Du, L; Feng, T; Zhao, B; Li, D; Cai, S; Zhu, T; Wang, F; Xiao, X; Gu, Q. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities. J. Antibiot 2010, 63, 165–170. [Google Scholar]
- Koizumi, Y; Arai, M; Tomoda, H; Omura, S. Oxaline, a fungal alkaloid, arrests the cell cycle in M phase by inhibition of tubulin polymerization. Biochim. Biophys. Acta 2004, 1693, 47–55. [Google Scholar]
- Kim, MY; Sohn, JH; Ahn, JS; Oh, H. Alternaramide, a cyclic depsipeptide from the marine-derived fungus Alternaria sp. SF-5016. J. Nat. Prod 2009, 72, 2065–2068. [Google Scholar]
- Volk, R-B. Screening of microalgae for species excreting norharmane, a manifold biologically active indole alkaloid. Microbiol. Res 2008, 163, 307–313. [Google Scholar]
- Rickards, RW; Rothschild, JM; Willis, AC; de Chazal, NM; Kirk, J; Kirk, K; Saliba, KJ; Smith, GD. Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron 1999, 55, 13513. [Google Scholar]
- Singh, S; Kate, BN; Banerjee, UC. Bioactive Compounds from Cyanobacteria and Microalgae: An Overview. Crit. Rev. Biotechnol 2005, 25, 73–95. [Google Scholar]
- Gao, C-H; Tian, X-P; Qi, S-H; Luo, X-M; Wang, P; Zhang, S. Antibacterial and antilarval compounds from marine gorgonian-associated bacterium Bacillus amyloliquefaciens SCSIO 00856. J. Antibiot 2010, 63, 191–193. [Google Scholar]
- Kamei, Y; Isnansetyo, A. Lysis of methicillin-resistant Staphylococcus aureus by 2,4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga. Int. J. Antimicrob. Agents 2003, 21, 71–74. [Google Scholar]
- Nicolaou, KC; Lim, YH; Becker, J. Total synthesis and absolute configuration of the bisanthraquinone antibiotic BE-43472B. Angew. Chem. Int. Ed 2009, 48, 3444–3448. [Google Scholar]
- Davidson, BS. New dimensions in natural products research: cultured marine microorganisms. Curr. Opin. Biotechnol 1995, 6, 284–291. [Google Scholar]
- Fuesetani, N. Fuesetani, M, Ed.; Drugs from the Sea; Karger publishers: Basel, Switzerland, 2000; Volume Chapter 1, pp. 1–5. [Google Scholar]
- Rajaganapathi, J; Kathiresan, K; Singh, TP. Purification of anti-HIV protein from purple fluid of the sea hare Bursatella leachii de Blainville. Mar. Biotechnol 2002, 4, 447–453. [Google Scholar]
- Wang, R; Du, Z-L; Duan, W-J; Zhang, X; Zeng, F-L; Wan, X-X. Antiviral treatment of hepatitis B virus-transgenic mice by a marine ascidian, Styela plicata. World J. Gastroenterol 2006, 12, 4038–4043. [Google Scholar]
- Kim, SK; Dominic Ravichandran, Y; Khan, SB; Kim, YT. Prospective of the Cosmeceuticals Derived from Marine Organisms. Biotechnol. Bioproc. Eng 2008, 13, 511–523. [Google Scholar]
- Ryu, B; Qian, ZJ; Kim, MM; Nam, KW; Kim, SK. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem 2009, 78, 98–105. [Google Scholar]
- Heo, SJ; Ko, SC; Cha, SH; Kang, DH; Park, HS; Choi, YU; Kim, D; Jung, WK; Jeon, YJ. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. in Vitro 2009, 23, 1123–1130. [Google Scholar]
- CORHH: Committee on the Ocean’s Role in Human Health, National Research Council, Monsoons to Microbes: Understanding the Ocean's Role in Human Health; The National Academies Press: Washington, D.C., USA, 1999; p. 78.
