Marine Antitumor Drugs: Status, Shortfalls and Strategies
Abstract
:1. Introduction
2. Marine Drugs against Major Players in Cancer
2.1. MMP Inhibitors
2.2. HIF Inhibitors
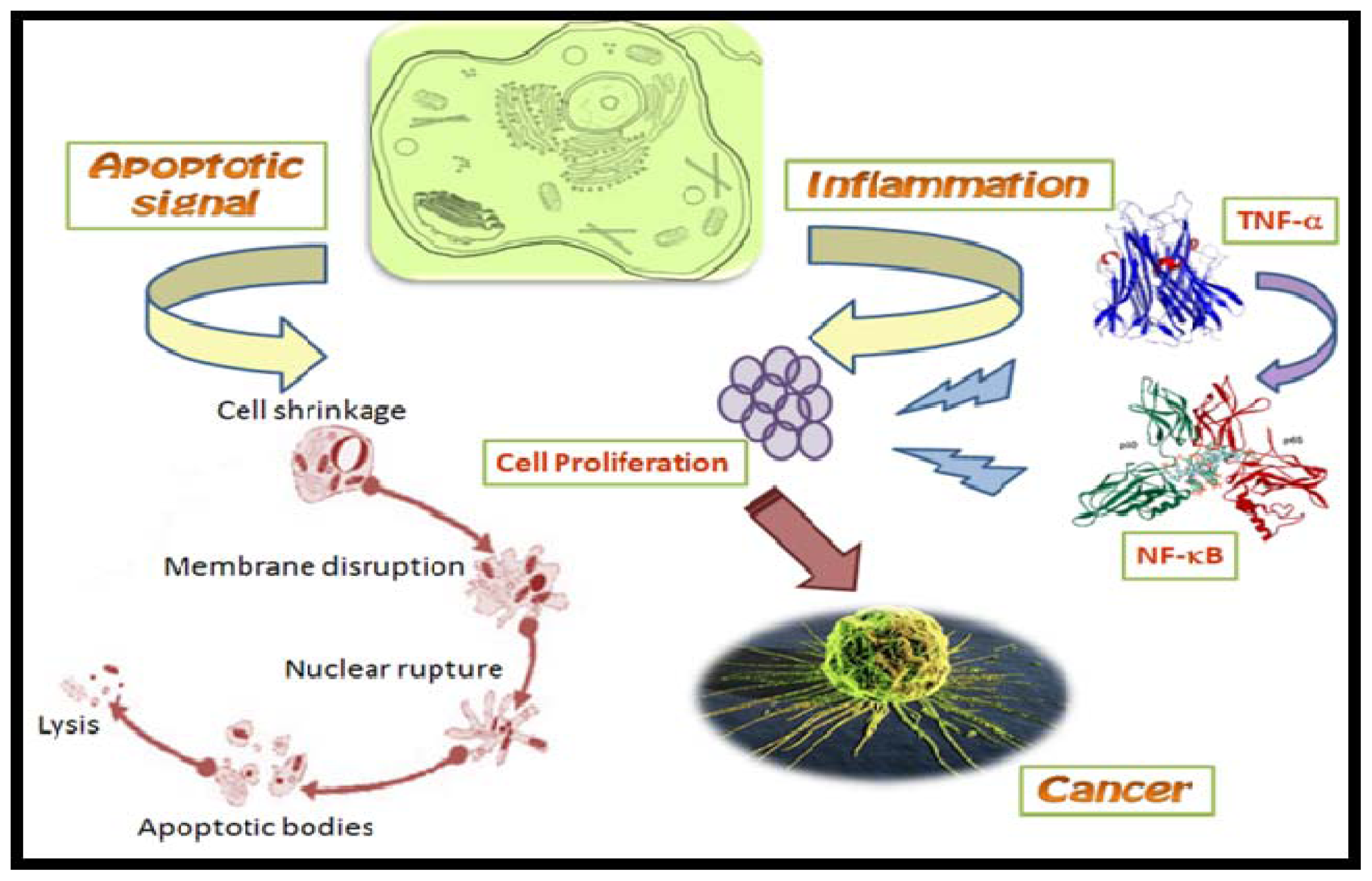
2.3. Nuclear Factor-κB Inhibitors
2.4. Topoisomerase Inhibitors
2.5. PKC Inhibitors
3. Clinical Status of Marine Derived Antitumor Drugs
4. Shortfalls in Marine Antitumor Drug Research
5. Strategies to Combat the Pitfalls
6. Conclusions
Acknowledgements
References
- da Rocha, AB; Lopes, RM; Schwartsmann, G. Natural products in anticancer therapy. Curr. Opin. Pharmacol 2001, 1, 364–369. [Google Scholar]
- McCullagh, M. Natural product pharmaceuticals-the third generation. Drug Disc. World. 2008. Available online: http://www.ddw-online.com/drug_discovery/258437/natural_product_pharmaceuticals_the_third_generation.html (accessed on 25 August 2009).