- Yayanos, AA. Microbiology to 10,500 meters in the deep sea. Annu. Rev. Microbiol 1995, 49, 777–805. [Google Scholar]
- Inagaki, F; Nunoura, T; Nakagawa, S; Teske, A; Lever, M; Lauer, A; Suzuki, M; Takai, K; Delwiche, M; Colwell, FS; Nealson, KH; Horikoshi, K; D’Hondt, S; J⊘rgensen, BB. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc. Natl. Acad. Sci. USA 2006, 103, 2815–2820. [Google Scholar]
- Fenical, W. Marine bacteria: Developing a new chemical resource. Chem. Rev 1993, 93, 1673–1683. [Google Scholar]
- Kobayashi, J; Ishibashi, M. Bioactive Metabolites of Symbiotic Marine Microorganisms. Chem. Rev 1993, 93, 1753–1769. [Google Scholar]
- Okami, YJ. The search for bioactive metabolites form marine bacteria. Mar. Biotechnol 1993, 1, 59–65. [Google Scholar]
- Pathirana, C; Jensen, PR; Fenical, W. Marinone and debromomarinone, antiobiotic sesquiterpenoid naphthoquinones of a new structure class from a marine bacterium. Tetrahedron Lett 1992, 33, 7663–7666. [Google Scholar]
- Trischman, JA; Jensen, PR; Fenical, W. Halobacillin, a cytotoxic cyclic acylpeptide of the iturin class produced by a marine bacillus. Tetrahedron Lett 1994a, 35, 5571–5574. [Google Scholar]
- Trischman, JA; Tapiolas, DM; Jensen, PR; Dwight, R; McKee, TC; Ireland, CM; Stout, TJ; Clardy, J. Salinamides A and B: Anti-inflammatory depsipeptides from a marine streptomycete. J. Am. Chem. Soc 1994b, 116, 757–758. [Google Scholar]
- Numata, A; Takahashi, C; Matsushita, T; Miyamoto, T; Kawai, K; Usami, Y; Matsumura, E; Inoue, M; Ohishi, H; Shingu, T. Fumiquinazolines, novel metabolites of a fungus isolated from a saltfish. Tetrahedron Lett 1992, 33, 1621–1624. [Google Scholar]
- Cheng, XC; Varoglu, M; Abrell, L; Crews, P; Lobkovsky, E; Clardy, J. Chloriolins A–C, chlorinated sesquiterpenes produced by fungal cultures separated from a Jaspis marine sponge. J. Org. Chem 1994, 59, 6344–6348. [Google Scholar]
- Kakeya, H; Takahashi, I; Okada, G; Isono, K; Osada, H. Epolactaene, a novel neuritogenic compound human neuroblastoma cells, produced by a marine fungus. J. Antibiot 1995, 48, 733–735. [Google Scholar]
- Takahashi, C; Takai, Y; Kimura, Y; Numata, A; Shigematsu, N; Tanaka, H. Cytotoxic metabolites from a fungal adherent of a marine alga. Phytochemistry 1995, 38, 155–158. [Google Scholar]
- Belofsky, GN; Jensen, PR; Renner, MK; Fenical, W. New cytotoxic sesquiterpenoid nitrobenzoyl esters from a marine isolate of the fungus Aspergillus versicolor. Tetrahedron 1998, 54, 1715–1724. [Google Scholar]
- Romanenko, LA; Uchino, M; Kalinovskaya, NI; Mikhailov, VV. Isolation, phylogenetic analysis and screening of marine mollusc-associated bacteria for antimicrobial, hemolytic and surface activities. Microbiol. Res 2008a, 163, 633–644. [Google Scholar]
- Isnansetyo, A; Kamei, Y. Bioactive substances produced by marine isolates of Pseudomonas. J. Ind. Microbiol. Biotechnol 2009a, 36, 1239–1248. [Google Scholar]
- Romanenko, LA; Uchino, M; Tanaka, N; Frolova, GM; Slinkina, NN; Mikhailov, VV. Occurrence and antagonistic potential of Stenotrophomonas strains isolated from deep-sea invertebrates. Arch. Microbiol 2008b, 189, 337–344. [Google Scholar]
- Romanenko, LA; Uchino, M; Tebo, BM; Tanaka, N; Frolova, GM; Mikhailov, VV. Pseudomonas marincola sp. nov., isolated from marine environments. Int. J. Syst. Evol. Microbiol 2008c, 58, 706–710. [Google Scholar]
- Bowman, JP. Bioactive Compound Synthetic Capacity and Ecological Significance of Marine Bacterial Genus Pseudoalteromonas. Mar. Drugs 2007, 5, 220–241. [Google Scholar]
- Isnansetyo, A; Kamei, Y. MC21-A, a bactericidal antibiotic produced by a new marine bacterium, Pseudoalteromonas phenolica sp. nov. O-BC30T against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother 2003, 47, 480–488. [Google Scholar]
- Kamei, Y; McCarthy, SA; Kakimoto, D; Johnson, R. Inhibition of Paramecium caudatum by an Alteromonas luteoviolacea antibiotic. Antimicrob. Agents Chemother 1986, 30, 301–303. [Google Scholar]
- Egan, S; James, S; Holmström, C; Kjelleberg, S. Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata. Env. Microbiol 2002, 4, 433–442. [Google Scholar]
- Franks, A; Haywood, P; Holmström, C; Egan, S; Kjelleberg, S; Kumar, N. Isolation and structure elucidation of a novel yellow pigment from the marine bacterium Pseudoalteromonas tunicata. Molecules 2005, 10, 1286–1291. [Google Scholar]
- Lindquist, N; Fenical, W. New tambjamine class alkaloids from the marine ascidian Atapozoa sp. and its nudibranch predators – origins of the tambjamines in atapozoa. Experientia 1991, 47, 504–508. [Google Scholar]
- König, G; Kehraus, S; Seibert, S; Abdel-Lateff, A; Müller, D. Natural products from marine organisms and their associated microbes. Chem. Biochem 2006, 7, 229–238. [Google Scholar]
- Burke, C; Thomas, T; Egan, S; Kjelleberg, S. The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata. Environ. Microbiol 2007, 9, 814–818. [Google Scholar]
- Satpute, SK; Banat, IM; Dhakephalkar, PK; Banpurkar, AG; Chopade, BA. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv 2007, 28, 436–450. [Google Scholar]
- Arena, A; Gugliandolo, C; Stassi, G; Pavone, B; Iannello, D; Bisignano, G; Maugeri, TL. An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: antiviral activity on immunocompetent cells. Immunol. Lett 2009, 123, 132–137. [Google Scholar]
- Das, P; Mukherjee, S; Sen, R. Improved bioavailability and biodegradation of a model polyaromatic hydrocarbon by a biosurfactant producing bacterium of marine origin. Chemosphere 2008, 72, 1229–1234. [Google Scholar]
- Das, P; Mukherjee, S; Sen, R. Biosurfactant of marine origin exhibiting heavy metal remediation properties. Bioresour. Technol 2009, 100, 4887–4890. [Google Scholar]
- Bhadury, P; Mohammad, BT; Wright, PC. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol 2006, 33, 325–337. [Google Scholar]