- Pallela, R; Yoon, N-Y; Kim, SK. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar]
- Mayer, AM; Rodríguez, AD; Berlinck, RG; Hamann, MT. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar]
- Hickford, SJ; Blunt, JW; Munro, MH. Antitumour polyether macrolides: Four new halichondrins from the New Zealand deep-water marine sponge Lissodendoryx sp. Bioorg. Med. Chem 2009, 17, 2199–2203. [Google Scholar]
- Aoki, S; Cao, L; Matsui, K; Rachmat, R; Akiyama, S-i; Kobayashi, M. Kendarimide A, a novel peptide reversing P-glycoprotein-mediated multidrug resistance in tumor cells, from a marine sponge of Haliclona sp. Tetrahedron 2004, 60, 7053–7059. [Google Scholar]
- Thomas, TRA; Kavlekar, DP; LokaBharathi, PA. Marine Drugs from Sponge-Microbe Association—A Review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar]
- Rodriguez, AD; Martinez, N. Marine antitumor agents: 14-deoxycrassin and pseudoplexaurol, new cembranoid diterpenes from the Caribbean gorgonian Pseudoplexaura porosa. Experientia 1993, 49, 179–181. [Google Scholar]
- Olano, C; Méndez, C; Salas, JA. Antitumor Compounds from Marine Actinomycetes. Mar. Drugs 2009, 7, 210–248. [Google Scholar]
- Hassan, HM; Khanfar, MA; Elnagar, AY; Mohammed, R; Shaala, LA; Youssef, DT; Hifnawy, MS; El Sayed, KA. Pachycladins A–E, prostate cancer invasion and migration inhibitory Eunicellin-based diterpenoids from the red sea soft coral Cladiella pachyclados. J. Nat. Prod 2010, 73, 848–853. [Google Scholar]
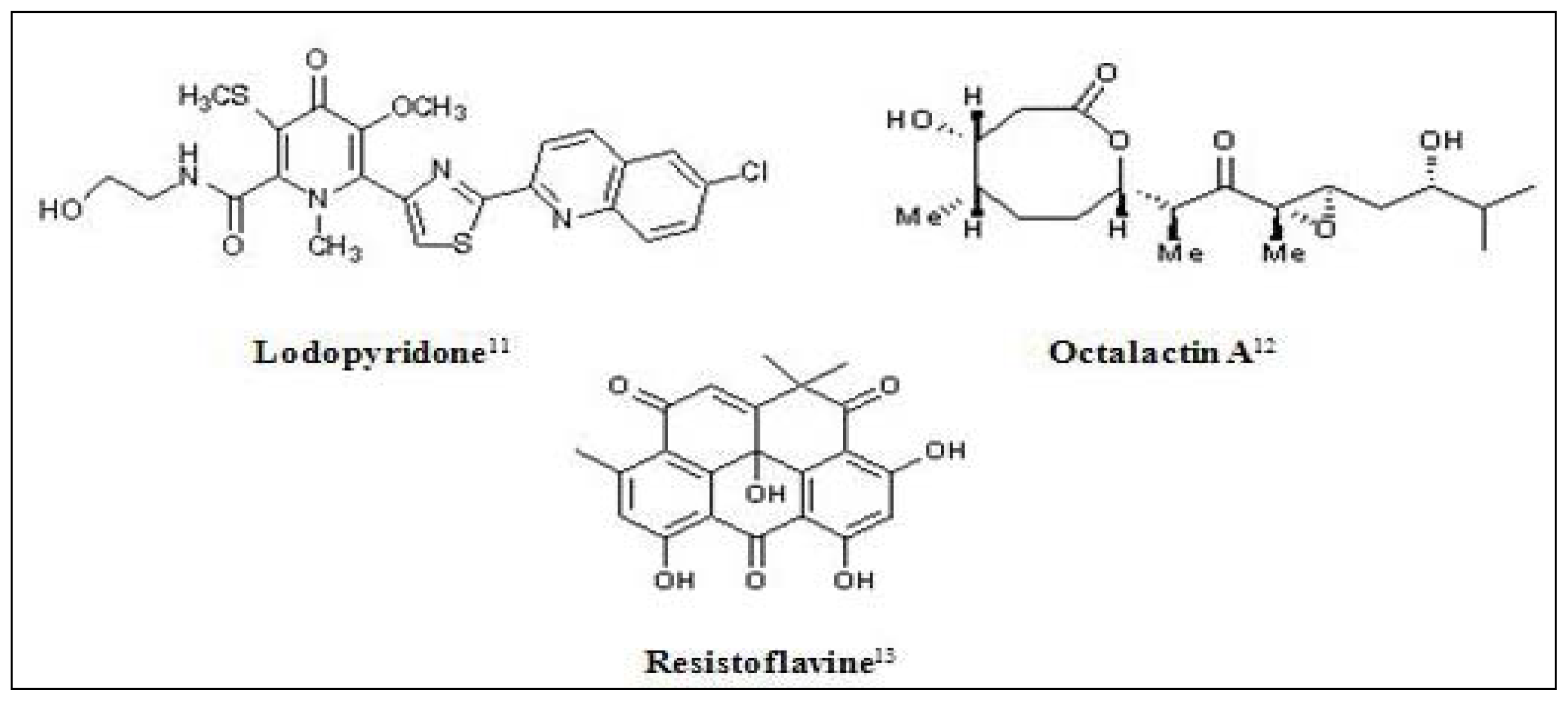
- Maloney, KN; Macmillan, JB; Kauffman, CA; Jensen, PR; Dipasquale, AG; Rheingold, AL; Fenical, W. Lodopyridone, a structurally unprecedented alkaloid from a marine actinomycete. Org. Lett 2009, 11, 5422–5424. [Google Scholar]
- Tapiolas, DM; Roman, M; Fenical, W; Stout, TJ; Clardy, J. Octalactins A and B: cytotoxic eight-membered-ring lactones from a marine bacterium, Streptomyces sp. J. Am. Chem. Soc 1991, 113, 4682–4683. [Google Scholar]
- Gorajana, A; Venkatesan, M; Vinjamuri, S; Kurada, VVSNB; Peela, S; Jangam, P; Poluri, E; Zeeck, A. Resistoflavine, cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. Microbiol. Res 2007, 162, 322–327. [Google Scholar]
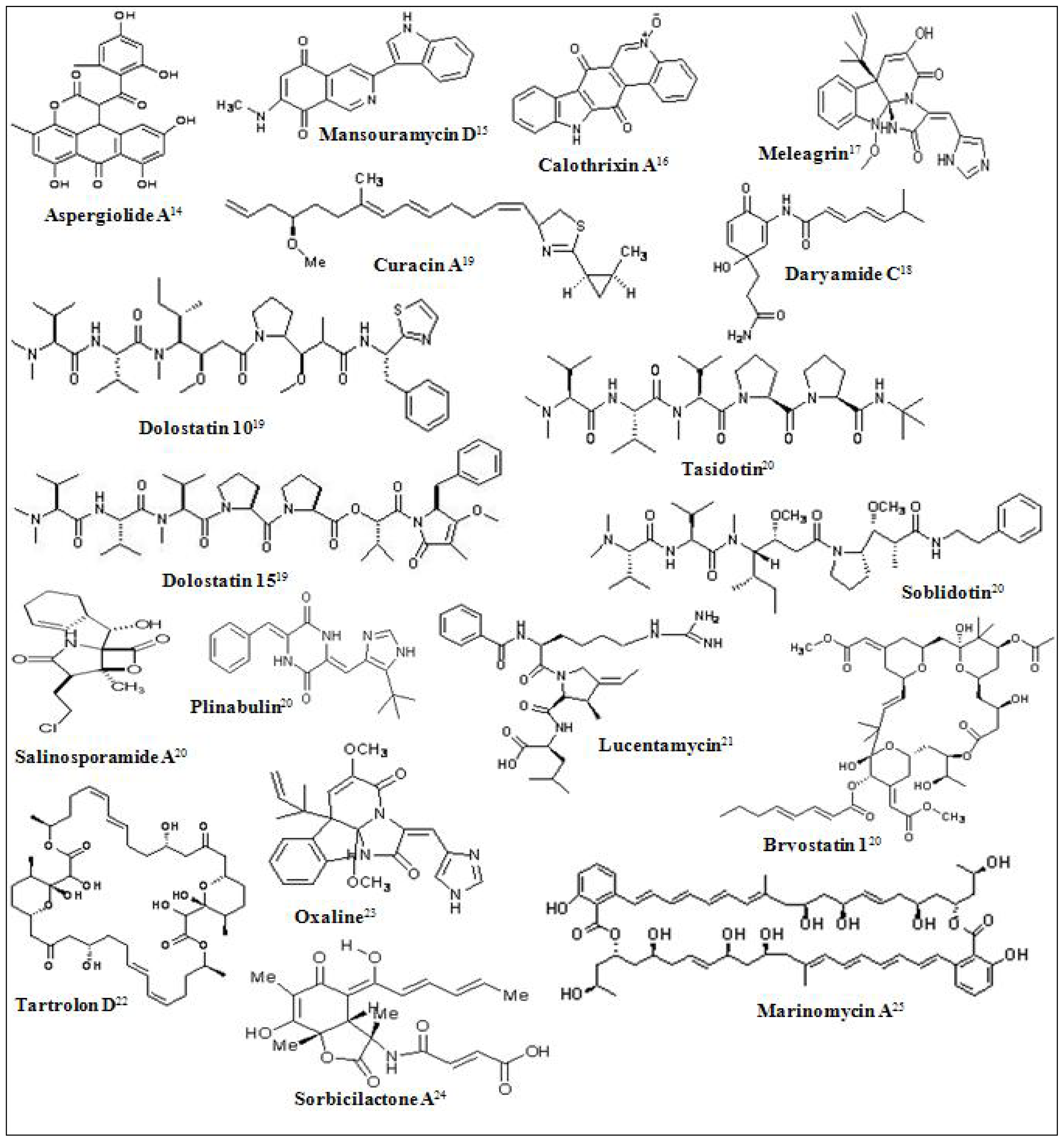
- Du, L; Zhu, T; Fang, Y; Liu, H; Gu, Q; Zhu, W. Aspergiolide A, a novel anthraquinone derivative with naphtho(1,2,3-de)chromene-2,7-dione skeleton isolated from a marine-derived fungus Aspergillus glaucus. Tetrahedron 2007, 63, 1085–1088. [Google Scholar]
- Hawas, UW; Shaaban, M; Shaaban, KA; Speitling, M; Maier, A; Kelter, G; Fiebig, HH; Meiners, M; Helmke, E; Laatsch, H. Mansouramycins A–D, cytotoxic isoquinolinequinones from a marine streptomycete. J. Nat. Prod 2009, 72, 2120–2124. [Google Scholar]
- Rickards, RW; Rothschild, JM; Willis, AC; de Chazal, NM; Kirk, J; Kirk, K; Saliba, KJ; Smith, GD. Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron 1999, 55, 13513. [Google Scholar]