- Newman, DJ; Hill, RT. New drugs from marine microbes: the tide is turning. J. Ind. Microbiol. Biotechnol 2006, 33, 539–544. [Google Scholar]
- Kjer, J; Debbab, A; Aly, AH; Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protocols 2010, 5, 479–490. [Google Scholar]
- Du, L; Zhu, T; Fang, Y; Liu, H; Gu, Q; Zhu, W. Aspergiolide A, a novel anthraquinone derivative with naphtho(1,2,3-de)chromene-2,7-dione skeleton isolated from a marine-derived fungus Aspergillus glaucus. Tetrahedron 2007, 63, 1085–1088. [Google Scholar]
- Kwong, TF; Miao, L; Li, X; Qian, PY. Novel Antifouling and Antimicrobial Compound from a Marine-Derived Fungus Ampelomyces sp. Mar. Biotechnol 2006, 8, 634–640. [Google Scholar]
- Xiong, H; Qi, S; Xu, Y; Li, M; Qian, P-Y. Antibiotic and antifouling compound production by the marine-derived fungus Cladosporium sp. F14. J. Hydro-Environ. Res 2009, 2, 264–270. [Google Scholar]
- Gai, Y; Zhao, LL; Hu, CQ; Zhang, HP. Fusarielin E, a new antifungal antibiotic from Fusarium sp. Chin. Chem. Lett 2007, 18, 954–956. [Google Scholar]
- Li, X; Kim, SK; Nam, KW; Kang, JS; Choi, HD; Son, BW. A new antibacterial dioxopiperazine alkaloid related to gliotoxin from a marine isolate of the fungus Pseudallescheria. J. Antibiot. (Tokyo) 2006, 59, 248–250. [Google Scholar]
- Zhang, D; Yang, X; Kang, JS; Choi, HD; Son, BW. Chlorohydroaspyrones A and B, antibacterial aspyrone derivatives from the marine-derived fungus Exophiala sp. J. Nat. Prod 2008, 71, 1458–1460. [Google Scholar]
- Stadler, M; Anke, H; Sterner, O. Metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. III. production of novel isocoumarin derivatives, isolation and biological activities. J. Antibiot 1995, 48, 261–266. [Google Scholar]
- Nenkep, VN; Yun, K; Li, Y; Choi, HD; Kang, JS; Son, BW. New production of haloquinones, bromochlorogentisylquinones A and B, by a halide salt from a marine isolate of the fungus Phoma herbarum. J. Antibiot 2010, 63, 199–201. [Google Scholar]
- Takahashi, Y; Omura, S. Isolation of new actinomycete strains for the screening of new bioactive compounds. J. Gen. Appl. Microbiol 2003, 49, 141–154. [Google Scholar]
- Olano, C; Méndez, C; Salas, JA. Antitumor compounds from marine actinomycetes. Mar. Drugs 2009, 7, 210–248. [Google Scholar]
- Prudhomme, J; McDaniel, E; Ponts, N; Bertani, S; Fenical, W; Jensen, P; Le Roch, K. Marine actinomycetes: a new source of compounds against the human malaria parasite. PLoS One 2008, 3, e2335. [Google Scholar]
- El-Gendy, MM; Hawas, UW; Jaspars, M. Novel bioactive metabolites from a marine derived bacterium Nocardia sp. ALAA 2000. J. Antibiot. (Tokyo) 2008, 61, 379–386. [Google Scholar]
- McArthur, KA; Mitchell, SS; Tsueng, G; Rheingold, A; White, DJ; Grodberg, J; Lam, KS; Potts, BC. Lynamicins A–E, chlorinated bisindole pyrrole antibiotics from a novel marine actinomycete. J. Nat. Prod 2008, 71, 1732–1737. [Google Scholar]
- Cho, JY; Williams, PG; Kwon, HC; Jensen, PR; Fenical, W. Lucentamycins A–D, cytotoxic peptides from the marine-derived actinomycete Nocardiopsis lucentensis. J. Nat. Prod 2007, 70, 1321–1328. [Google Scholar]
- Hawas, UW; Shaaban, M; Shaaban, KA; Speitling, M; Maier, A; Kelter, G; Fiebig, HH; Meiners, M; Helmke, E; Laatsch, H. Mansouramycins A–D, cytotoxic isoquinolinequinones from a marine streptomycete. J. Nat. Prod 2009, 72, 2120–2124. [Google Scholar]
- Pérez, M; Crespo, C; Schleissner, C; Rodríguez, P; Zúñiga, P; Reyes, F. Tartrolon D, a cytotoxic macrodiolide from the marine-derived actinomycete Streptomyces sp. MDG-04-17-069. J. Nat. Prod 2009, 72, 2192–2194. [Google Scholar]
- Maloney, KN; Macmillan, JB; Kauffman, CA; Jensen, PR; Dipasquale, AG; Rheingold, AL; Fenical, W. Lodopyridone, a structurally unprecedented alkaloid from a marine actinomycete. Org. Lett 2009, 11, 5422–5424. [Google Scholar]
- Carlson, JC; Li, S; Burr, DA; Sherman, DH. Isolation and Characterization of Tirandamycins from a Marine-Derived Streptomyces sp. J. Nat. Prod 2009, 72, 2076–2079. [Google Scholar]
- Maskey, RP; Helmke, E; Kayser, O; Fiebig, HH; Maier, A; Busche, A; Laatsch, H. Anticancer and antibacterial trioxacarcins with high anti-malaria activity from a marine Streptomycete and their absolute stereochemistry. J. Antibiot. (Tokyo) 2004, 57, 771–779. [Google Scholar]
- Xu, Y; He, H; Schulz, S; Liu, X; Fusetani, N; Xiong, H; Xiao, X; Qian, PY. Potent antifouling compounds produced by marine Streptomyces. Biores. Technol 2010, 101, 1331–1336. [Google Scholar]
- Iwata, F; Sato, S; Mukai, T; Yamada, S; Takeo, J; Abe, A; Okita, T; Kawahara, H. Lorneic Acids, Trialkyl-Substituted Aromatic Acids from a Marine-Derived Actinomycete. J. Nat. Prod 2009, 72, 2046–2048. [Google Scholar]
- Lopes, VR; Fernández, N; Martins, RF; Vasconcelos, V. Primary screening of the bioactivity of brackish water cyanobacteria: toxicity of crude extracts to Artemia salina larvae and Paracentrotus lividus embryos. Mar. Drugs 2010, 8, 471–482. [Google Scholar]
- Patterson, GML; Larsen, LK; Moore, RE. Bioactive natural products from blue-green algae. J. Appl. Phycol 1994, 6, 151–157. [Google Scholar]
- Falch, B. What remains of cyanobacteria? Pharm. i. u. Zeit 1996, 25, 311–319. [Google Scholar]
- Luesch, H; Harrigan, GG; Goetz, G; Horgen, FD. The cyanobacterial origin of potent anticancer agents originally isolated from sea hares. Curr. Med. Chem 2002, 9, 1791–1806. [Google Scholar]
- Sivonen, K; Leikoski, N; Fewer, DP; Jokela, J. Cyanobactins-ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol 2010, 86, 1213–1225. [Google Scholar]
- Simmons, TL; Engene, N; Ureña, LD; Romero, LI; Ortega-Barría, E; Gerwick, L; Gerwick, WH. Viridamides A and B, lipodepsipeptides with antiprotozoal activity from the marine cyanobacterium Oscillatoria nigro-Viridis. J. Nat. Prod 2008, 71, 1544–1550. [Google Scholar]
- Allmendinger, A; Spavieri, J; Kaiser, M; Casey, R; Hingley-Wilson, S; Lalvani, A; Guiry, M; Blunden, G; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of twenty-three British and Irish red algae. Phytother. Res 2010, 24, 1099–1103. [Google Scholar]