- Du, L; Feng, T; Zhao, B; Li, D; Cai, S; Zhu, T; Wang, F; Xiao, X; Gu, Q. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities. J. Antibiot 2010, 63, 165–170. [Google Scholar]
- Asolkar, RN; Jensen, PR; Kauffman, CA; Fenical, W. Daryamides A–C, Weakly Cytotoxic Polyketides from a Marine-Derived Actinomycete of the Genus Streptomyces Strain CNQ-085. J. Nat. Prod 2006, 69, 1756–1759. [Google Scholar]
- Tan, LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar]
- Mayer, AMS; Glaser, KB; Cuevas, C; Jacobs, RS; Kem, W; Little, RD; McIntosh, JM; Newman, DJ; Potts, BC; Shuster, DE. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol. Sci 2010, 31(6), 255–265. [Google Scholar]
- Cho, JY; Williams, PG; Kwon, HC; Jensen, PR; Fenical, W. Lucentamycins A-D, cytotoxic peptides from the marine-derived actinomycete Nocardiopsis lucentensis. J. Nat. Prod 2007, 70, 1321–1328. [Google Scholar]
- Pérez, M; Crespo, C; Schleissner, C; Rodríguez, P; Zúñiga, P; Reyes, F. Tartrolon D, a cytotoxic macrodiolide from the marine-derived actinomycete Streptomyces sp. MDG-04-17-069. J. Nat. Prod 2009, 72, 2192–2194. [Google Scholar]
- Koizumi, Y; Arai, M; Tomoda, H; Omura, S. Oxaline, a fungal alkaloid, arrests the cell cycle in M phase by inhibition of tubulin polymerization. Biochim. Biophys. Acta 2004, 1693, 47–55. [Google Scholar]
- Bringmann, G; Gulder, TA; Lang, G; Schmitt, S; Stöhr, R; Wiese, J; Nagel, K; Imhoff, JF. Large-scale biotechnological production of the antileukemic marine natural product sorbicillactone A. Mar. Drugs 2007, 5, 23–30. [Google Scholar]
- Kwon, HC; Kauffman, CA; Jensen, PR; Fenical, W. Marinomycins A–D, Antitumor- Antibiotics of a New Structure Class from a Marine Actinomycete of the Recently Discovered Genus “Marinispora”. J. Am. Chem. Soc 2006, 128, 1622–1632. [Google Scholar]
- Lee, M; Murphy, G. Matrix metalloproteinases at a glance. J. Cell Sci 2004, 117, 4015–4016. [Google Scholar]
- Gill, S; Parks, W. Metalloproteinases and their inhibitors: regulators of wound healing. Int. J. Biochem. Cell Biol 2008, 40, 1334–1347. [Google Scholar]
- Egeblad, M; Werb, Z. New functions for the matrix metalloproteinase in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar]
- Overall, CM; Lopez-Otin, C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar]
- Chen, Z; Kim, SK. Matrix Metalloproteinase Inhibitors (MMPIs) from Marine Natural Products: the Current Situation and Future Prospects. Mar. Drugs 2009, 7, 71–84. [Google Scholar]
- Kim, MM; Kim, SK. Chitooligosaccharides inhibit activation and expression of matrix metalloproteinase-2 in human dermal fibroblasts. FEBS Lett 2006, 580, 2661–2666. [Google Scholar]