- Wiktionary–Awiki based open content dictionary database. Available online: http://en.wiktionary.org/wiki/diatom/ (accessed on 01 June 2010).
- Desbois, AP; Mearns-Spragg, A; Smith, VJ. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol 2009, 11, 45–52. [Google Scholar]
- Bernan, VS; Greenstein, M; Maise, WM. Marine microorganisms as a source of new natural products. Adv. Appl. Microbiol 1997, 43, 57–90. [Google Scholar]
- Pietra, F. Secondary metabolites from marine microorganisms bacteria, protozoa, algae and fungi-achievements and prospectives. J. Nat. Prod 1997, 4, 453–464. [Google Scholar]
- Faulkner, DJ; Harper, MK; Haygood, MG; Salomon, CE; Schmidt, EW. Fusetani, N, Ed.; Symbiotic bacteria in sponges: sources of bioactive substances. In Drugs from the Sea; Karger Publishers: Basel, Switzerland, 2000; pp. 107–119. [Google Scholar]
- Jensen, PR; Fenical, W. Fusetani, N, Ed.; Marine microorganisms and drug discovery: current status and future potential. In Drugs from the Sea; Karger Publishers: Basel, Switzerland, 2000; pp. 6–29. [Google Scholar]
- Thomas, TRA; Kavlekar, DP; LokaBharathi, PA. Marine Drugs from Sponge-Microbe Association–A Review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar]
- Baker, PW; Kennedy, J; Dobson, ADW; Marchesi, JR. Phylogenetic Diversity and Antimicrobial Activities of Fungi Associated with Haliclona simulans Isolated from Irish Coastal Waters. Mar. Biotechnol 2009, 11, 540–547. [Google Scholar]
- Gandhimathi, R; Arunkumar, M; Selvin, J; Thangavelu, T; Sivaramakrishnan, S; Kiran, GS; Shanmughapriya, S; Natarajaseenivasan, K. Antimicrobial potential of sponge associated marine actinomycetes. J. Mycol. Méd 2008, 18, 16–22. [Google Scholar]
- Selvin, J; Shanmughapriya, S; Gandhimathi, S; Kiran, GS; Ravji, TR; Natarajaseenivasan, K; Hema, TA. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl. Microbiol. Biotechnol 2009, 83, 435–445. [Google Scholar]
- Byun, HG; Zhang, H; Mochizuki, M; Adachi, K; Shizuri, Y; Lee, WJ; Kim, SK. Novel antifungal diketopiperazine from marine fungus. J. Antibiot. (Tokyo) 2003, 56, 102–106. [Google Scholar]
- Kalinovskaya, NI; Ivanova, EP; Alexeeva, YV; Gorshkova, NM; Kuznetsova, TA; Dmitronek, AD; Nicolau, DV. Low-molecular-weight, biologically active compounds from marine Pseudoalteromonas species. Curr. Microbiol 2004, 48, 441–446. [Google Scholar]
- Radjasa, OK. Marine invertebrate-associated bacteria in coral reef ecosystems as a new source of bioactive compounds. J. Coast. Dev 2004, 7, 65–70. [Google Scholar]
- Tapiolas, DM; Roman, M; Fenical, W; Stout, TJ; Clardy, J. Octalactins A and B: cytotoxic eight-membered-ring lactones from a marine bacterium, Streptomyces sp. J. Am. Chem. Soc 1991, 113, 4682–4683. [Google Scholar]
- Zheng, L; Han, X; Chen, H; Lin, W; Yan, X. Marine bacteria associated with marine macroorganisms: the potential antimicrobial resources. Ann. Microbiol 2005, 55, 119–124. [Google Scholar]
- Wicklow, DT; Joshi, BK; Gamble, WR; Gloer, JB; Dowd, PF. Antifungal Metabolites (Monorden, Monocillin IV, and Cerebrosides) from Humicola fuscoatra Traaen NRRL 22980, a Mycoparasite of Aspergillus flavus Sclerotia. Appl. Environ. Microbiol 1998, 64, 4482–4484. [Google Scholar]
- Joshi, BK; Gloer, JB; Wicklow, DT; Dowd, PF. New Verticillin and Glisoprenin Analogues from Gliocladium catenulatum, a Mycoparasite of Aspergillus flavus Sclerotia. J. Nat. Prod 1999, 62, 730–733. [Google Scholar]
- Soman, AG; Gloer, JB; Wicklow, DT. Antifungal and Antibacterial Metabolites from a Sclerotium-Colonizing Isolate of Mortierella vinacea. J. Nat. Prod 1999, 62, 386–388. [Google Scholar]
- Soman, AG; Gloer, JB; Angawi, RF; Wicklow, DT; Dowd, PF. Vertilecanins: New Phenopicolinic Acid Analogs from Verticillium lecanii. J. Nat. Prod 2001, 64, 189–192. [Google Scholar]
- Che, Y; Gloer, JB; Wicklow, DT. Phomadecalins A-D and Phomapentenone A: New Bioactive Metabolites from Phoma sp. NRRL 25697, a Fungal Colonist of Hypoxylon Stromata. J. Nat. Prod 2002, 65, 399–402. [Google Scholar]
- Zhang, L; An, R; Wang, J; Sun, N; Zhang, S; Hu, J; Kuai, J. Exploring novel bioactive compounds from marine microbes. Curr. Opin. Microbiol 2005, 8, 276–281. [Google Scholar]
- Mayer, AMS; Glaser, KB; Cuevas, C; Jacobs, RS; Kem, W; Little, RD; McIntosh, JM; Newman, DJ; Potts, BC; Shuster, DE. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol. Sci 2010, 31, 255–265. [Google Scholar]
- Nereus Pharmaceuticals homepage. Available online: http://www.nereuspharm.com/overview.shtml (accessed on 02 July 2010).
- Feling, RH; Buchanan, GO; Mincer, TJ; Kauffman, CA; Jensen, PJ; Fenical, W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem 2003, 42, 355–357. [Google Scholar]
- Davidson, SK; Allen, SW; Lim, GE; Anderson, CM; Haygood, MG. Evidence for the biosynthesis of bryostatins by the bacterial symbiont “Candidatus Endobugula sertula” of the bryozoan Bugula neritina. Appl. Environ. Microbiol 2001, 67, 4531–4537. [Google Scholar]
- Bringmann, G; Gulder, TA; Lang, G; Schmitt, S; Stöhr, R; Wiese, J; Nagel, K; Imhoff, JF. Large-scale biotechnological production of the antileukemic marine natural product sorbicillactone A. Mar. Drugs 2007, 5, 23–30. [Google Scholar]
- Thakur, NL; Thakur, AL. Marine Biotechnology: an Overview. Ind. J. Biotechnol 2006, 5, 263–268. [Google Scholar]
- Schwartsmann, G; Da Rocha, AB; Berlinck, RG; Jimeno, J. Marine organisms as a source of new anticancer agents. Lancet Oncol 2001, 2, 221–225. [Google Scholar]
- Kelecom, A. Secondary metabolites from marine microorganisms. Anais da Academia Brasileira de Ciências 2002, 74, 151–170. [Google Scholar]
- Ni, X; Yue, L; Chi, Z; Li, J; Wang, X; Madzak, C. Alkaline Protease Gene Cloning from the Marine Yeast Aureobasidium pullulans HN2-3 and the Protease Surface Display on Yarrowia lipolytica for Bioactive Peptide Production. Mar. Biotechnol 2009, 11, 81–89. [Google Scholar]