- Van Ta, Q; Kim, MM; Kim, SK. Inhibitory effect of chitooligosaccharides on matrix metalloproteinase-9 in human fibrosarcoma cells (HT1080). Mar. Biotechnol 2006, 8, 593–599. [Google Scholar]
- Rajapakse, N; Kim, MM; Mendis, E; Huang, RH; Kim, SK. Carboxylated chitooligosaccharides (CCOS) inhibit MMP-9 expression in human fibrosarcoma cells via downregulation of AP-1. Biochim. Biophys. Acta 2006, 1760, 1780–1788. [Google Scholar]
- Wang, SB; Cheng, YN; Wang, FS; Sun, LR; Liu, CH; Chen, GJ; Li, YH; Ward, SG; Qu, XJ. Inhibition activity of sulfated polysaccharide of Sepiella maindroni ink on matrix metalloproteinase (MMP)-2. Biomed. Pharmacother 2008, 62, 297–302. [Google Scholar]
- Kim, MM; van Ta, Q; Mendis, E; Rajapakse, N; Jung, WK; Byun, HG; Jeon, YJ; Kim, SK. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci 2006, 79, 1436–1443. [Google Scholar]
- Semenza, GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol. Med 2002, 8(Suppl), S62–S67. [Google Scholar]
- Hockel, M; Vaupel, P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst 2001, 93, 266–276. [Google Scholar]
- Semenza, GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol. Med 2001, 7, 345–350. [Google Scholar]
- Kung, AL; Wang, S; Klco, JM; Kaelin, WG; Livingston, DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat. Med 2000, 6, 1335–1340. [Google Scholar]
- Mohammed, KA; Hossain, CF; Zhang, L; Bruick, RK; Zhou, YD; Nagle, DG. Laurenditerpenol, a New Diterpene from the Tropical Marine Alga Laurencia intricata that Potently Inhibits HIF-1 Mediated Hypoxic Signaling in Breast Tumor Cells. J. Nat. Prod 2004, 67, 2002–2007. [Google Scholar]
- Liu, R; Liu, Y; Zhou, YD; Nagle, DG. Molecular-Targeted Antitumor Agents 15: Neolamellarins from the Marine Sponge Dendrilla nigra Inhibit Hypoxia-Inducible Factor-1 (HIF-1) Activation and Secreted Vascular Endothelial Growth Factor (VEGF) Production in Breast Tumor Cells. J. Nat. Prod 2007, 70, 1741–1745. [Google Scholar]
- Liu, Y; Liu, R; Mao, SC; Morgan, JB; Jekabsons, MB; Zhou, YD; Nagle, DG. Molecular- Targeted Antitumor Agents. 19. Furospongolide from a Marine Lendenfeldia sp. Sponge Inhibits Hypoxia-Inducible Factor-1 Activation in Breast Tumor Cells. J. Nat. Prod 2008, 71, 1854–1860. [Google Scholar]
- Dai, J; Liu, Y; Jia, H; Zhou, YD; Nagle, DG. Benzochromenones from the marine crinoid Comantheria rotula inhibit hypoxia-inducible factor-1 (HIF-1) in cell-based reporter assays and differentially suppress the growth of certain tumor cell lines. J. Nat. Prod 2007, 70, 1462–1466. [Google Scholar]
- Hodges, TW; Hossain, CF; Kim, YP; Zhou, YD; Nagle, DG. Molecular-Targeted Antitumor Agents: The Saururus cernuus Dineolignans Manassantin B and 4-O-Demethylmanassantin B Are Potent Inhibitors of Hypoxia-Activated HIF-1. J. Nat. Prod 2004, 67, 767–771. [Google Scholar]
- Nagle, DG; Zhou, YD. Marine natural products as inhibitors of hypoxic signaling in tumors. Phytochem. Rev 2009, 8, 415–429. [Google Scholar]
- Keutgens, A; Robert, I; Viatour, P; Chariot, A. Deregulated NF-kB activity in haemological malignancies. Biochem. Pharmacol 2006, 72, 1069–1080. [Google Scholar]
- Baldwin, AS. The NF-κB and I κB proteins: new discoveries and insights. Annu. Rev. Immunol 1996, 14, 649–683. [Google Scholar]
- Folmer, F; Jaspars, M; Dicato, M; Diederich, M. Marine natural products as targeted modulators of the transcription factor NF-kappa B. Biochem. Pharmacol 2008, 75, 603–617. [Google Scholar]
- Feling, RH; Buchanan, GO; Mincer, TJ; Kauffman, CA; Jensen, PJ; Fenical, W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem 2003, 42, 355–357. [Google Scholar]
- Asolkar, RN; Freel, KC; Jensen, PR; Fenical, W; Kondratyuk, TP; Park, EJ; Pezzuto, JM. Arenamides A-C, cytotoxic NFkappaB inhibitors from the marine actinomycetes Salinispora arenicola. J. Nat. Prod 2009, 72, 396–402. [Google Scholar]
- Nam, SJ; Gaudêncio, SP; Kauffman, CA; Jensen, PR; Kondratyuk, TP; Marler, LE; Pezzuto, JM; Fenical, W. Fijiolides A and B, inhibitors of TNF-alpha-induced NFkappaB activation, from a marine-derived sediment bacterium of the genus Nocardiopsis. J. Nat. Prod 2010, 73, 1080–1086. [Google Scholar]
- Schumacher, M; Cerella, C; Eifes, S; Chateauvieux, S; Morceau, F; Jaspars, M; Dicato, M; Diederich, M. Heteronemin, a spongean sesterterpene, inhibits TNF alpha-induced NF-kappa B activation through proteasome inhibition and induces apoptotic cell death. Biochem. Pharmacol 2010, 79, 610–622. [Google Scholar]
- Champoux, JJ. DNA Topoisomerases: Structure, Function, and Mechanism. Ann. Rev. Biochem 2001, 70, 369–413. [Google Scholar]
- Dias, N; Vezin, H; Lansiaux, A; Bailly, C. Topoisomerase Inhibitors of Marine Origin and Their Potential Use as Anticancer Agents. Top. Curr. Chem 2005, 253, 89–108. [Google Scholar]
- Bonnard, I; Bontemps, N; Lahmy, S; Banaigs, B; Combaut, G; Francisco, C; Colson, P; Houssier, C; Waring, MJ; Bailly, C. Binding to DNA and cytotoxic evaluation of ascididemin, the major alkaloid from the Mediterranean ascidian Cystodytes dellechiajei. Anticancer Drug Des 1995, 10, 333–346. [Google Scholar]
- Dassonneville, L; Wattez, N; Baldeyrou, B; Mahieu, C; Lansiaux, A; Banaigs, B; Bonnard, I; Bailly, C. Inhibition of topoisomerase II by the marine alkaloid ascididemin and induction of apoptosis in leukemia cells. Biochem Pharmacol 2000, 60(4), 527–537. [Google Scholar]
- Tardy, C; Facompré, M; Laine, W; Baldeyrou, B; García-Gravalos, D; Francesch, A; Mateo, C; Pastor, A; Jiménez, J.A; Manzanares, I; Cuevas, C; Bailly, C. Topoisomerase I-mediated DNA cleavage as a guide to the development of antitumor agents derived from the marine alkaloid lamellarin D: triester derivatives incorporating amino acid residues. Bioorg. Med. Chem 2004, 12(7), 1697–1712. [Google Scholar]