- Qian, P-Y; Lau, SCK; Dahms, H-U; Dobretsov, S; Harder, T. Marine Biofilms as Mediators of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Mar. Biotechnol 2007, 9, 399–410. [Google Scholar]
- Olano, C; Lombo, F; Mendez, C; Salas, JA. Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metabol. Eng 2008, 10, 281–292. [Google Scholar]

| Microorganism | Compound | Biological activity | Reference |
|---|---|---|---|
| Cyanobacteria | |||
| Dolastatin 10 |  | Antimicrotubule; and the synthetic analogue, TZT-1027, as antitumor | |
| Dolastatin 15 | 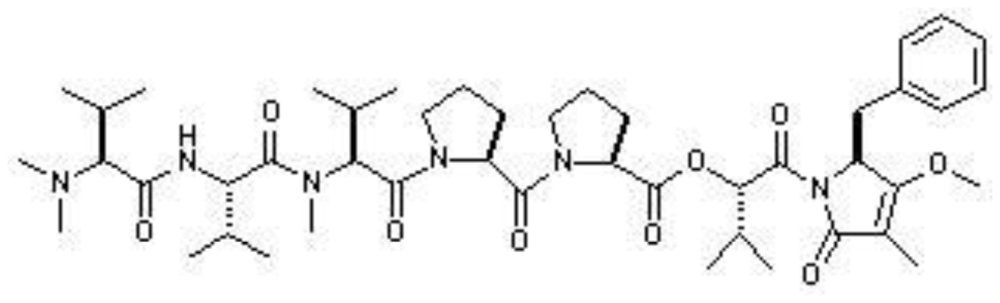 | Antimicrotubule; and the synthetic analogue, ILX-651, as antitumor | [7] |
| Curacin A |  | Antimicrotubule | |
| Toyocamycin | 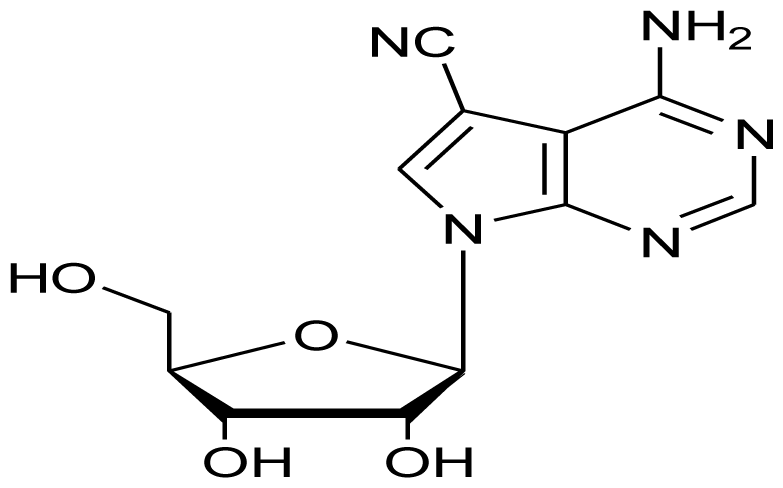 | Antifungal | [8] |
| Actinomycetes | |||
| Resistoflavine |  | Anticancerous and antibacterial | [9] |
| Marinomycin A | 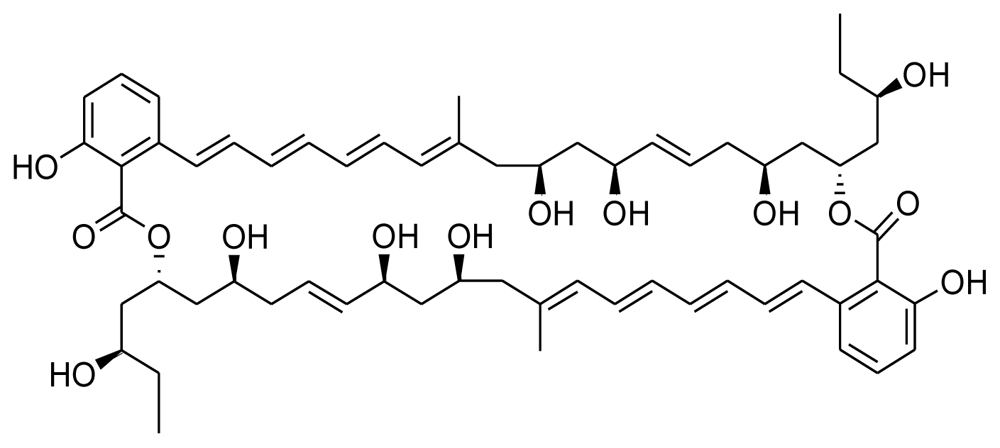 | Antitumor and antibiotic | [10] |
| Daryamide C |  | Antitumor | [11] |
| Violacein | 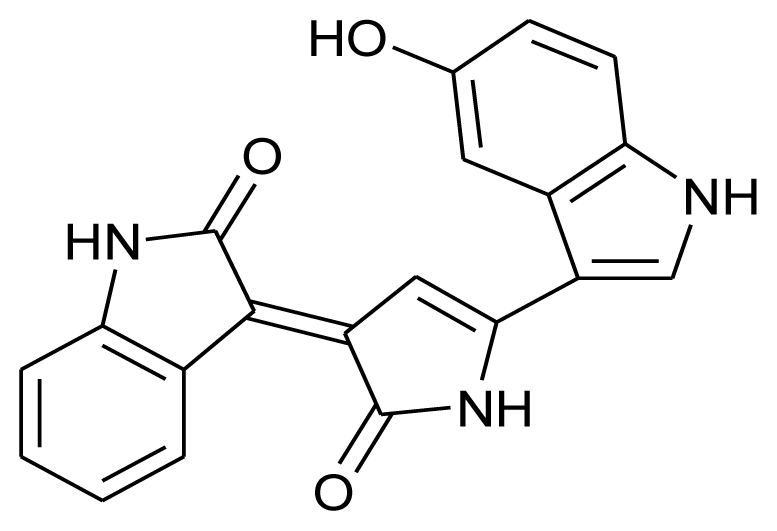 | Antiprotozoal | [12] |
| Bacteria | |||
| Macrolactin S | 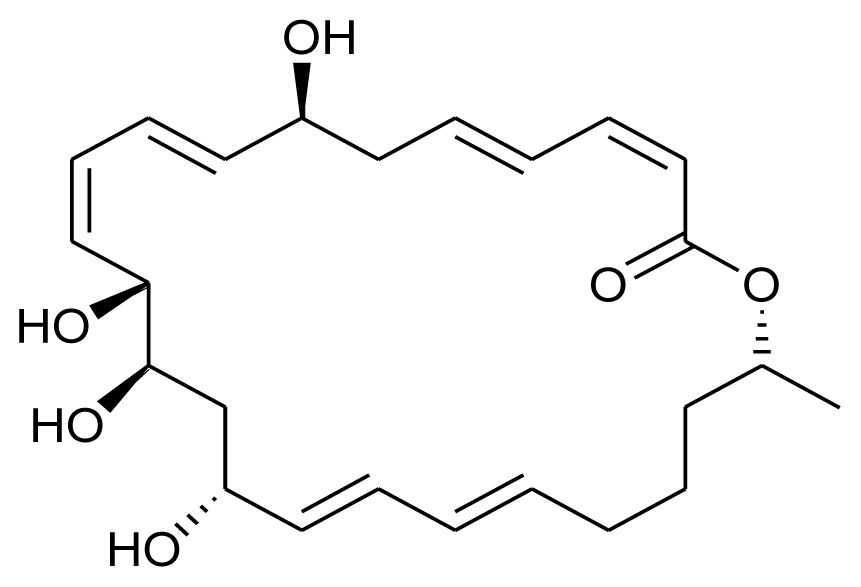 | Antibacterial | [13] |
| Pyrone I and II | 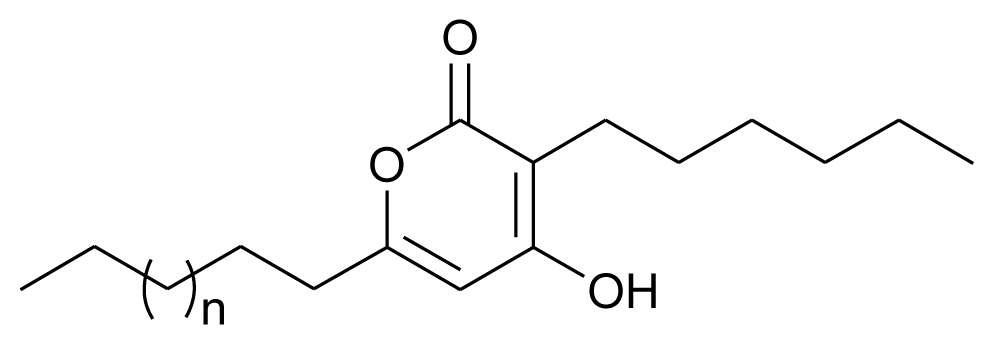 | Antibacterial | [14] |
| MC21-B |  | Antibacterial | [15] |
| Fungi | |||
| Meleagrin | 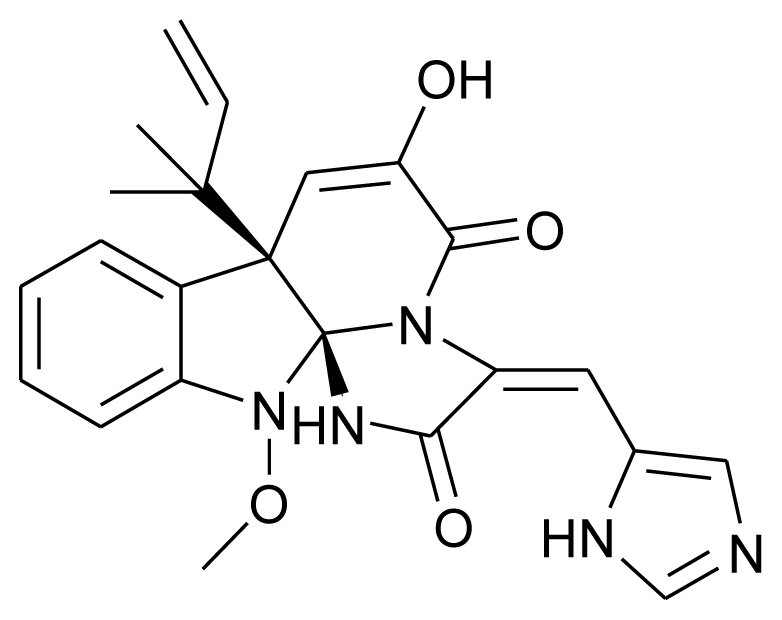 | Antitumor | [16] |
| Oxaline |  | Antitumor | [17] |
| Alternaramide |  | Antibacterial | [18] |
| Algae | |||
| Norharman | 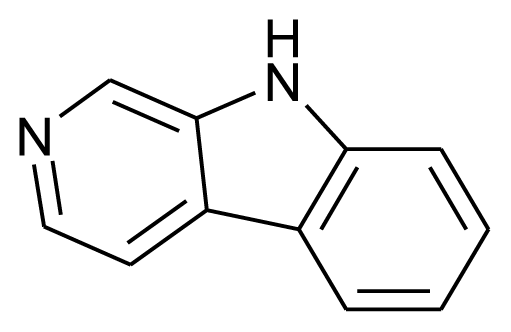 | Enzyme inhibitor | [19] |
| Calothrixin-A |  | Antimalarial and anticancerous | [20] |
| Eicosapentanoic acid (EPA) |  | Treats heart disease, Anti inflammatory agent (rheumatoid arthritis and immunodeficiency diseases) | [21] |
| Symbiotic microbes | |||
| Macrolactin V | 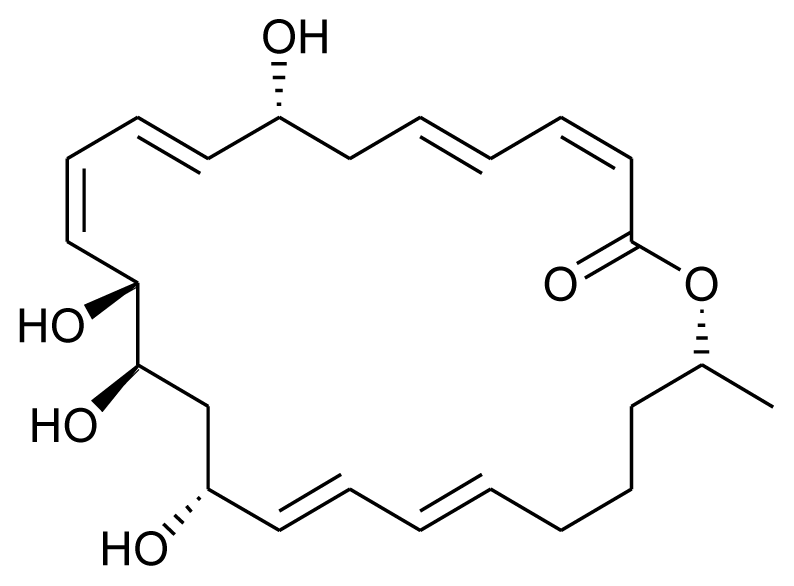 | Antibacterial and antilarval | [22] |
| DAPG | 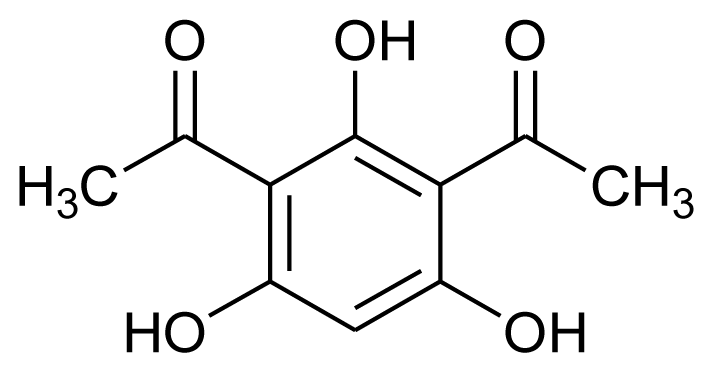 | Antibacterial (anti-MRSA, anti-VRSA and anti-VRE) | [23] |
| BE-43472B | 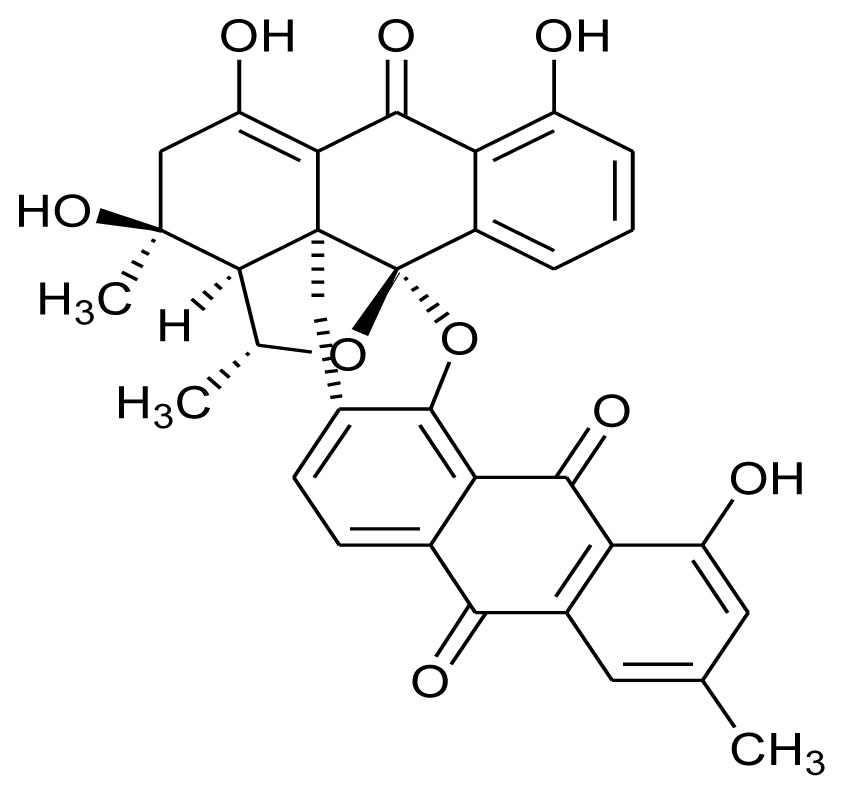 | Antibacterial (anti-MRSA and anti-VRE) | [24] |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bhatnagar, I.; Kim, S.-K. Immense Essence of Excellence: Marine Microbial Bioactive Compounds. Mar. Drugs 2010, 8, 2673-2701. https://doi.org/10.3390/md8102673
Bhatnagar I, Kim S-K. Immense Essence of Excellence: Marine Microbial Bioactive Compounds. Marine Drugs. 2010; 8(10):2673-2701. https://doi.org/10.3390/md8102673
Chicago/Turabian StyleBhatnagar, Ira, and Se-Kwon Kim. 2010. "Immense Essence of Excellence: Marine Microbial Bioactive Compounds" Marine Drugs 8, no. 10: 2673-2701. https://doi.org/10.3390/md8102673
APA StyleBhatnagar, I., & Kim, S.-K. (2010). Immense Essence of Excellence: Marine Microbial Bioactive Compounds. Marine Drugs, 8(10), 2673-2701. https://doi.org/10.3390/md8102673