- Gorin, MA; Pan, Q. Protein kinase Cε: an oncogene and emerging tumor biomarker. Mol. Cancer 2009, 8, 9. [Google Scholar]
- Pettit, GR; Herald, CL; Doubek, DL; Herald, DL; Arnold, E; Clardy, J. Isolation and structure of bryostatin 1. J. Am. Chem. Soc 1982, 104, 6846–6848. [Google Scholar]
- Mutter, R; Wills, M. Chemistry and Clinical Biology of the Bryostatins. Bioorg. Med. Chem 2000, 8, 1841–1860. [Google Scholar]
- Kortmansky, J; Schwartz, GK. Bryostatin-1: A novel PKC inhibitor in clinical development. Cancer Invest 2003, 21, 924–936. [Google Scholar]
- Keck, GE; Poudel, YB; Welch, DS; Kraft, MB; Truong, AP; Stephens, JC; Kedei, N; Lewin, NE; Blumberg, PM. Substitution on the A-ring confers to bryopyran analogues the unique biological activity characteristic of bryostatins and distinct from that of the phorbol esters. Org. Lett 2009, 11, 593–596. [Google Scholar]
- Jackson, KL; Henderson, JA; Phillips, AJ. The Halichondrins and E7389. Chem. Rev 2009, 109(7), 3044–3079. [Google Scholar]
- Okouneva, T; Azarenko, O; Wilson, L; Littlefield, BA; Jordan, MA. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol. Cancer Ther 2008, 7, 2003–2011. [Google Scholar]
- Watanabe, J; Natsume, T; Kobayashi, M. Comparison of the antivascular and cytotoxic activities of TZT-1027 (Soblidotin) with those of other anticancer agents. Anticancer Drugs 2007, 18, 905–911. [Google Scholar]
- Mitsiades, CS; Ocio, EM; Pandiella, A; Maiso, P; Gajate, C; Garayoa, M; Vilanova, D; Montero, J; Mitsiades, N; McMullan, CJ; Munshi, NC; Hideshima, T; Chauhan, D; Aviles, P; Otero, G; Faircloth, G; Mateos, MV; Richardson, PG; Mollinedo, F; San-Miguel, JF; Anderson, KC. Aplidin, a marine organism-derived compound with potent antimyeloma activity in vitro and in vivo. Cancer Res 2008, 68, 5216–5225. [Google Scholar]
- Kanoh, K; Kohno, S; Asari, T; Harada, T; Katada, J; Muramatsu, M; Kawashima, H; Sekiya, H; Uno, I. (−)-Phenylahistin: A new mammalian cell cycle inhibitor produced by Aspergillus ustus. Bioorg. Med. Chem. Lett 1997, 7, 2847–2852. [Google Scholar]
- Nicholson, B; Lloyd, GK; Miller, BR; Palladino, MA; Kiso, Y; Hayashi, Y; Neuteboom, ST. NPI-2358 is a tubulin-depolymerizing agent: in-vitro evidence for activity as a tumor vascular-disrupting agent. Anticanc. Drugs 2006, 17, 25–31. [Google Scholar]
- Davidson, SK; Allen, SW; Lim, GE; Anderson, CM; Haygood, MG. Evidence for the biosynthesis of bryostatins by the bacterial symbiont "Candidatus Endobugula sertula" of the bryozoan Bugula neritina. Appl. Environ. Microbiol 2001, 67, 4531–4537. [Google Scholar]
- Thakur, NL; Thakur, AL. Marine Biotechnology: an overview. Ind. J. Biotechnol 2006, 5, 263–268. [Google Scholar]
- Nereus Pharmaceuticals homepage. Available online: http://www.nereuspharm.com/overview.shtml (accessed on 02 July 2010).
- Schwartsmann, G; Da Rocha, AB; Berlinck, RG; Jimeno, J. Marine organisms as a source of new anticancer agents. Lancet Oncol 2001, 2, 221–225. [Google Scholar]
- Wang, Y; Lyu, YL; Wang, JC. Dual localization of human DNA topoisomerase IIIalpha to mitochondria and nucleus. Proc. Natl. Acad. Sci. USA 2002, 99, 12114–12119. [Google Scholar]
- Goulaouic, H; Roulon, T; Flamand, O; Grondard, L; Lavelle, F; Riou, JF. Purification and characterization of human DNA topoisomerase III alpha. Nucleic Acids Res 1999, 27, 2443–2450. [Google Scholar]
- Swami, M. Hypoxia: The HIF2α puzzle. Nat. Rev. Cancer 2010, 10, 603. [Google Scholar]
- Lam, KS. Discovery of novel metabolites from marine actinomycetes. Curr. Opin. Microbiol 2006, 9, 245–251. [Google Scholar]
- Bull, AT; Ward, AC; Goodfellow;, M. Search and discovery strategies for biotechnology: the paradigm shift. Microbiol. Mol. Biol. Rev 2000, 64, 573–606. [Google Scholar]
- Hanash, S; Taguchi, A. The grand challenge to decipher the cancer proteome. Nat. Rev. Cancer 2010, 10, 652–660. [Google Scholar]
- Chittiboyina, AG; Kumar, GM; Carvalho, PB; Liu, Y; Zhou, YD; Nagle, DG; Avery, MA. Total synthesis and absolute configuration of laurenditerpenol: a HIF-1 activation inhibitor. J. Med. Chem 2007, 50, 6299–6302. [Google Scholar]
- Jung, ME; Im, GYJ. Convergent total synthesis of the racemic HIF-1 inhibitor laurenditerpenol. Tetrahedron Lett 2008, 49, 4962–4964. [Google Scholar]
- Bhadury, P; Mohammad, BT; Wright, PC. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol 2006, 33, 325–337. [Google Scholar]
- Newman, DJ; Hill, RT. New drugs from marine microbes: the tide is turning. J. Ind. Microbiol. Biotechnol 2006, 33, 539–544. [Google Scholar]
- Belofsky, GN; Jensen, PR; Fenical, W. Sansalvamide: A new cytotoxic cyclic depsipeptide produced by a marine fungus of the genus Fusarium. Tetrahedron Lett 1999, 40, 2913–2916. [Google Scholar]
- Raghukumar, C. Marine fungal biotechnology: an ecological perspective. Fungal Divers 2008, 31, 19–35. [Google Scholar]
- Moradali, MF; Mostafavi, H; Ghods, S; Hedjaroude, GA. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharmacol 2007, 7, 701–724. [Google Scholar]
- Xie, S; Xiao, J; Xu, J. Advance in microbial ribosome engineering. Wei Sheng Wu Xue Bao 2009, 49, 981–986. [Google Scholar]
- Andersen, JN; Sathyanarayanan, S; Di Bacco, A; Chi, A; Zhang, T; Chen, AH; Dolinski, B; Kraus, M; Roberts, B; Arthur, W; Klinghoffer, RA; Gargano, D; Li, L; Feldman, I; Lynch, B; Rush, J; Hendrickson, RC; Blume-Jensen, P; Paweletz, CP. Pathway-based identification of biomarkers for targeted therapeutics: Personalized oncology with PI3K pathway inhibitors. Sci. Transl. Med 2010, 2, 43ra55. [Google Scholar]
- Borkakoti, N. Matrix metalloprotease inhibitors: design from structure. Biochem. Soc. Transactions 2004, 32, 17–20. [Google Scholar]
 represents Topoisomerase enzyme and
represents Topoisomerase enzyme and  represents Topoisomerase inhibitor.
represents Topoisomerase inhibitor.
 represents Topoisomerase enzyme and
represents Topoisomerase enzyme and  represents Topoisomerase inhibitor.
represents Topoisomerase inhibitor.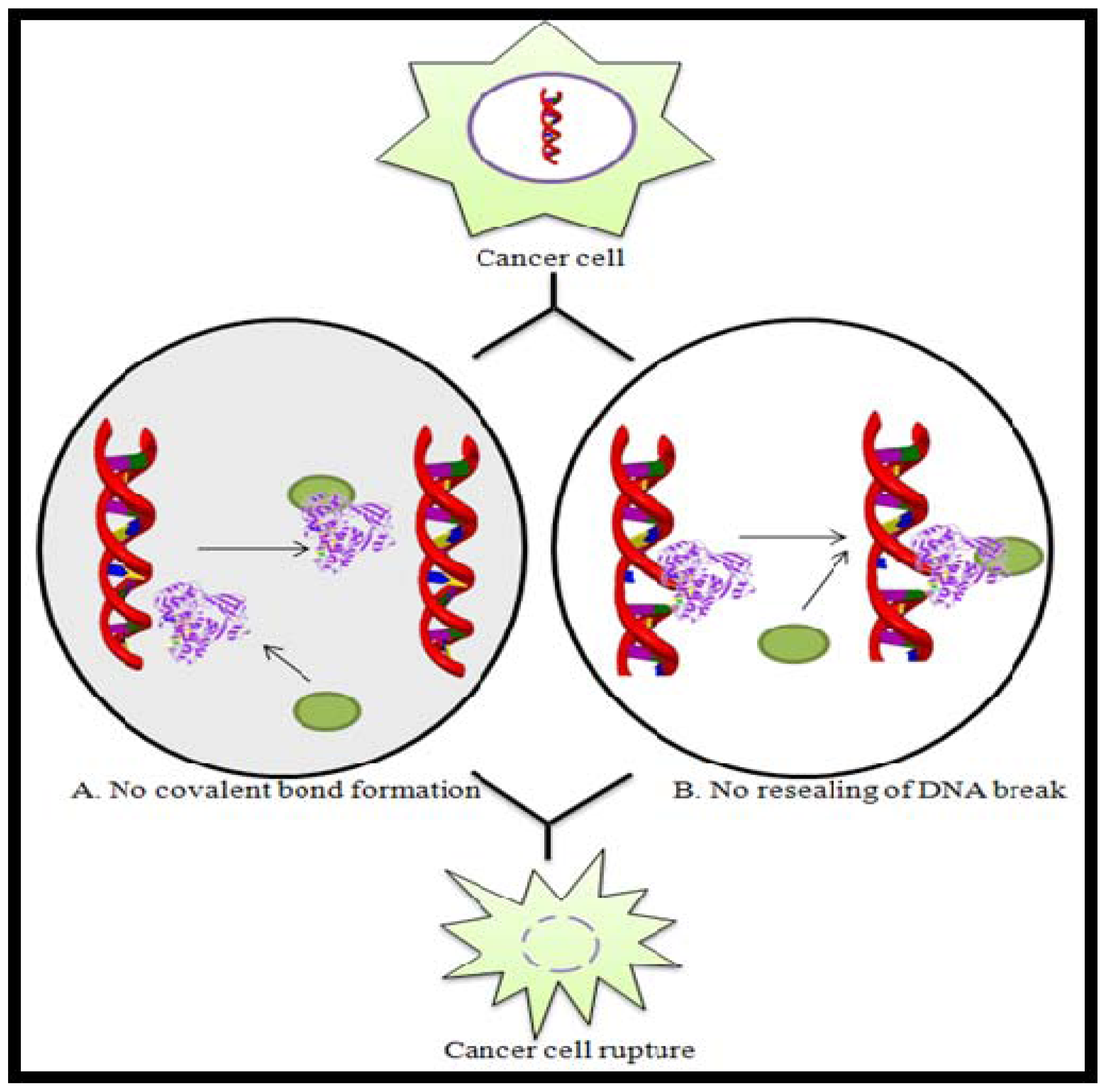
| Compound Name | Chemical Class | Organism | Mode of action | Company | Status |
|---|---|---|---|---|---|
| Cytarabine, Ara-C | Nucleoside | Sponge | DNA Polymerase Inhibitor | Bedford, Enzon | Approved |
| Trabectedin (ET-743) | Alkaloid | Tunicate | Cell cycle arrest | PharmaMar | Approved |
| Eribulin Mesylate (E7389) | Macrolide | Sponge | Microtubule interfering agent | Eisai Inc. | Phase III |
| Soblidotin (TZT 1027) | Peptide | Bacterium | Microtubule interfering and vascular disrupting agent | Aska Pharmaceuticals | Phase III |
| Squalamine lactate | Aminosteroid | Shark | Calcium binding protein antagonist | Genaera | Phase II |
| Cemadotin | Peptide | Sea slug | Microtubule interfering agent | Knoll | Phase II |
| Plinabulin (NPI-2358) | Diketopiperazine | Fungus | Vascular disrupting agent | Nereus Pharmaceuticals | Phase II |
| Plitidepsin | Depsipeptide | Tunicate | Apoptosis inducer | PharmaMar | Phase II |
| Elisidepsin | Depsipeptide | Mollusc | - | PharmaMar | Phase II |
| Zalypsis | Alkaloid | Nudibranch | Cell cycle arrest | PharmaMar | Phase II |
| Tasidotin, Synthadotin (ILX-651) | Peptide | Bacterium | Microtubule interfering agent | Genzyme Corporation | Phase II |
| Discodermolide | Polyketide | Sponge | Microtubule interfering agent | Novartis | Phase I |
| HT1286 | Dipeptide | Sponge | Microtubule interfering agent | Wyeth | Phase I |
| LAF389 | Amino acid derivative | Sponge | Methionine aminopeptidase inhibitor | Novartis | Phase I |
| Kahalalide F | Cyclic depsipeptide | Sea slug/alga | Lysosomotropic | PharmaMar | Phase I |
| KRN7000 | α-galactosylceramide | Sponge | Immunostimulatory | Kirin | Phase I |
| Bryostatin 1 | Polyketide | Bacterium/Bryozoa | PKC isozyme inhibitor | National Cancer Institute | Phase I |
| Hemiasterlin (E7974) | Tripeptide | Sponge | Microtubule interfering agent | Eisai Inc. | Phase I |
| Marizomib, Salinosporamide A; NPI-0052) | Beta-lactone-gamma lactam | Bacterium | Proteasome inhibitor | Nereus Pharmaceuticals | Phase I |
| LY355703, CRYPTO 52 | Cryptophycin | Cyanobacterium | Microtubule interfering agent | - | Preclinical |
| Depsipeptide (NSC 630176) | Bicyclic peptide | Cyanobacterium | Histone deacetylase inhibitor | - | Preclinical |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bhatnagar, I.; Kim, S.-K. Marine Antitumor Drugs: Status, Shortfalls and Strategies. Mar. Drugs 2010, 8, 2702-2720. https://doi.org/10.3390/md8102702
Bhatnagar I, Kim S-K. Marine Antitumor Drugs: Status, Shortfalls and Strategies. Marine Drugs. 2010; 8(10):2702-2720. https://doi.org/10.3390/md8102702
Chicago/Turabian StyleBhatnagar, Ira, and Se-Kwon Kim. 2010. "Marine Antitumor Drugs: Status, Shortfalls and Strategies" Marine Drugs 8, no. 10: 2702-2720. https://doi.org/10.3390/md8102702
APA StyleBhatnagar, I., & Kim, S.-K. (2010). Marine Antitumor Drugs: Status, Shortfalls and Strategies. Marine Drugs, 8(10), 2702-2720. https://doi.org/10.3390/md8102702





